Abstract
Purpose
The identification and discovery of prognostic markers for colorectal cancer (CRC) are of great clinical significance. CCBE1 is expressed in various tumors and its expression correlates with lymphangiogenesis and angiogenesis. However, the association between CCBE1 expression and CRC outcome has not been reported. The aim of this study was to investigate clinical significance of CCBE1 expression in CRC.
Patients and methods
CCBE1 expression was examined in 30 pairs of fresh CRC tissues and compared with adjacent normal (AN) tissues using quantitative real-time PCR (qRT-PCR), Western blotting and immunohistochemistry (IHC) staining. Tissue microarray immunohistochemical staining was used to study the CCBE1 expression characteristics of 204 CRC patient samples collected from January 2002 to December 2007, and the relationship of CCBE1 with clinicopathological features and prognosis of CRC was analyzed.
Results
CCBE1 was highly expressed in CRC tissues compared with matched AN tissues (P=0.001). Moreover, high expression of CCBE1 was significantly associated with tumor differentiation, lymph node metastasis, vascular invasion, liver metastasis and TNM stage in CRC patients (P≤0.01). Kaplan–Meier survival analysis revealed that high CCBE1 expression, poor tumor differentiation, lymph node metastasis and vascular invasion were significantly associated (all P<0.001) with poor prognosis for patients. Furthermore, univariate and multivariate Cox analysis revealed that high CCBE1 expression, poor tumor differentiation, lymph node metastasis and vascular invasion were independent risk factors for both overall survival (OS) and disease-free survival (DFS) of CRC patients (all P<0.05). OS and DFS of 267 CRC patients from The Cancer Genome Atlas (TCGA) database showed the same trend (log-rank P=6e-04, HR [high] =2.4; log-rank P=0.0081, HR [high] =1.9).
Conclusion
High levels of CCBE1 contribute to the aggressiveness and poor prognosis of CRC. CCBE1 can serve as a novel potential biomarker to predict CRC patients’ prognosis.
Introduction
Colorectal cancer (CRC) is the third most common malignant cancer and one of the main causes of cancer-related death worldwide. There are approximately 1.4 million newly diagnosed CRC patients each year, and their 5-year survival rate is not ideal.Citation1 The bad prognosis of patients with CRC is largely due to the metastatic progression.Citation2 Metastasis is a multi-link, multifactorial, continuous and complex process that is one of the basic biological characteristics of malignant tumors and a key factor in determining the prognosis of cancer.Citation3–Citation5 In general, the metastatic process involves the spread of tumor cells, vascular invasion, lymph node metastasis and growth of new cancer cell colonies.Citation6,Citation7 CRC is one of the five leading causes of cancer-related death among both men and women in China.Citation8 Currently, the incidence of CRC is still increasing in China, and approximately 25% of CRC patients have metastasized tumors prior to diagnosis.Citation9,Citation10 Therefore, it is necessary to identify effective predictors associated with CRC progression and metastasis, which may help patients choose appropriate treatments and monitoring.
CCBE1, which is located in human chromosomal region 18q21.32, encodes a highly conserved protein with EGF-like domain.Citation11 Early studies found that CCBE1 not only regulated extracellular matrix remodeling and migration and multicellular organism developmentCitation12,Citation13 but also played an important role in the development of lymphatic vessels, angiogenic sprouting and lymphangiogenic budding from venous endothelium.Citation14–Citation20 In the past few years, the role of CCBE1 in cancers is beginning to be reported. However, reports indicate that CCBE1 expression has different effects in different malignancies. Barton et alCitation21 and Li et alCitation22 supported CCBE1 as a potential tumor suppressor in ovarian cancer and lung cancer. Mesci et alCitation23 and Mesci and LiuCitation24 showed that in breast cancer CCBE1 is targeted by miR-330-3p, resulting in a more aggressive phenotype. However, Tian et alCitation25 described CCBE1 as a tumor promoter in gastrointestinal stromal tumors (GISTs), which enhanced tumor angiogenesis. In addition, Guo et alCitation26 and Zhang and LiuCitation27 indicated that the increased expression of SLP-2 promoted the formation of lymph vessels and exacerbated lymphatic metastasis of rectal cancer via upregulation of CCBE1. Lymphatic metastasis and vascular invasion are the main means of CRC spread and can significantly affect the prognosis of patients.Citation28–Citation31 However, less information was available for the prognostic value of CCBE1 in CRC.
In this study, we detected the expression of CCBE1 in 30 cases of fresh CRC tissues and paired adjacent normal (AN) tissues and tissue samples from 204 CRC cases analyzed for correlation between CCBE1 expression and clinicopathological features. Further, whether CCBE1 could be used as a potential prognostic biomarker for primary CRC patients after surgical resection was assessed.
Patients and methods
Patients and samples
Thirty cases of fresh CRC tissues and matched AN tissues were collected from the Department of General Surgery from January to December 2017. All tissues were freshly frozen in liquid nitrogen until quantitative real-time PCR (qRT-PCR) and Western blotting test. Furthermore, from January 2002 to September 2007, a total of 204 patients’ CRC samples with detailed clinical records were collected and reassembled into multiple tissue arrays in our retrospective study. All samples were collected without chemotherapy, immunotherapy and radiotherapy before surgery from Xiangya Hospital. All cases were diagnosed independently by two pathologists and followed up regularly until December 2017. This study was approved by the ethics committee of Xiangya Hospital of Central South University. According to the Declaration of Helsinki, all patients and their families provided written informed consent and agreed to use their tissue samples in the study. Follow-up of all the patients was carried out based on the surveillance suggested in the guidelines.
RNA extraction and gene expression by qRT-PCR
Total RNA from fresh CRC and AN tissues was extracted with the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. RNA quantity and quality were evaluated using a Nanodrop spectrophotometer (Thermo Fisher Scientific). RNA was reverse transcribed into cDNA by BeyoRT™ II First-Strand cDNA Synthesis Kit (Beyotime, Shanghai, China), and the expression of CCBE1 was measured using SYBR Green Master Mix (Beyotime) on the Applied Biosystems Quant-Studio™ 3 & 5 Real-Time PCR System (Thermo Fisher Scientific). The sequences of the primers used were listed as follows: CCBE1-F: 5′-TACCGATATGACCGGGAGAG-3′ and CCBE1-R: 5′-AGCTGCCCAAGGTATTGATG-3′; GADPH-F: 5′-GTCTCCTCTGACTTCAACAGCG-3′ and GADPH-R: 5′-ACCACCCTGTTGCTGTAGCCAA-3′. The experiments were repeated three times. The data were normalized to GAPDH expression and calculated as 2−ΔΔCT.
Western blot
Proteins were extracted from fresh CRC and AN tissues using RIPA lysis buffer with protease inhibitors and quantified by BCA Protein Assay Kit (Beyotime). An amount of 30 µg per sample was separated by SDS-PAGE and then transferred to the polyvinylidene difluoride (PVDF) membrane (Hoff-man-La Roche Ltd., Basel, Switzerland). The membranes were blocked with 5% skimmed milk and incubated with CCBE1 (Affinity, Cincinnati, OH, USA; diluted 1:1,000) and GAPDH (Affinity; diluted 1:1,000) antibodies overnight at 4°C. The antigen–antibody complex on the membrane was detected with enhanced chemiluminescence reagents (Thermo Fisher Scientific).
Immunohistochemistry (IHC)
Tissue microarrays were constructed from a representative core from each CRC tissue and AN tissue. Tissue sections with diameters of 1.5 mm and 4 µm thicknesses were placed on slides coated with 3-aminopropyltriethoxysilane. Immunohistochemical staining for tissue was performed using the polymer horseradish peroxidase detection system (Zhongshan Goldenbridge Biotechnology, Beijing, China). All tissue microarrays were incubated with the CCBE1 antibody (diluted 1:200) overnight at 4°C. After incubation with secondary biotinylated antibody, sections were stained, the same length, with diaminobenzidine (DAB) and hematoxylin. The immunohistochemical staining intensity of CCBE1 was scored as negative (0), weak (1), moderate (2) and strong (3), and the percentage of positive cells was scored as 5% (0), 5–30% (1), 31–50% (2) and >50% (3). The scores were calculated by multiplying these two values (ranging from 0 to 9). The protein expression in CRC specimens was divided into the low expression group (<4) and the high expression group (≥4) for further analysis.Citation32,Citation33
Statistical analyses
All data were analyzed with IBM SPSS Statistics 22 Software (IBM Corporation, Armonk, NY, USA). Data were expressed as mean ± standard error of the mean (SEM) for at least three independent experiments. Quantitative data between groups were compared using the Student’s t-test. Correlations between different CCBE1 expression levels were determined using Pearson’s chi-squared test. Overall survival (OS) and disease-free survival (DFS) analyses were performed by the Kaplan–Meier method and log-rank test. Univariate and multivariate analyses were analyzed with Cox proportional hazard regression model to verify the independent risk factors. P-value of <0.05 was defined as statistically significant.
Results
Expression levels of CCBE1 in CRC patients
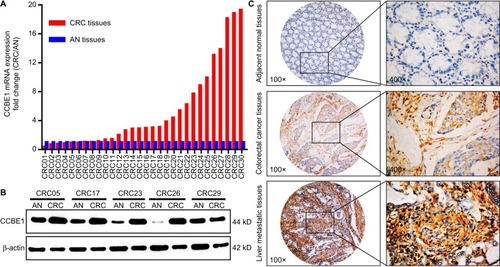
The expression of CCBE1 in 30 cases of fresh CRC tissues and matched AN tissues was detected by qRT-PCR, Western blotting and IHC. The qRT-PCR results indicated that CCBE1 mRNA level was significantly higher in CRC tissues compared with matched AN tissues ([fold change {CRC/AN} >2] in 63.3% cases [19/30]; ). Meanwhile, Western blotting results revealed that CCBE1 protein was highly expressed in most primary CRC tissues than in the matched AN tissues (). In addition, the immunohistochemical staining intensity of CCBE1 in liver metastatic CRC tissues is much stronger than that in paired primary CRC tissues and AN tissues (). These data indicate that CCBE1 is highly expressed in CRC tissues and may be associated with its invasion and metastasis.
Figure 1 CCBE1 expression in CRC patients.
Note: The expression of CCBE1 was detected in 30 fresh CRC tissues and matched AN tissues by qRT-PCR (A), Western blotting (B) and IHC (C).
Abbreviations: AN, adjacent normal; CRC, colorectal cancer; IHC, immunohistochemistry; qRT-PCR, quantitative real-time PCR.
CCBE1 expression correlates with clinicopathological features of CRC patients
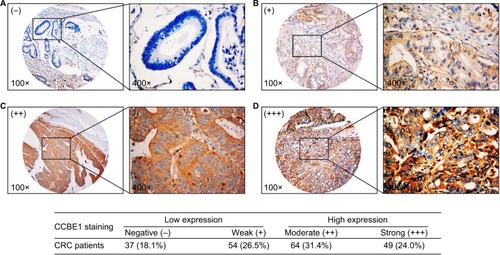
To analyze the association between CCBE1 and the clinicopathological features of CRC, we examined the expression of CCBE1 in 204 CRC tissue samples by IHC. As shown in , the ICH staining intensity of CCBE1 was scored as negative (−), weak (+), moderate (++) and strong (+++), and the percentage of positive cells was scored as 5% (−), 5 −30% (+), 31 −50% (++) and >50% (+++). The scores were calculated by multiplying these two values (ranging from 0 to 9). The protein expression in CRC specimens was divided into the low expression group (<4) and the high expression group (≥4) for further analysis.Citation32,Citation33 The protein expression in CRC specimens was divided into the low expression group (91, 44.6%) and the high expression group (113, 55.4%). Pearson’s chi-squared test analysis showed that CCBE1 expression was significantly correlated with tumor differentiation (P=0.003), TNM stage (P=0.009), lymph node metastasis (P<0.001), vascular invasion (P=0.006), liver metastasis (P=0.010) and serum carcinoembryonic antigen (CEA; P=0.015) expression, but was not associated with gender, age, primary tumor location, tumor size, serum CA19-9 expression, or postoperative chemotherapy (; P>0.05).
Table 1 Correlations of CCBE1 expression with clinicopathological characteristics of CRC patients
Figure 2 CCBE1 expression was detected in CRC patients by immunohistochemical staining.
Notes: (A) Thirty-seven cases of CCBE1 negative staining of CRC tissues accounted for 18.1%. (B) Fifty-four cases of CCBE1 weak staining (+) of CRC tissues accounted for 26.5%. (C) Sixty-four cases of CCBE1 moderate staining of CRC tissues accounted for 31.4%. (D) Forty-nine cases of CCBE1 strong staining of CRC tissues accounted for 24.0%. The protein expression in CRC specimens was divided into the low expression group (91, 44.6%) and the high expression group (113, 55.4%).
Abbreviation: CRC, colorectal cancer.
High CCBE1 expression correlates with poor survival of CRC patients
The correlation between CCBE1 expression and patients’ OS and DFS was estimated by Kaplan–Meier analyses. Kaplan–Meier analyses showed that CRC patients with higher expression of CCBE1 had poor OS and DFS compared with patients with lower expression (P<0.001 for both OS and DFS; ); Moreover, tumor differentiation, lymph node metastasis, vascular invasion and liver metastasis were confirmed to be associated with patients’ OS and DFS ( and ; ; P<0.01). Univariate and multivariate analyses were performed to determine independent prognostic factors for CRC patients after surgery. Univariate analyses showed that CCBE1 expression (P<0.001 for OS and DFS), tumor differentiation (P<0.001 for OS and DFS), lymph node metastasis (P<0.001 for OS and DFS), vascular invasion (P<0.001 for OS and DFS), liver metastasis (P<0.001 for OS and DFS) and TNM stage (P=0.012 for OS, P=0.005 for DFS) were prognostic factors, and multivariate analyses showed that CCBE1 expression (P<0.001 for OS, P=0.028 for DFS), tumor differentiation (P=0.001 for OS, P<0.001 for DFS), lymph node metastasis (not significant [NS] for OS, P=0.044 for DFS), vascular invasion (P<0.001 for OS, NS for DFS) were independent prognostic factors in CRC patients after surgery ( and ). In The Cancer Genome Atlas (TCGA) CRC data, the patients were divided into the low expression group and the high expression group according to the same median value. In TCGA database analysis, patients with high CCBE1 levels had a worse prognosis than patients with low CCBE1 levels (OS: log-rank P=6e-04, HR [high] =2.4, P [HR] =0.00084; DFS: log-rank P=0.0081, HR [high] =1.9, P [HR] =0.0093; ). These results fully demonstrated that CCBE1 expression was closely correlated with poor survival and could be used as a novel independent prognostic biomarker for CRC patients after surgery.
Table 2 Univariable and multivariable analysis of OS and clinicopathological variables of CRC patients
Table 3 Univariable and multivariable analysis of DFS and clinicopathological variables of CRC patients
Figure 3 Kaplan–Meier OS and DFS analyses of CRC.
Note: Results indicated that CCBE1 expression (A), tumor differentiation (B), lymph node metastasis (C) and vascular invasion (D) were associated with CRC patients’ prognosis (all P<0.001).
Abbreviations: CRC, colorectal cancer; DFS, disease-free survival; OS, overall survival.
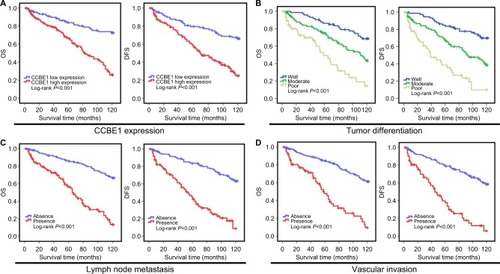
Figure 4 Kaplan–Meier OS (A) and DFS (B) curves of the TCGA CRC cohort show that patients with high CCBE1 level had a worse prognosis than patients with low CCBE1 level (OS: log-rank P=6e-04, HR [high] =2.4, P [HR] =0.00084; DFS: log-rank P=0.0081, HR [high] =1.9, P [HR] =0.0093).
Abbreviations: CRC, colorectal cancer; DFS, disease-free survival; OS, overall survival; TCGA, The Cancer Genome Atlas; TPM, transcripts per million.
![Figure 4 Kaplan–Meier OS (A) and DFS (B) curves of the TCGA CRC cohort show that patients with high CCBE1 level had a worse prognosis than patients with low CCBE1 level (OS: log-rank P=6e-04, HR [high] =2.4, P [HR] =0.00084; DFS: log-rank P=0.0081, HR [high] =1.9, P [HR] =0.0093).Abbreviations: CRC, colorectal cancer; DFS, disease-free survival; OS, overall survival; TCGA, The Cancer Genome Atlas; TPM, transcripts per million.](/cms/asset/ce647538-afa0-480d-b1e3-596eace8b803/dcmr_a_12185865_f0004_c.jpg)
Discussion
CRC is the third most common malignant cancer and one of the main causes of cancer-related death worldwide.Citation1 The bad prognosis of patients with CRC is largely due to the metastatic progression.Citation2 In general, the metastatic process involves the spread of tumor cells, vascular invasion, lymph node metastasis and growth of new cancer cell colonies.Citation2,Citation4,Citation5,Citation7,Citation8,Citation34 Therefore, it is necessary to identify effective predictors to help patients choose appropriate treatments and monitoring. As a newly discovered important lymphangiogenesis and pro-angiogenic factor, CCBE1 has received increasing attention in cancer research.Citation21,Citation23–Citation27 In this study, data analyzed from 30 fresh CRC tissues and paired AN tissues indicated that CCBE1 is highly expressed in CRC tissues and may be associated with tumor malignancy, invasion and metastasis. This result is consistent with the findings of Zhang and LiuCitation27 and Tian et al,Citation25 which showed that the upregulation of CCBE1 aggravates lymphatic metastasis of rectal cancer and enhances tumor angiogenesis of GISTs. However, CCBE1 is similar to other oncogenes, has tissue specificity and has different expression levels and effects in different tumors. For instance, Barton et alCitation21 supported CCBE1 as a potential tumor suppressor in ovarian cancer, and Mesci et alCitation23 showed that CCBE1 exerts a tumor suppressive effect in breast cancer by in vitro invasion assay of human breast cancer cell lines. In addition, lymphatic metastasis and vascular invasion are the main means of CRC spread and can significantly affect the prognosis of patients.Citation35 Previous studies found that CCBE1 is a secreted molecule involved in lymphangiogenesis,Citation14,Citation36 and congenital CCBE1 mutations have been shown to cause Hennekam syndrome.Citation36,Citation37 To confirm the relationship between CCBE1 expression and prognosis and clinicopathological features of CRC, 204 cases of CRC tissues were stained to analyze. IHC results showed that high expression of CCBE1 was observed in 55.4% (113/204) CRC patients. Pearson’s chi-squared test analysis showed that CCBE1 expression was significantly correlated with tumor differentiation, TNM stage, lymph node metastasis, vascular invasion, liver metastasis and serum CEA, but was not associated with gender, age, primary tumor location, tumor size, serum CA19-9 level or postoperative chemotherapy. The abovementioned results indicated that the expression level of CCBE1 was closely related to CRC invasion and metastasis. It is well known that tumor differentiation, lymph node metastasis, vascular invasion, organ metastasis and TNM staging are key factors influencing the progression and survival prognosis of CRC patients.Citation2,Citation30,Citation34,Citation38 In the present study, our data also indicated that tumor differentiation, lymph node metastasis, vascular invasion and liver metastasis were associated with patients’ poor OS and DFS. Most importantly, we found that high expression of CCBE1 was significantly associated with CRC patients’ poor OS and DFS, and OS analysis of 267 CRC patients from the TCGA database showed the same trend. Previous studies showed that the upregulation of CCBE1 was correlated with poorer survival of rectal cancerCitation26 and GISTs,Citation25 similar to our results. However, the tumor-promoting or tumor suppressor function of CCBE1 has background specificity, even in cancers of the same tissue origin. Mesci et alCitation23 provided the first report on the loss of CCBE1 in breast cancer, and Barton et alCitation21 described the loss of CCBE1 in ovarian cancers. Their results showed a correlation with poorer survival with lower CCBE1 expression, which is contrary to our results. Univariate and multivariate analyses showed that CCBE1 expression, tumor differentiation, lymph node metastasis and vascular invasion were independent prognostic factors in CRC patients after surgery. These results fully demonstrated that the presence of CCBE1 was closely correlated with poor survival and could be used as a novel independent prognostic biomarker for CRC patients after surgery. However, our study only initially assessed the clinical value of CCBE1 in CRC patients. To confirm the prognostic value of CCBE1 in CRC patients, further multicenter studies are needed to validate our observations.
Conclusion
Our study suggests that CCBE1 is a highly expressed oncogene in CRC patients. High expression of CCBE1 is significantly related to tumor differentiation, TNM stage, lymph node metastasis, vascular invasion, liver metastasis, serum CEA and poor prognosis. In addition, our findings indicated that CCBE1 was closely correlated with poor OS and DFS and could be used as a novel independent prognostic biomarker for CRC patients after surgery. Of course, further investigations are needed to validate our findings.
Acknowledgments
The authors would like to thank TCGA and all the patients and their families who agreed to participate in this study.
Author contributions
CGY conceived and designed the study. YRZ and HL performed the experiments and YRZ wrote the manuscript. LMX and CGY assisted with experimental performance. YRZ, CGJ and ZPZ assisted with the data analysis. YRZ and CGY modified the manuscript. All the authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- SunZQMaSZhouQBPrognostic value of lymph node metastasis in patients with T1-stage colorectal cancer from multiple centers in ChinaWorld J Gastroenterol201723488582859029358866
- McallisterSSWeinbergRAThe tumour-induced systemic environment as a critical regulator of cancer progression and metastasisNat Cell Biol201416871772725082194
- ValastyanSWeinbergRATumor metastasis: molecular insights and evolving paradigmsCell2011147227529222000009
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- LiuHLiuZLiKTBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancerJ Gastroenterol Hepatol20173291570158028127799
- ShibueTWeinbergRAMetastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environmentSemin Cancer Biol20112129910621145969
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- ChenWCancer statistics: updated cancer burden in ChinaChin J Cancer Res2015271125717219
- YouWCJinFDevesaSRapid increase in colorectal cancer rates in urban Shanghai, 1972-97, in relation to dietary changesJ Cancer Epidemiol Prev20027314314612665213
- YamamotoFYamamotoMScanning copy number and gene expression on the 18q21-qter chromosomal region by the systematic multiplex PCR and reverse transcription-PCR methodsElectrophoresis200728121882189517523142
- FurtadoJBentoMCorreiaEInácioJMBeloJAExpression and function of Ccbe1 in the chick early cardiogenic regions are required for correct heart developmentPLoS One2014912e11548125545279
- ZouZEnisDRBuiHThe secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesisBlood2013121163228323623426945
- HoganBMBosFLBussmannJCcbe1 is required for embryonic lymphangiogenesis and venous sproutingNat Genet200941439639819287381
- BosFLCauntMPeterson-MaduroJCCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivoCirc Res2011109548649121778431
- HägerlingRPollmannCAndreasMA novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultra-microscopyEMBO J201332562964423299940
- WeijtsBGvan ImpelASchulte-MerkerSde BruinAAtypical E2fs control lymphangiogenesis through transcriptional regulation of Ccbe1 and Flt4PLoS One201389e7369324069224
- AstinJWHaggertyMJOkudaKSVegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sproutingDevelopment2014141132680269024903752
- JeltschMJhaSKTvorogovDCCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activationCirculation2014129191962197124552833
- PollmannCHägerlingRKieferFVisualization of lymphatic vessel development, growth, and functionAdv Anat Embryol Cell Biol201421416718624276894
- BartonCAGlossBSQuWCollagen and calcium-binding EGF domains 1 is frequently inactivated in ovarian cancer by aberrant promoter hypermethylation and modulates cell migration and survivalBr J Cancer20101021879619935792
- LiPCongZQiangYClinical significance of CCBE1 expression in lung cancerMol Med Rep20181722107211229207117
- MesciAHuangXTaebSTargeting of CCBE1 by miR-330-3p in human breast cancer promotes metastasisBr J Cancer2017116101350135728419078
- MesciALiuSKCollagen and Calcium Binding EGF Domains 1 (CCBE1) in cancer - a new role past lymphatics?Oncoscience2017411–1216816929344552
- TianGAZhuCCZhangXXCCBE1 promotes GIST development through enhancing angiogenesis and mediating resistance to imatinibSci Rep201663107127506146
- GuoRLWangXRWangQGThe regulatory role of SLP-2 and mechanism on CCBE1 gene expression in rectal carcinoma and adjacent lymphatic tube tissuesEur Rev Med Pharmacol Sci2018221879429364474
- ZhangLLiuFJExpression of SLP-2 gene and CCBE1 are associated with prognosis of rectal cancerEur Rev Med Pharmacol Sci20172161214121828387911
- PeiQZhuHTanFIntravascular emboli is an independent risk factor for the prognosis of stage III colorectal cancer patients after radical surgeryOncotarget2016735572685727627528226
- GuDSzallasiAThrombocytosis Portends Adverse Prognosis in Colorectal Cancer: A Meta-Analysis of 5,619 Patients in 16 Individual StudiesAnticancer Res20173794717472628870890
- CronerRSFörtschTBrücklWMMolecular signature for lymphatic metastasis in colorectal carcinomasAnn Surg2008247580381018438117
- SunZOuCRenWXieXLiXLiGDownregulation of long non-coding RNA ANRIL suppresses lymphangiogenesis and lymphatic metastasis in colorectal cancerOncotarget2016730475364755527286457
- TerpeHJStörkelSZimmerUExpression of CD44 isoforms in renal cell tumors. Positive correlation to tumor differentiationAm J Pathol199614824534638579108
- TaoYMHuangJLZengSBTB/POZ domain-containing protein 7: epithelial-mesenchymal transition promoter and prognostic biomarker of hepatocellular carcinomaHepatology20135762326233723325674
- NaxerovaKReiterJGBrachtelEOrigins of lymphatic and distant metastases in human colorectal cancerScience20173576346556028684519
- TacconiCCorrealeCGandelliAVascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasionGastroenterology20151481451714381451
- AldersMHoganBMGjiniEMutations in CCBE1 cause generalized lymph vessel dysplasia in humansNat Genet200941121272127419935664
- JacksonCCBestLLorenzoLA Multiplex Kindred with Hennekam Syndrome due to Homozygosity for a CCBE1 Mutation that does not Prevent Protein ExpressionJ Clin Immunol2016361192726686525
- ArteagaALeachSDHarariPMSpreading Colon Cancer Can Bypass Lymph NodesCancer Discov201779924925
