Abstract
Purpose
Health-related quality of life (HRQoL) is a significant prognostic factor for overall survival in hepatocellular carcinoma (HCC) patients, and this is independent of stage and liver function. Inflammation plays a significant role in HCC development and progression. It was hypothesized that the inflammatory status of HCC patients may affect their HRQoL. The relationship between HRQoL and inflammatory status was explored using indicators IL-8 level and modified inflammation-based index (mIBI, based on IL-8, C-reactive protein, and albumin).
Methods
From 2007–2011, HCC patients were enrolled prospectively. Baseline HRQoL assessment utilized the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-HCC18; clinical and laboratory data were collected at diagnosis. Two summary indices, C30 and HCC18 index-scores, were calculated. Correlation analyses were performed between HRQoL and inflammatory markers.
Results
In the 445 patients studied, significant correlations were found between IL-8 levels and EORTC QLQ-C30, QLQ-HCC18, C30, and HCC18 index-scores. The strongest correlated factors were those reflective of constitutional symptoms, namely QLQ-C30 “appetite loss” (with Pearson’s correlation coefficient, r=0.322, P<0.0001); QLQ-C30 “fatigue” (r=0.311, P<0.0001); QLQ-C30 “role functioning” (r=−0.305, P<0.0001); QLQ-HCC18 “nutrition” (r=0.317, P<0.0001); and QLQ-HCC18 “fatigue” (r=0.306, P<0.0001). In addition, moderate but significant correlations were also observed with HCC18 index score (r=0.321, P<0.0001), and C30 index score (r=0.306, P<0.0001). HRQoL factors were also significantly correlated with mIBI.
Conclusion
Baseline HRQoL using the conventional assessments of EORTC QLQ-C30 and QLQ-HCC18, as well as C30 and HCC18 index-scores, significantly correlated with inflammatory indicators (IL-8 level and mIBI) in HCC patients. Among the strongest correlations were those between IL-8 level and the two index-scores, as well as HRQoL aspects that represent constitutional symptoms. When paralleled with molecular findings, traditional HRQoL assessment in HCC has gained a new level of understanding: pattern recognition within an HRQoL instrument could potentially identify patients with more severe inflammatory state.
Introduction
Hepatocellular carcinoma (HCC) is an aggressive cancer, which carries a high morbidity and mortality. It is the fifth commonest cancer and the second leading cause of cancer death in the world.Citation1 In early days, when HCC treatment was limited, the prognosis of HCC patients was poor, with median survival of 2–4 months, albeit with a wide survival range from 0.1–65 months.Citation2–Citation5 Various factors have been reported to be of prognostic significance for HCC. Of these, two emerging factors are health-related quality of life (HRQoL)Citation6–Citation10 and inflammatory markers.Citation11
Inflammation plays a significant role in HCC development and progression.Citation12 In HCC, an immunosuppressive environment has been demonstrated.Citation12 Tumor-promoting inflammatory cells, including myeloid-derived suppressive cells (MDSC) and regulatory T cells, and tumor-promoting microenvironment have been reported to foster cancer cells immune evasion, proliferation, invasiveness, and angiogenesis.Citation13 Predominant Th2-like cytokines (IL-4, IL-8, IL-10, and IL-5) compared to Th1-like cytokines (IL-1α, IL-1β, IL-2, tumor necrosis factor α) in the tumor microenvironment was associated with a more aggressive form of HCC.Citation14 HCC patients with higher MDSC counts were found to have higher serum IL-10, IL-13, and vascular endothelial growth factor levels. These patients have been reported to have more advanced stage HCC, poorer liver functional reserve, and shorter overall survival (OS).Citation15 On the contrary, HCC patients with an inflammatory microenvironment with the presence of immune cells infiltration and an expression of innate immune response genes within their resected tumors were shown to have more favorable prognosis with longer survival than those without such an inflammatory microenvironment.Citation16
It is unknown if tumor-promoting or tumor-inhibitory inflammatory responses would result in systemic manifestation along with elevation in inflammatory markers including serum cytokine levels, thereby affecting patients’ constitutional symptoms. For instance, a higher serum IL-6 level has been associated with shorter survival in one study;Citation17 however, another study has reported conflicting results.Citation18 On the other hand, higher serum levels of IL-8, IL-35, fibroblast growth factor 2, growth-regulated oncogene, interferon gamma-induced protein 10, vascular endothelial growth factor, as well as lower serum levels of interferon alpha-2 have also been reported to be associated with shorter relapse-free and OS in HCC patients.Citation19,Citation20 Various studies have explored the prognostic significance of a panel of cytokines in HCC patients, one of these studies was from our group, who evaluated 42 cytokines. IL-8 has been consistently found to be the only significant prognostic cytokine for survival.Citation21–Citation23
HRQoL is a multifaceted and complex assessment of human life with special attention paid to healthcare-related issues. Earlier reports, as well as more recent studies, have shown HRQoL to be a prognostic factor for patients with early as well as advanced stage HCC.Citation6–Citation9 The incorporation of HRQoL data into existing staging systems has been suggested to further enhance their prognostication powers.Citation7,Citation8,Citation24 In our recently reported cohort, the prognostic significance of baseline HRQoL at HCC presentation was independent of clinical variables including the stage of HCC, liver function, and patient’s demographic data.Citation9 The HRQoL factors that were analyzed included a general HRQoL instrument for cancer patients, the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30, as well as HCC-specific HRQoL instrument, QLQ-HCC18. Apart from conventional assessment using these instruments, our proposed C30 index score and HCC18 index score, which were, respectively, summative scores of all the domains and items of QLQ-C30 and QLQ-HCC18, were also found to be significant prognosticators.Citation9 These index scores have the advantage of transforming complex HRQoL data into simpler tools that are applicable to routine daily clinical practice.
A few recent reports have suggested that correlations exist between HRQoL and cytokine levels in patients with advanced malignancies.Citation25–Citation29 Although the reason for such a correlation has not yet been elucidated, cancer-induced cytokine release as a result of inflammatory response has been implicated in cachexia.Citation30 Alongside functional impairment, cachexia is associated with a variety of symptoms including malaise, anorexia, and weight loss. We hypothesized that HRQoL impairment in HCC patients may be correlated with their status of inflammation. In this study among HCC patients, the objectives were to determine the correlations between HRQoL and inflammatory status of individual patients. The latter was determined by IL-8 level and the modified inflammation-based index (mIBI), which have recently been identified to be prognostic markers in patients with HCC.Citation23
Methods
Patients
From January 2007 to December 2011, newly diagnosed HCC patients were invited to the study.
Patients were eligible for the study if they had newly diagnosed HCC (based either on histology, typical findings from radiological and biochemical tests, or typical imaging findings in two radiological modalities), had not received any treatment for HCC, and were able to read and comprehend Chinese. Patients were excluded from the study if they had a history of other malignancies, encephalopathy, or cognitive impairment.
HRQoL assessment
Individual patients completed HRQoL assessment at study entry using self-administered EORTC QLQ-C30Citation31 and QLQ-HCC18Citation32 questionnaires (Chinese versions). C30 and HCC18 index-scores were calculated as previously published.Citation9 Detailed descriptions of these assessments are listed in .
Table 1 HRQoL instruments used in the study.
Inflammatory markers, clinical factors, and follow-up
Baseline blood taken was used to assess the serum levels of IL-8, C-reactive protein (CRP), and albumin. mIBI is an inflammation-based score derived from IL-8, CRP, and albumin levels. To calculate mIBI, a score is assigned according to the level of log10 IL-8 (0 for <1.8; 1 for ≥1.8), CRP (0 for <10 mg/dL; 1 for ≥10 mg/dL), and albumin (0 for ≥35 g/L; 1 for <35 g/L), respectively. mIBI score is the summation of these three scores. The cut-off of log10 IL-8 at 1.8 was determined by a receiver operator characteristic curve in the prognostic analysis, which has been reported previously.Citation23 Demographic and clinical data were collected. Patients were followed up until death.
Statistical analyses
Patient characteristics were assessed by standard descriptive analyses. Correlation between continuous variable (log IL-8 level) and continuous HRQoL factors was analyzed using Pearson’s correlation analysis. Patients were dichotomized into mIBI scores of 2–3 vs 0–1 and into log IL8 of ≥1.8 vs <1.8 for correlative analyses. Correlations between the dichotomized variables and continuous HRQoL factors were analyzed by Student’s t-test and/or logistic regressions. The statistical analyses were performed using statistical software (SAS version 9.3; SAS Institute, Cary, NC). A P-value of <0.05 was regarded as statistically significant.
Ethics approval and informed consents
This study was approved by the regional ethics committee (Joint Clinical Research Ethics Committee of the Chinese University of Hong Kong and New Territories East Cluster of Hospital Authority). Written informed consent was obtained from all study patients.
Results
Patient characteristics
Four hundred and forty-five patients had complete HRQoL data and information on their inflammatory indicators. and show the patients’ baseline clinical characteristics, inflammatory indicators, and HRQoL. The median age at diagnosis was 60 years. The majority of patients (94%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Fifty-eight percent had cirrhosis, 81% had hepatitis B, while 6% had hepatitis C infection. Fourteen percent belonged to Barcelona Clinic Liver Cancer (BCLC) stage A, 24% stage B, 56% stage C, and 5% stage D. With regards to first-line treatment, 51 patients (12%) had surgery, 28 (6%) had local ablative therapy, 110 (25%) had trans-arterial therapy, 85 (19%) had systemic therapy, and 171 (38%) had best supportive care alone. The mean C30 index score was 30.4 (±19.5), and the mean HCC18 index score was 25.1 (±17.1). The median follow-up duration was 30.5 (95% CI=27.5–33.7) months. The median OS was 8.9 (95% CI=7.3–10.4) months.
Table 2 Baseline clinical and laboratory characteristics of HCC patients.
Table 3 Health-related QoL characteristics of the 455 HCC patients based on EORTC QLQ-C30 and EORTC QLQ-HCC18 assessment
Correlation between HRQoL and IL-8 level
The mean IL-8 level was 128.4 (±171.7) pg/ml, and the mean log IL-8 value was 1.84 (±0.51) pg/ml. HRQoL was significantly correlated with log IL-8 level (see ). Log IL-8 had positive and significant correlations with C30 index score, HCC18 index score, QLQ-C30 “fatigue”, “nausea and vomiting”, “pain”, “dyspnea”, “insomnia”, “appetite loss”, “diarrhea”, and “financial difficulty”, QLQ-HCC18 “fatigue”, “body image”, “jaundice”, “nutrition”, “pain”, “sex life”, and “abdominal swelling” (P<0.05). Log IL-8 had a negative and significant correlation with QLQ-C30 “physical functioning”, “role functioning”, “emotional functioning”, “social functioning”, and “global QoL”. That is, a higher log IL-8 level was correlated with worse HRQoL. The strongest correlations were detected with HCC18 index score, where the Pearson’s correlation coefficient was r=0.321 (P<0.0001); the corresponding result for C30 index score was 0.306 (P<0.0001); for QLQ-C30 “appetite loss”, r=0.322 (P<0.0001); for QLQ-C30 “fatigue”, r=0.311 (P<0.0001); for QLQ-C30 “role functioning”, r=−0.305 (P<0.0001); for QLQ-HCC18 “nutrition”, r=0.317 (P<0.0001); and for QLQ-HCC18 “fatigue”, r=0.306 (P<0.0001). Scatter plots for C30 and HCC18 index scores against log IL-8 are shown in and , respectively. Additional analyses were conducted using logistic regression, and findings of univariate and multivariate analyses support the above findings (see and ).
Figure 1 Scatter plot of C30 index scores against log IL-8 levels of the 455 HCC patients.
Abbreviation: HCC, hepatocellular carcinoma.
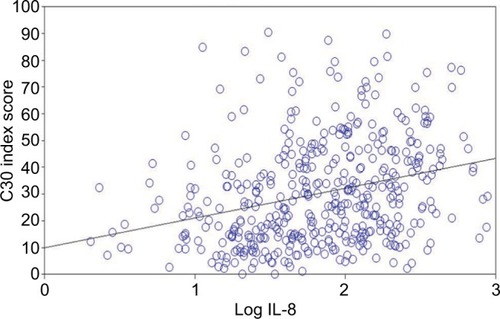
Figure 2 Scatter plot of HCC18 index scores against log IL-8 levels of the 455 HCC patients.
Abbreviation: HCC, hepatocellular carcinoma.
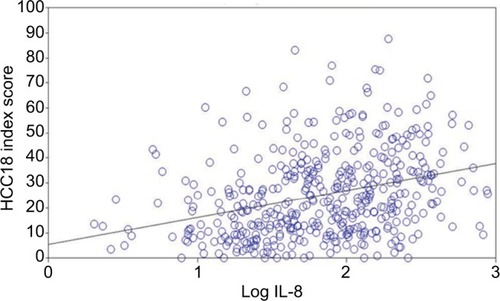
Table 4 Pearson’s correlation analyses between log IL-8 and HRQoL variables.
Correlation between HRQoL and mIBI
HRQoL domains/items were significantly correlated with mIBI (see ). Two hundred and nine patients (47%) had mIBI 2–3. Compared to patients with mIBI 0–1, those with mIBI 2–3 had significantly worse mean C30 index score and HCC18 index score (Student’s t-test P<0.0001). mIBI 2–3 was also significantly associated with HRQoL domain/item scores in QLQ-C30 “physical functioning”, “role functioning”, “social functioning”, “global QoL”, “fatigue”, “nausea and vomiting”, “pain”, “dyspnea”, “insomnia”, “appetite loss”, “diarrhea”, and “financial difficulty” (Student’s t-test P<0.001). Finally, on analysis with QLQ-HCC18, mIBI was significantly associated with “fatigue”, “body image”, “nutrition”, “pain”, “fever”, “sex life”, and “abdominal swelling” (Student’s t-test P<0.03). In summary, patients with higher mIBI score (ie, higher inflammatory status) had worse HRQoL. Additional analyses were conducted using logistic regression, and findings of univariate and multivariate analyses support the above findings (see and ).
Table 5 Student’s t-test for HRQoL scores according to mIBI scores 0–1 vs 2–3.
Discussion
In the present study, the correlations of HRQoL with inflammatory markers were investigated. C30 and HCC18 index scores, as well as the majority of the domains and items in the EORTC QLQ-C30 and QLQ-HCC18, were significantly correlated with IL-8 level and mIBI. These suggest the potential associations between HRQoL and inflammation, and since IL-8 and mIBI have both been previously shown to be prognosticators, it may help explain previous reports in which HRQoL was found to be a significant prognostic factor for survival in HCC patients that was independent of stage and liver function.Citation6–Citation9
C30 and HCC18 index scores were the strongest prognostic HRQoL factors for OS in HCC patients in a previous study;Citation9 the underlying reason has been suggested to be due to their representativeness of all factors within their respective HRQoL instruments.Citation9 In the current analysis, the performance of these two index scores remained better than the majority of individual HRQoL factors. This further supports the representative power of these two index scores. As mentioned previously, the simplicity of a single index score, in contrast to a large collection of scores in an HRQoL instrument, enables convenient utilization of complex HRQoL data for routine clinical use. Since QoL index scores have a significant correlation with IL-8 level and mIBI, we tentatively suggest that the inflammatory status of an individual patient may affect his/her HRQoL.
Although the majority of factors in QLQ-C30 and QLQ-HCC18 have significant correlations with IL-8 level, it is noteworthy that quite a number of these correlations, which were mainly liver-disease related, were only modest. Among the strongest correlations with IL-8 were QLQ-C30 fatigue, QLQ-HCC18 fatigue, QLQ-C30 appetite loss, QLQ-HCC18 nutrition, and QLQ-C30 role functioning. These factors reflect constitutional symptoms, eg, malaise, anorexia, weight loss, and cachexia. Inflammatory response, as represented by IL-8 level, could potentially cause constitutional symptoms, thereby resulting in QoL impairment that was captured by the present study. In other words, the inflammatory status of HCC patients was associated with a specific pattern of HRQoL impairment, predominantly involving adverse effects on constitutional symptoms but not on factors associated with liver disease-related symptoms. When paralleled with molecular findings, traditional HRQoL assessment has gained a new level of understanding: pattern recognition within an HRQoL instrument could identify patients with more severe inflammatory state. From another perspective, since measurement of cytokines is not routinely available, assessing HRQoL may be a good indicator of the inflammatory status of an individual patient.
The present study is limited by having analyzed only two inflammatory indicators, IL8 and mIBI. Nonetheless, data suggests that the inflammatory process could potentially be a contributing factor to poor HRQoL in patients with HCC. Further studies with other inflammatory indicators are needed.
It has been demonstrated that using interventional therapies such as exercise programs or anti-inflammatory medication could lower certain cytokine levels, thereby improving the HRQoL of cancer patients.Citation33–Citation36 Findings from the current study support further investigation to determine the role of therapeutic intervention against inflammatory processes in alleviating constitutional symptoms, thereby improving patient’s HRQoL.
Conclusion
Baseline HRQoL using conventional assessment of EORTC QLQ-C30 and QLQ-HCC18, as well as C30 and HCC18 index scores, significantly correlated with inflammatory indicators (IL-8 level and mIBI) in HCC patients. Among the strongest correlations were those between IL-8 level and the two index scores, as well as HRQoL aspects that represent constitutional symptoms within the EORTC HRQoL instruments. The current findings bring forth insights to traditional HRQoL assessment, and could potentially revolutionize the understanding and utility of HRQoL in HCC patients: pattern recognition within a HRQoL instrument could help to identify patients with more severe inflammatory state.
Author contributions
Leung Li, Winnie Yeo, Stephen L Chan, Frankie Mo, Edwin P Hui, Jane Koh, Allen KC Chan, Nelson LS Tang, Kit F Lee, Paul BS Lai, and Simon CH Yu contributed to the following: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Winnie Yeo provided general supervision to the research group.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S1 Univariate logistic regressions of HRQoL variables for log IL-8 ≥1.8
Table S2 Multiple logistic regressions of HRQoL variables for log IL-8 ≥1.8
Table S3 Univariate logistic regressions of HRQoL variables for mIBI 2–3
Table S4 Multiple logistic regressions of HRQoL variables for mIBI 2–3
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- KimUBDooCJBaekSHNatural history and prognostic factors of primary hepatocellular carcinoma: study of 70 untreated patientsKorean J Intern Med1989421361422562101
- CalvetXBruixJGinésPPrognostic factors of hepatocellular carcinoma in the West: a multivariate analysis in 206 patientsHepatology1990124 Pt 17537602170267
- PawarodeAVoravudNSriuranpongVKullavanijayaPPattYZNatural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patientsAm J Clin Oncol19982143863919708639
- OkudaKOhtsukiTObataHNatural history of hepatocellular carcinoma and prognosis in relation to treatment study of 850 patientsCancer19855649189282990661
- YeoWMoFKKohJQuality of life is predictive of survival in patients with unresectable hepatocellular carcinomaAnn Oncol20061771083108916600982
- BonnetainFPaolettiXColletteSQuality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trialsQual Life Res200817683184318618292
- DioufMFilleronTBarbareJCThe added value of quality of life (QOL) for prognosis of overall survival in patients with palliative hepatocellular carcinomaJ Hepatol201358350952123178978
- LiLMoFKChanSLPrognostic values of EORTC QLQ-C30 and QLQ-HCC18 index-scores in patients with hepatocellular carcinoma - clinical application of health-related quality-of-life dataBMC Cancer2017171828052758
- LiLYeoWValue of quality of life analysis in liver cancer: a clinician’s perspectiveWorld J Hepatol201792086788328804570
- GretenTFDuffyAGKorangyFHepatocellular carcinoma from an immunologic perspectiveClin Cancer Res201319246678668524030702
- WanSKuoNKryczekIZouWWellingTHMyeloid cells in hepatocellular carcinomaHepatology20156241304131225914264
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- Hernandez-GeaVToffaninSFriedmanSLLlovetJMRole of the microenvironment in the pathogenesis and treatment of hepatocellular carcinomaGastroenterology2013144351252723313965
- AriharaFMizukoshiEKitaharaMIncrease in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosisCancer Immunol Immunother20136281421143023764929
- ChewVTowCTeoMInflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patientsJ Hepatol201052337037919720422
- JangJWOhBSKwonJHSerum IL-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinomaCytokine201260368669322906998
- ChoHJKimSSAhnSJLow serum IL-6 levels as a predictive marker of recurrence in patients with hepatitis B virus related hepatocellular carcinoma who underwent curative treatmentCytokine201573224525225797190
- QiuXWangXSongYChenLPlasma level of IL-35 as an independent prognostic indicator in hepatocellular carcinomaDig Dis Sci201661123513352127699510
- ChenZYWeiWGuoZXUsing multiple cytokines to predict hepatocellular carcinoma recurrence in two patient cohortsBr J Cancer2014110373374024495874
- WellingTHFuSWanSZouWMarreroJAElevated serum IL-8 is associated with the presence of hepatocellular carcinoma and independently predicts survivalCancer Invest2012301068969723072563
- KimSSChoHJWonJHIL-8 level as a prognostic marker in patients with hepatitis B virus-associated hepatocellular carcinoma treated with transarterial chemoembolizationCytokine201576244945726163999
- ChanSLChanAWChanAKSystematic evaluation of circulating inflammatory markers for hepatocellular carcinomaLiver Int201737228028927501075
- Sternby EilardMHagströmHMortensenKEQuality of life as a prognostic factor for survival in hepatocellular carcinomaLiver Int201838588589428941130
- Oliveira MirandaDSoares de LimaTARibeiro AzevedoLFeresORibeiro da RochaJJPereira-da-SilvaGProinflammatory cytokines correlate with depression and anxiety in colorectal cancer patientsBiomed Res Int20142014515
- KaoSCVardyJHarvieRHealth-related quality of life and inflammatory markers in malignant pleural mesotheliomaSupport Care Cancer201321369770522936495
- Lynch KellyDLyonDEAmeringerSAElswickRKSymptoms, cytokines, and quality of life in patients diagnosed with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantationOncol Nurs Forum201542326527525901378
- PanjuAHDaneshAMindenMDKelvinDJAlibhaiSMAssociations between quality of life, fatigue, and cytokine levels in patients aged 50+ with acute myeloid leukemiaSupport Care Cancer200917553954618931862
- PaulsenØLairdBAassNThe relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancerPLoS One2017125e017762028542626
- ChibaFSodaKYamadaSThe importance of tissue environment surrounding the tumor on the development of cancer cachexiaInt J Oncol201444117718624247481
- AaronsonNKAhmedzaiSBergmanBThe European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncologyJ Natl Cancer Inst19938553653768433390
- BlazebyJMCurrieEZeeBCDevelopment of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18Eur J Cancer200440162439244415519517
- OhBButowPMullanBImpact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trialAnn Oncol201021360861419880433
- SprodLKJanelsinsMCPaleshOGHealth-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuanJ Cancer Surviv20126214615422160628
- HojanKKwiatkowska-BorowczykELeporowskaEMileckiPInflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12-month exercise programPol Arch Intern Med20171271253528075422
- PanahiYSaadatABeiraghdarFSahebkarAAdjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trialPhytother Res201428101461146724648302
