Abstract
Background
Celecoxib has previously been shown to be effective in reducing recurrent colorectal adenomas, but its long-term effects are unknown. In addition, safety issues are of major concern. Therefore, we examined the efficacy and safety of celecoxib as a chemopreventive agent along with its posttreatment effect.
Methods
We performed a meta-analysis based on a systematic review of randomized controlled trials (RCTs) comparing celecoxib at various doses (400 mg once daily, 200 mg twice daily, and 400 mg twice daily) vs placebo in persons with history of colorectal adenomas. Several databases were searched from inception up to April 2018. Long-term follow-ups of RCTs were also included to evaluate posttreatment effect. Primary outcome was the incidence of recurrent colorectal adenomas. Various safety outcomes were evaluated, especially cardiovascular (CV) events. Risk–benefit integrated analyses were also performed.
Results
A total of three RCTs (4,420 patients) and three post-trial studies (2,159 patients) were included in the analysis. Use of celecoxib at any dose for 1–3 years significantly reduced the incidence of recurrent advanced adenomas (risk ratio, 0.42 [95% CI, 0.34–0.53]) and any adenomas (0.67 [95% CI, 0.62–0.72]) compared with placebo. Subgroup analysis on different dosing suggested a greater effect with 400 mg twice daily. However, celecoxib 400 mg twice daily significantly increased the risk of serious adverse (1.2 [95% CI, 1.0–1.5]) and CV events (3.42 [95% CI, 1.56–7.46]), while celecoxib at 400 mg/day, especially with once daily dosing, did not increase CV risk (1.01 [95% CI, 0.70–1.46]). Analysis of post-trial studies indicated that the treatment effect disappeared (1.15 [95% CI, 0.88–1.49]) after discontinuing celecoxib for >2 years.
Conclusion
Celecoxib 400 mg once daily dosing could potentially be considered as a viable chemopreventive option in patients with high risk of adenomas but with low CV risk. Long-term trials on celecoxib at a dose of ≤400 mg either once or twice daily are warranted.
Background
Colorectal cancer (CRC) is the third most common cancer worldwide, with over 1.4 million new cases estimated to have occurred in 2012.Citation1 It is widely accepted that adenomas/polyps are well-known precursors of sporadic CRCs.Citation2 Early detection and removal of adenomatous polyps by colonoscopic screening has been showed to reduce mortality from CRC.3 Unfortunately, surveillance colonoscopic screening is underutilized.Citation4–Citation6 A variety of reasons, including suboptimal adherence to screening, availability, and cost, may play a part in this problem. For those who undergo polypectomy, the recurrence rate is still relatively high.2,3,7 Therefore, the use of chemoprevention strategies to complement surveillance screening may have a potential to further reduce CRC morbidity and mortality among those with adenomatous polyps.
Protective effect of non-aspirin nonsteroidal antiinflammatory drugs (NSAIDs) on colorectal adenomas have been documented in previous systematic reviews.Citation8–Citation12 However, concerns about cardiovascular (CV) safety and risk of serious bleeding events hamper the acceptance of these strategies for secondary prevention of CRC.Citation11,Citation13 Cyclooxygenase-2 (COX-2) inhibitorsCitation14 selectively interfere with COX-2 enzyme and have been shown to cause less major bleeding compared with traditional NSAIDs.Citation15–Citation18 For CV safety, most non-aspirin NSAIDs and COX-2 inhibitors have been shown to increase the risk of thrombotic CV events.Citation15,Citation19–Citation21 However, the risk of these events may be a result of complex interplay among a specific drug molecule, dose, and baseline CV risk.Citation16,Citation22 Rofecoxib, which was withdrawn from the market, was shown to have a much higher risk compared with celecoxib.Citation21 For celecoxib, the risk appeared to be dose dependent and was evident among patients with high CV risk at baseline.Citation19 In addition, available evidence suggested that twice daily dosing, not once daily dosing, was associated with increased CV risk.Citation19,Citation23,Citation24 Recently, celecoxib at approved doses (200–400 mg/day), was found to be noninferior to ibuprofen or naproxen with regard to CV safety in a large, randomized, controlled trial with over 24,000 patients.Citation25 As a result, celecoxib at approved doses could be a viable option for patients with history of adenomas where the risk of CRC may outweigh the risk of CV events.Citation8,Citation26 We, therefore, conducted a systematic review and meta-analysis to evaluate efficacy and safety of celecoxib in patients with a history of adenomas. We performed risk–benefit integrated analysis to comprehensively evaluate celecoxib’s multidimensional effects in this setting. Moreover, we also investigated whether the adenoma-preventive effect of celecoxib waned after withdrawal.
Methods
Study design
This study was performed as part of a systematic review that has been previously registered (PROSPERO CRD42015025849)Citation27 and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.Citation28
Search strategy and study selection
We identified relevant studies through a systematic search of Medline and EMBASE until April 2018. In addition, we searched published systematic reviews for additional studies. The search strategy is provided in Table S1. Studies included were randomized controlled trials (RCTs) and long-term follow-up of RCTs that met the following inclusion criteria: participants were adults (aged ≥18 years) at an increased risk due to a previous history of adenomas who underwent polypectomy and with a documented clean colon before randomization; intervention was celecoxib at any dose; the comparator was placebo or control; and the outcome was the proportion of subjects who developed colorectal neoplasia, described as either colorectal adenomas or advanced adenomas.
Outcomes of interest
Primary efficacy outcomes of interest were the incidence of recurrent colorectal adenomas (advanced adenomas and any adenomas). Advanced adenoma was defined by one or more of the following features: 1 cm or larger, with villous or tubulovillous histology, with high-grade dysplasia, and/or with intramucosal carcinoma or invasive cancer.Citation29 Any adenomas include both advanced and nonadvanced adenomas (defined as one or two small [<1 cm] tubular adenomas or serrated polyps without cytologic dysplasia)Citation29 and invasive cancers.
Safety outcomes were the incidence of CRC, mortality due to any causes, serious adverse events, serious CV events, and renal and hypertensive disorders reported on any followup after randomization. Serious adverse events were defined as events resulting in death, hospital admission because of an adverse event, severe gastrointestinal bleeding, CV or non-CV complications, or discontinuation of intervention due to an adverse event or events that were defined as serious or severe by study authors. Serious CV events were defined as the composite of CV death, myocardial infarction, stroke, heart failure, thromboembolic event, or defined as serious CV event by the study investigators. Renal and hypertensive disorders included reports of elevated serum creatinine levels, fluid retention and edema, hypertension, proteinuria, and renal failure. We also evaluated the posttreatment effects of celecoxib on the incidence of recurrent colorectal neoplasia after discontinuing the intervention for >2 years.
Data extraction and quality assessment
Two reviewers (SKV and KGL) screened the relevant publications and then extracted data on the study, participants, and treatment-related characteristics onto a standardized form, and discrepancies were resolved by another author after group discussion. Data on efficacy outcomes were extracted with modified intention-to-treat analysis (ie, subjects who received at least one dose of celecoxib at any dose and had at least one colonoscopy after randomization). Data on safety outcomes were extracted by intention-to-treat principle, using the initial number of randomized participants allocated to each trial arm. Participants who were lost to follow-up were considered free of adverse events.
Previous evidence suggested that the effects of NSAIDs on adenoma recurrence may not be sustained after treatment cessation.Citation30–Citation32 Hence, we abstracted efficacy outcomes measured cumulatively at two time points, within 1 year of discontinuing intervention (primary efficacy analysis) and ≥2 years after discontinuing intervention (posttreatment effect analysis). Two reviewers (SKV and SMC) independently assessed the risk of bias (ROB) in the context of the primary outcome by using the revised Cochrane risk of bias tool (RoB 2.0).Citation33
Data synthesis and statistical analysis
Meta-analysis was performed with DerSimonian and Laird random-effects model to estimate pooled risk ratios and 95% confidence intervals incorporating heterogeneity within and between studies, with Stata version 14.0 (StataCorp, College Station, TX, USA).Citation34 Statistical heterogeneity between trials was assessed for primary outcomes using I2 statistics, with values >50% indicating substantial levels of heterogeneity.Citation35,Citation36 Publication bias could not be assessed due to the small number of included studies, which limited the power to distinguish between finding by chance and real asymmetry.Citation37 Subgroup analyses were performed for different dosings of celecoxibCitation38 including 200 mg twice daily (400 mg/day), 400 mg once daily, and 400 mg twice daily (800 mg/day). Sensitivity analyses were performed based on the use of surveillance colonoscopy per protocol completer analysis (outcomes included only those subjects who underwent colonoscopy surveillance at the prespecified time period per protocol and excluded subjects who underwent a colonoscopic surveillance assessment before the expected surveillance interval), fixed-effect model, and trials with low ROB.
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence (high, moderate, low, and very low) of estimates derived from meta-analyses using GRADEpro version 3.6.1 (McMaster University, 2014).Citation39
Risk–benefit integrated analysis
Similar to approaches used in previous meta-analysis,Citation8 we used risk–benefit integrated analysis to review the potential benefits (prevention of recurrent advanced adenomas) and risks (serious adverse events and CV events) of celecoxib at 400 and 800 mg/day. To understand potential benefits at the population level, we used risk ratios derived from the placebo comparisons of celecoxib in the meta-analysis to estimate absolute risk of advanced adenomas with intervention. We used published pooled estimates from the National Cancer Institute pooling project to estimate population-level risks of advanced adenomas as assumed control risk (7.4% in low-risk group or population with history of nonadvanced adenomas and 16.3% in high-risk group or population with history of advanced adenomas).Citation40 Similarly, to understand the potential risks, we used the pooled risk of serious adverse events in placebo groups in the meta-analysis (estimated using metaprop command in STATA) as a measure of baseline risk. We then used risk ratios derived from the placebo comparisons of celecoxib in the meta-analyses for serious adverse events and CV events to estimate absolute risk associated with celecoxib. We then presented excess benefit and risk of serious adverse events (over placebo) per 1,000 individuals who received treatment. Estimates of absolute risk were generated with the GRADEpro version 3.6.1 (McMaster University, 2014).Citation39
Results
Study selection
We identified 391 records in which 11 potentially eligible articles were reviewed in full text. Of these, six articles were excluded mostly due to the lack of eligible population. Therefore, a total of five studies were included in our review. Among these five studies, three trialsCitation23,Citation24,Citation41 met the eligibility criteria for the quantitative analysis of primary outcomes. Post-trial results from three studiesCitation30,Citation31,Citation41 were included for the analysis of posttreatment effect on the incidence of recurrent adenomas. The PRISMA flow diagram depicting the search and selection process for the primary outcomes is displayed in Figure S1.
Characteristics of the included studies
describes the characteristics of three RCTsCitation23,Citation24,Citation41 which reported the incidence of recurrent colorectal adenomas. A total of 4,420 participants with a previous history of adenomas who underwent polypectomy and with documented clean colon before randomization were included in the analysis. All trials were double-blinded and placebo-controlled. The treatment duration was 3 years in two trialsCitation23,Citation24 and 1 year in one trial.Citation41 Postrandomization colonoscopy was performed within 1 year of discontinuing intervention in all trials. The dose per day of celecoxib used in two trials was 400 mg once daily,Citation23,Citation41 and the remaining trialCitation24 tested both 400 mg (200 mg twice daily) and 800 mg (400 mg twice daily) doses. A detailed description of ROB assessment among included RCTs is presented in Table S2. Among three RCTs, one trialCitation41 was judged to be at high ROB and the remaining two trialsCitation23,Citation24 were judged to be at low ROB in all domains.
Post-trial results from three studiesCitation30,Citation31,Citation41 were available to investigate the effect of celecoxib withdrawal on incidence of recurrent colorectal adenomas. describes the identified studies. A total of 2,159 participants who completed follow-up colonoscopy after discontinuing intervention for 2–4 years were included in the analysis.
Table 1 RCTs reporting incidence of recurrent colorectal adenomas
Table 2 Studies reporting posttreatment effect of celecoxib on incidence of recurrent colorectal adenomas after 2 years of off-treatment
Effects on the primary efficacy outcomes
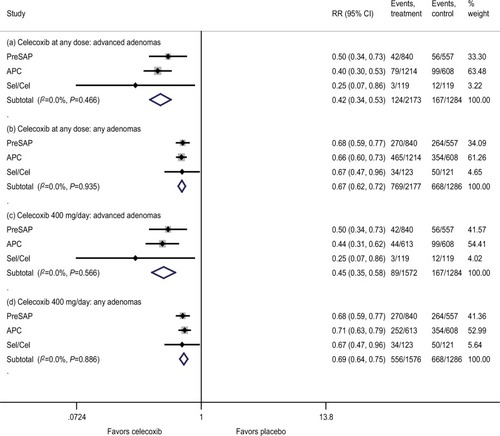
Based on the results from the meta-analyses, use of celecoxib at any dose (400–800 mg/day) for a duration of approximately 1–3 years showed a statistically significant reduction in the recurrence of advanced colorectal adenomas (RR, 0.42 [95% CI, 0.34–0.53]) and any adenomas (RR, 0.67 [95% CI, 0.62–0.72]) compared with placebo, with no heterogeneity (I2=0%; ). In the sensitivity analyses (Figures S2–S4), findings were robust and consistent with the primary analysis. A subgroup analysis of celecoxib at 400 mg/day demonstrated similar effects on advanced adenomas (RR, 0.45 [95% CI, 0.35–0.58]) and any adenomas (RR, 0.69 [95% CI, 0.64–0.75]), with no heterogeneity (I2=0%; ). Both 400 mg once daily and 200 mg twice daily dosing regimens provided similar effect size (Figure S5). For celecoxib at 800 mg/day, RR for advanced adenomas was 0.34 [95% CI, 0.24–0.50] and 0.55 [95% CI, 0.48–0.64] for any adenomas.
Figure 1 Effects on the primary efficacy outcomes.
Notes: Efficacy outcomes measured cumulatively from baseline, on postrandomization colonoscopy performed within 1 year of discontinuing intervention. Celecoxib at any dose: 400–800 mg/day.
Abbreviations: APC, Adenoma Prevention with Celecoxib trial; PreSAP, Prevention of Colorectal Sporadic Adenomatous Polyps study; Sel/Cel, Selenium and Celecoxib (Sel/Cel) Trial.
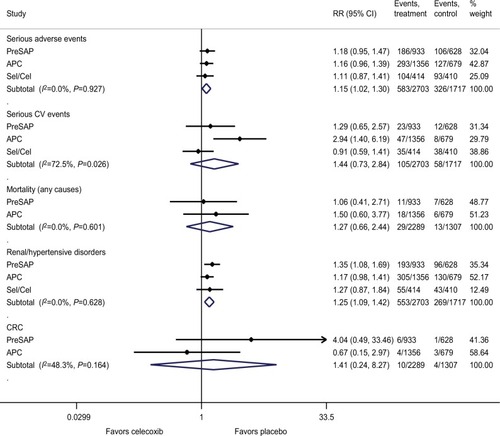
Effects on the safety outcomes
Results from meta-analyses () showed that celecoxib at any dose for a duration of approximately 1–3 years significantly increases the risk of serious adverse events (RR, 1.15 [95% CI, 1.02–1.30]) and renal-hypertensive disorders (RR, 1.25 [95% CI, 1.09–1.42]) compared with placebo. Other safety outcomes including serious CV events (RR, 1.44 [95% CI, 0.73–2.84]), all-cause mortality (RR, 1.27 [95% CI, 0.66–2.44]), and CRCs (RR, 1.41 [95% CI, 0.24–8.27]) were not significantly increased compared to placebo. Subgroup analysis based on different dosing regimens showed that celecoxib 400 mg twice daily (800 mg/day) significantly increased the risk of serious adverse (RR, 1.2 [95% CI, 1.0–1.5]) and CV events (RR, 3.42 [95% CI, 1.56–7.46]). Interestingly, for celecoxib at 400 mg/day, a significant increase in CV risk was observed only with 200 mg twice daily regimen (RR, 2.48 [95% CI, 1.10–5.59]) but not with 400 mg once daily regimen (RR, 1.01 [95% CI, 0.70–1.46]), compared with placebo (Figures S6 and S7).
Grade summary of evidence
Our application of GRADE methodology led us to conclude that the accumulated evidence for celecoxib (at any dose and 400 mg/day) is of high quality for adenoma prevention. Detailed information on GRADE summary of evidence is presented in Table S3.
Risk–benefit integrated analysis
Based on integrated analysis, we estimated that the use of celecoxib at 800 mg/day compared with placebo may lead to 108 (95% CI, 82–124) and 49 (95% CI, 30–56) fewer advanced adenomas in 1,000 patients with high-risk and low-risk adenomas, respectively. On the other hand, this would lead to an excess of 38 (95% CI, 0–95) serious adverse events and 82 (95% CI, 19–186) CV events compared with placebo. As for celecoxib at 400 mg/day, there would be 90 (95% CI, 68–106) and 41 (95% CI, 32–48) fewer advanced adenomas in persons with high-risk and low-risk adenomas, respectively. As for harm, this intervention would lead to an excess of 25 (95% CI, 2 fewer to 53 more) serious adverse events and 11 (95% CI, 8 fewer to 44 more) CV events, per 1,000 patients treated compared with placebo. The risk– benefit balance may also be different when different dosing regimens of 400 mg/day are used. Interestingly, celecoxib at 400 mg once daily may be associated with less harm since this dosing regimen would only lead to 30 excess harm ful events (29 serious adverse events and one CV event) compared with 65 events (15 serious adverse events and 50 CV events) with 200 mg twice daily regimen. Based on this analysis, the risk–benefit balance of celecoxib at 400 mg/day, especially with 400 mg once daily dosing may be acceptable especially among patients at high risk of CRC and at low CV risk (Table S4).
Posttreatment effect on efficacy outcomes
Meta-analyses of post-trial studies demonstrated no effect on the recurrence of advanced colorectal adenomas (RR, 1.15 [95% CI, 0.67–1.99]) and any adenomas (RR, 1.15 [95% CI, 0.88–1.49]) after discontinuing celecoxib for more than 2 years (Figure S8). There was moderate to substantial level of heterogeneity observed in both analyses.
Discussion
Previous meta-analyses suggested a substantial protective effect of non-aspirin NSAIDs on colorectal adenoma recurrence.Citation8–Citation12 Although the exact mechanism of action remains to be elucidated, both COX-dependent and COX-independent mechanisms have been shown to contribute to the antitumor effects. Despite such promising data, use of long-term NSAIDs for cancer prevention is not very well received due to their significant toxicity, especially CV toxicity.Citation8,Citation11 However, CV toxicity of NSAIDs is a complicated phenomenon, which is a result of a complex interplay mainly between baseline CV risk and the nature of each NSAID along with its dose. Celecoxib, a selective COX-2 inhibitor, is among a few NSAIDs that have been tested in a number of studies for CRC prevention. Although its efficacy in this indication looked initially promising, CV toxicity shown from cancer prevention trials thwarted the interest of the medical community.
Nevertheless, a previous review has suggested that moderate dose of celecoxib (≤400 mg/day) might not be associated with an increased CV risks.Citation26 Recently, the PRECISION trialCitation25 which was a large, randomized, CV safety trial comparing celecoxib at ≤400 mg/day with ibuprofen and naproxen suggested that celecoxib at this dose may not confer unacceptable CV risk along with other serious adverse events. As a result, a comprehensive evaluation to understand the totality of its risk–benefit in this important indication may be warranted. Our study was designed to shed some light into this important question. To the best of our knowledge, this study combined the entire body of relative and absolute efficacy and safety of celecoxib at different doses. We also tried to gage the magnitude of risk and benefit in patients with varying risk of adenomas to increase understanding of the risk–benefit equation, which may vary with different levels of risk. Thus, a clearer picture of an agent with multidimensional effects can be seen.
The results of our study showed that celecoxib demonstrated a dose-dependent effect in the reduction of adenoma recurrence and the risks of serious adverse events during a follow-up of up to 3 years. Our findings on celecoxib are consistent with those from previous meta-analysesCitation8–Citation10 regarding the benefits of non-aspirin NSAIDs for the prevention of recurrent adenomas. For risk–benefit balance, we found that benefit of celecoxib at 400 mg/day may outweigh the risks of adverse events in patients with high-risk adenomas. It is important to note that the absolute risk of serious CV events associated with NSAIDs was greatest among individuals with high CV risk at baseline.Citation13,Citation26,Citation42 Our analysis demonstrated no significant risks of CV events in patients on celecoxib 400 mg/day for 1–3 years despite that 45% of the study population were at high CV risk. This finding is consistent with the previous meta-analysis of randomized trials which reported no significant increase in CV risk with celecoxib 400 mg/day.Citation19,Citation43 Data from other observational studies also support such findings.Citation26 As a result, celecoxib 400 mg/day could potentially be considered as a viable chemopreventive agent in patients with high risk of adenomas but with low CV risk. In addition, subgroup analysis based on different dosing regimens suggested that celecoxib 400 mg once daily may have a much better safety profile on CV events compared with 200 mg twice daily. The finding that twice daily dosing may confer greater CV risk than once daily dosing of celecoxib in our study is consistent with previous meta-analysis along with other mechanistic studies.Citation19 Twice daily dosing of celecoxib has been shown to increase blood pressure more than that in once daily dosing.Citation44 A previous pharmacodynamic study conducted in healthy volunteers suggested that prostacyclin levels can recover to normal levels 12 hours after a single daily dose of celecoxib.Citation45 This raised a possibility of a more complete prostacyclin inhibition with twice daily than once daily dosing which ultimately leads to higher CV risk. Several other hypotheses have been proposed to explain differences in types and dosing of NSAIDs vs CV risk including different levels of inhibition of endothelial NO synthaseCitation46 and differential effects on the enhancement of methylarginines formation.Citation47 However, the exact mechanism of this difference remains unknown.
For high-dose celecoxib (800 mg/day), despite yielding slightly higher efficacy (18 more cases of advanced adenoma prevented compared with 400 mg/day), the overall risk was most likely unacceptable. With 108 advanced adenomas prevented, the trade-off was 82 excess CV events per 1,000 patients treated with 800 mg/day of celecoxib. This finding is very much consistent with previous meta-analysis, RCTs, and observational studies which indicated a high risk of CV events with a high dose of celecoxib. As a result, celecoxib at 800 mg/day is clearly not a viable option for this indication. Of interest is the fact that no trial has ever been conducted to evaluate celecoxib at a dose of 100–200 mg/day as a chemopreventive agent. Although the efficacy of this low dose is uncertain, adverse effects of celecoxib at ≤200 mg/day would most likely be less than 400 mg/day. This may allow celecoxib to be more acceptable for long-term use if it can be demonstrated to show some efficacy for this indication at this low level of dosing.
The preventive effect of celecoxib, as shown in our analysis, waned after ≥2 years of treatment cessation. The lack of a sustained clinical effect may reflect a rebound of COX-2 expression, or cessation of an alternative mechanism independent of COX-2 inhibition, as described previously.Citation30,Citation48 This is consistent with an increased risk of adenomas after 2 years of celecoxib discontinuation in the PreSAP trial,Citation30 which is similar to the APPROVe study with rofecoxib.Citation32 These data suggested that celecoxib may need to be employed on a long-term basis. Since both efficacy and adverse events can be cumulative, future study of celecoxib may need to be long term to fully elucidate the true balance on risk and benefit of this agent.
Our study has several important limitations. First, the limited number of trials along with their short duration, some of which were terminated early, may not provide sufficient data to genuinely represent the long-term risk and benefit of celecoxib. Second, by using a pooled estimate of population data from the National Cancer Institute pooling project, our analysis may be limited by the nature of the source data that were derived mainly from a Caucasian population and a specific geographical area. Since significant differences exist among various subtypes of CRC across the world, the applicability of such data to other parts of the world may be limited. This is also the case for pooled estimate on CV events where different rates of CV events are seen among different racial groups and geographical areas. Third, we did not analyze the impact of aspirin use on the risk–benefit of celecoxib. This was due to the fact that we were unable to obtain patient-level data of these included trials. Aspirin could potentially alter the risk–benefit balance of celecoxib if used concomitantly. This is due to the fact that aspirin has a modest protective effect on CRC and CV events, yet possesses gastrointestinal toxicity along with major bleedings.Citation10,Citation49,Citation50 As a result, readers should be aware of this limitation. Lastly, based on these limitations, our data are useful only for hypothesis generation and cannot be considered definitive. Future research on celecoxib as a chemo-preventive agent may potentially be considered but must be employed at a dose of ≤400 mg either once or twice daily.
Conclusion
In this comprehensive evaluation with risk–benefit integrated analysis, celecoxib at the dose of ≤400 mg/day could potentially be considered as a viable chemopreventive option, especially with a 400 mg once daily regimen. This may be particularly attractive in patients with high risk of adenomas but with low CV risk. Example of this patient group is a patient who has a history of high-risk adenomas and also has <5% of a 10-year risk of developing atherosclerotic CV disease. Celecoxib at a higher dose should be discouraged due to the unacceptable high level of risk compared with small benefit gained by increasing the dose beyond 400 mg/day. However, more long-term trials on celecoxib at a dose of ≤400 mg either once or twice daily are warranted to fully elucidate the true balance on risk and benefit of this agent.
Transparency declaration
The corresponding authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Data sharing statement
Technical appendix and dataset are available from the corresponding author.
Acknowledgments
The authors wish to thank Professor Brian L Furman, Strathclyde Institute of Pharmacy and Biomedical Sciences, Glasgow, UK, for his valuable comments and support which helped to improve the manuscript, and Mr Razman Shah Mohd Razali, reference librarian, International Medical University, for providing the full-text articles whenever needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- StrumWBColorectal adenomasN Engl J Med2016374111065107526981936
- LiebermanDARexDKWinawerSJGiardielloFMJohnsonDALevinTRGuidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal CancerGastroenterology2012143384485722763141
- HooverSSubramanianSTangkaFKLPatients and caregivers costs for colonoscopy-based colorectal cancer screening: experience of low-income individuals undergoing free colonoscopiesEval Program Plann201762818628153341
- SubramanianSBobashevGMorrisRJWhen budgets are tight, there are better options than colonoscopies for colorectal cancer screeningHealth Aff201029917341740
- NgSCWongSHColorectal cancer screening in AsiaBr Med Bull2013105294223299409
- CottetVJoosteVFournelIBouvierAMFaivreJBonithon-KoppCLong-term risk of colorectal cancer after adenoma removal: a population-based cohort studyGut20126181180118622110052
- DulaiPSSinghSMarquezEChemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysisBMJ2016355i618827919915
- VeettilSKTeerawattanapongNChingSMEffects of chemo-preventive agents on the incidence of recurrent colorectal adenomas: a systematic review with network meta-analysis of randomized controlled trialsOnco Targets Ther2017102689270028579807
- VeettilSKLimKGChingSMSaokaewSPhisalprapaPChaiyakunaprukNEffects of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs on the incidence of recurrent colorectal adenomas: a systematic review with meta-analysis and trial sequential analysis of randomized clinical trialsBMC Cancer201717176329137605
- RostomADubéCLewinGNonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task ForceAnn Intern Med2007146537638917339623
- DubéCRostomALewinGThe use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task ForceAnn Intern Med2007146536537517339622
- BelloAEHoltRJCardiovascular risk with non-steroidal anti-inflammatory drugs: clinical implicationsDrug Saf2014371189790225079141
- BresalierRSSandlerRSQuanHCardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trialN Engl J Med2005352111092110215713943
- BombardierCLaineLReicinAComparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study GroupN Engl J Med2000343211520152811087881
- SilversteinFEFaichGGoldsteinJLGastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety StudyJAMA2000284101247125510979111
- ChanFKLLanasAScheimanJBergerMFNguyenHGoldsteinJLCelecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trialLancet2010376973617317920638563
- AschenbrennerDSCardiovascular risk of celecoxib no worse than that of ibuprofen or naproxenAm J Nurs20181181019
- SolomonSDWittesJFinnPVCardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysisCirculation2008117162104211318378608
- KearneyPMBaigentCGodwinJHallsHEmbersonJRPatronoCDo selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trialsBMJ200633275531302130816740558
- GrahamDCampenDHuiRRisk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control studyLancet2005365945847548115705456
- MeekILMartAFJ van de LaarVonkemanHENon-steroidal anti-inflammatory drugs: an overview of cardiovascular risksPharmaceuticals2010372146216227713346
- ArberNEagleCJSpicakJCelecoxib for the prevention of colorectal adenomatous polypsN Engl J Med2006355988589516943401
- BertagnolliMMEagleCJZauberAGCelecoxib for the prevention of sporadic colorectal adenomasN Engl J Med2006355987388416943400
- NissenSEYeomansNDSolomonDHCardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritisN Engl J Med2016375262519252927959716
- McgettiganPHenryDCardiovascular risk and inhibition of cyclo-oxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase2JAMA2006296131633164416968831
- VeettilSKSaokaewSLimKGChingSMPhisalprapaPChaiyakunaprukNComparative effectiveness of chemopreventive interventions for colorectal cancer: protocol for a systematic review and network meta-analysis of randomised controlled trialsJ Gastrointest Oncol20167459560227563450
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med20091514W64264269
- ShortMWLaytonMCTeerBNDomagalskiJEColorectal cancer screening and surveillanceAm Fam Physician20159129310025591210
- ArberNSpicakJRáczIFive-year analysis of the prevention of colorectal sporadic adenomatous polyps trialAm J Gastroenterol201110661135114621503000
- BertagnolliMMEagleCJZauberAGFive-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib TrialCancer Prev Res200924310321
- BaronJASandlerRSBresalierRSA randomized trial of rofecoxib for the chemoprevention of colorectal adenomasGastroenterology200613161674168217087947
- HigginsJPTSterneJACSavovicJA revised tool for assessing risk of bias in randomized trialsCochrane Database Syst Rev201610Suppl 13
- DersimonianRLairdNMeta-analysis in clinical trials revisitedContemp Clin Trials201545Pt A13914526343745
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- MelsenWGBootsmaMCRoversMMBontenMJThe effects of clinical and statistical heterogeneity on the predictive values of results from meta-analysesClin Microbiol Infect201420212312924320992
- SterneJASuttonAJIoannidisJPRecommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trialsBMJ2011343d400221784880
- AntmanEMBennettJSDaughertyAUse of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart AssociationCirculation2007115121634164217325246
- BrozekJLAlkEAAlonso-CoelloPGrading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventionsAllergy200964566967719210357
- MartínezMEBaronJALiebermanDAA pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomyGastroenterology2009136383284119171141
- ThompsonPAAshbeckELRoeDJCelecoxib for the prevention of colorectal adenomas: results of a suspended randomized controlled trialJ Natl Cancer Inst201610812djw15127530656
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trialsLancet2013382989476977923726390
- GunterBRButlerKAWallaceRLSmithSMHarirforooshSNon-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysisJ Clin Pharm Ther2017421273828019014
- SolomonSDPfefferMAMcmurrayJJVEffect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomasCirculation2006114101028103516943394
- GrosserTFriesSFitzgeraldGABiological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunitiesJ Clin Invest2006116141516395396
- YuYRicciottiEScaliaRVascular COX-2 modulates blood pressure and thrombosis in miceSci Transl Med20124132132ra54
- GrosserTRicciottiEFitzgeraldGAThe cardiovascular pharmacology of nonsteroidal anti-inflammatory drugsTrends Pharmacol Sci201738873374828651847
- HanifRPittasAFengYEffects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathwayBiochem Pharmacol19965222372458694848
- Bibbins-DomingoKAspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation StatementAnn Intern Med20161641283684527064677
- VeettilSKJinatongthaiPNathisuwanSEfficacy and safety of chemopreventive agents on colorectal cancer incidence and mortality: systematic review and network meta-analysisClin Epidemiol2018101433144530349391