Abstract
Background
Non-small-cell lung cancer (NSCLC) is a global public health problem, and brain is a common metastatic site in advanced NSCLC. Currently, whole-brain radiotherapy (WBRT) remains a major treatment for brain metastases, while EGFR-tyrosine kinase inhibitor (TKI) is the standard treatment for advanced NSCLC harboring EGFR mutations, which is also effective for brain metastases. However, whether EGFR-TKIs plus radiotherapy is superior to EGFR-TKIs alone for the treatment of advanced EGFR-mutant NSCLS with brain metastases remains controversial. This study aimed to compare the efficacy of concurrent EGFR-TKIs and WBRT vs EGFR-TKI alone in a retrospective cohort of advanced EGFR-mutant NSCLS with brain metastases.
Patients and methods
The medical records of 104 treatment-naïve, advanced EGFR-mutant NSCLC patients with brain metastases were retrospectively reviewed, and there were 56 patients undergoing concurrent EGFR-TKI and WBRT, and 48 patients given EGFR-TKI alone, including 20 cases with salvage WBRT upon brain metastasis progression. The survival prognosis was compared between the two cohorts.
Results
The baseline clinicopathologic factors were balanced between the two cohorts. After a median follow-up of 23 months, 35.6% of the study subjects survived. Concurrent EGFR-TKI and WBRT significantly improved the median intracranial PFS (iPFS) compared with EGFR-TKI alone (17.7 vs 11.0 months, P=0.015); however, no significant difference was seen in median overall survival between the two cohorts (28.1 vs 24.0 months, P=0.756). In addition, the median iPFS was found to significantly vary in the number of brain metastases (≤3 vs>3 metastases: 18.0 vs 12.5 months, P=0.044). Subgroup analysis showed that concurrent EGFR-TKI and WBRT improved median iPFS compared with EGFR-TKI alone in patients with more than three brain metastases (P=0.001); however, no significant difference was observed between the two regimens in patients with three or less brain metastases (P=0.526).
Conclusion
Our data demonstrate that concurrent EGFR-TKI and WBRT achieves longer iPFS than EGFR-TKI alone in advanced EGFR-mutant NSCLC with brain metastases. In advanced EGFR-mutant NSCLC with three or less brain metastases, EGFR-TKI alone may be an option as a first-line therapy.
Introduction
Lung cancer, the most common type of cancer and the leading cause of cancer-related deaths worldwide, remains a great threat to public health across the world.Citation1 Each year, >1 million new cases are diagnosed with lung cancer, and over 1 million deaths are attributed to this malignancy.Citation2 As a major type of lung cancer, non-small-cell lung cancer (NSCLC) accounts for ~85% of all lung cancers.Citation3
Metastasis determines the design of the treatment regimen and prognosis in NSCLC patients.Citation4,Citation5 Brain is a common metastatic site in patients with NSCLC, and ~20%–25% of the NSCLC patients at initial diagnosis are estimated to have brain metastasis.Citation6,Citation7 It has been shown that the NSCLC patients with brain metastases have a poor prognosis, low quality of life, and short survival, with a median survival period of only 1–2 months in untreated patients.Citation8–Citation11
Currently, the treatment options for NSCLC with brain metastasis mainly include stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), surgery, chemotherapy, targeted therapy, and symptomatic and supportive treatment.Citation12–Citation14 WBRT and SRS remain the standard treatment for brain metastases in NSCLC; however, such treatments exhibit limited effects on patients’ survival, which only prolong the survival period of 4–6 months.Citation15,Citation16 Conventional therapies, such as chemotherapy, have shown an unsatisfactory efficacy in the treatment of brain metastases in NSCLC due to the presence of blood–brain barrier (BBB).Citation17 A search for treatments that may greatly prolong the survival and improve the prognosis has, therefore, been paid much attention in NSCLC patients with brain metastases.
Driver gene-targeted therapy achieves better survival benefits for the patients with advanced NSCLC, and EGFR is a predominant driver oncogene in NSCLC.Citation18 EGFR-tyrosine kinase inhibitors (TKIs) have been accepted as the initial therapy for advanced NSCLC patients with EGFR-sensitive mutations, and EGFR-TKI treatment was reported to achieve an overall response rate (ORR) of 55%–85%, progression-free survival (PFS) of ~12 months, and overall survival (OS) of 24–36 months in advanced EGFR-mutant NSCLC patients.Citation19–Citation21 Data from Phase 3 clinical trials have shown that EGFR-TKI treatment may increase the objective response rate (RR), prolong the PFS, and cause significantly lower incidence of adverse reactions in NSCLC patients relative to in platinum-containing double-drug chemotherapy.Citation22,Citation23
It has been found that the NSCLC patients harboring an EGFR mutation have a higher incidence of brain metastasis (30%–70%) than those without EGFR mutations, and the management of brain metastasis is, therefore, critical to antitumor therapy in NSCLC patients harboring an EGFR mutation.Citation24–Citation27 Data from clinical trials have demonstrated that EGFR-TKIs may cause regression of established brain metastases from NSCLC, with an intracranial RR of 75% in treatment-naïve, advanced EGFR-mutant NSCLC patients.Citation28–Citation30 To date, the optimal timing of brain radiotherapy, however, remains controversial in EGFR-mutant lung cancer patients with brain metastasis in the presence of well-controlled intracranial and extracranial lesions by EGFR-TKIs. The present study was, therefore, designed to compare the efficacy of concurrent EGFR-TKIs and WBRT vs EGFR-TKI alone in a retrospective cohort of advanced EGFR-mutant NSCLS with brain metastases, so as to provide insights into the optimal timing of brain radiotherapy.
Patients and methods
Study subjects
The medical records of 104 treatment-naïve, advanced EGFR-mutant NSCLC patients with brain metastases admitted to Fujian Provincial Cancer Hospital (Fuzhou, People’s Republic of China) during the period from January 1, 2012 to June 30, 2017 were retrospectively reviewed. All NSCLCs were definitively diagnosed with pathologic examinations, and EGFR mutation analysis was performed using amplification refractory mutation system PCR or next-generation sequencing. There were 56 patients undergoing concurrent EGFR-TKI and WBRT, and 48 patients given EGFR-TKI alone, including 20 cases with salvage WBRT upon brain metastasis progression.
Treatment regimens
All patients received an initial MRI scan of the brain prior to administration of EGFR-TKIs and every 2–3 months post-treatment with EGFR-TKIs. All subjects were given 150 mg erlotinib (Roche, Basel, Switzerland) once a day (QD), 250 mg gefitinib (AstraZeneca Pharmaceuticals, Waltham, MA, USA) QD or 150 mg icotinib (Betta Pharmaceuticals Co., Ltd., Hangzhou, People’s Republic of China) three times a day, and WBRT was delivered at a dose of 30 Gy divided in ten fractions for 5 days a week, given at a total of 2 weeks. The response to therapy was evaluated according to the Response Evaluation Criteria in Solid Tumor version 1.1, 1 month after the initial treatment with EGFR-TKIs,Citation31 followed by once every 2–3 months, and the adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0.Citation32
Survival analysis
Intracranial PFS (iPFS) was defined as the survival from initial EGFR-TKIs treatment to intracranial progression following WBRT. OS was estimated from the date of initial diagnosis until the date of death or the last follow-up.
Statistical analysis
All data were entered into Microsoft Excel 2007 (Microsoft, Inc., Redmond, WA, USA), and all statistical analyses were performed using the statistical software SPSS version 23.0 (IBM Corporation, Armonk, NY, USA). Chi-squared test or Fisher’s exact test was used to compare the clinicopathologic characteristics between the two cohorts. Survival curves were generated using the Kaplan–Meier method, and the survival probability was compared with the log-rank test. A Cox regression model was employed for univariate and multivariate analyses to evaluate the corresponding 95% CIs and HRs. A P value of <0.05 was considered statistically significant.
Informed consent
All participants signed the informed consent pertaining to targeted therapy or brain radiotherapy. All subjects involved in this study agreed to publish related demographic and clinical features.
Ethical approval
The study protocol was reviewed and approved by the Ethical Review Committee of Fujian Provincial Cancer Hospital (approval no. FJZLYY2015-00179). All experimentations described in this study were conducted in accordance with the Declaration of Helsinki.
Results
Clinicopathologic characteristics of the study subjects
The study subjects included 45 men and 59 women, and had a median age of 59 years (range, 23–79 years) at diagnosis. Of all subjects, 97.1% were diagnosed with adenocarcinoma and 88.5% had an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 0 or 1. There were 83 cases with extracranial metastases and 48 cases with symptomatic brain metastases initially. In addition, there were 39 cases harboring an EGFR exon 19 deletion mutation, 39 cases harboring an EGFR exon 21L858R mutation, 5 cases harboring an EGFR exon 21L861Q mutation, 4 cases harboring an EGFR exon 18G719X mutation, 4 cases harboring combined EGFR exon 21L858R and exon 20 T790M mutations, 4 cases harboring combined EGFR exon 19 deletion mutation and exon 20 T790M mutation, while the mutation sites of the other 9 cases were unclear. The baseline clinicopathologic features were balanced between the two cohorts ().
Table 1 Clinicopathologic characteristics of the study subjects
Intracranial progression
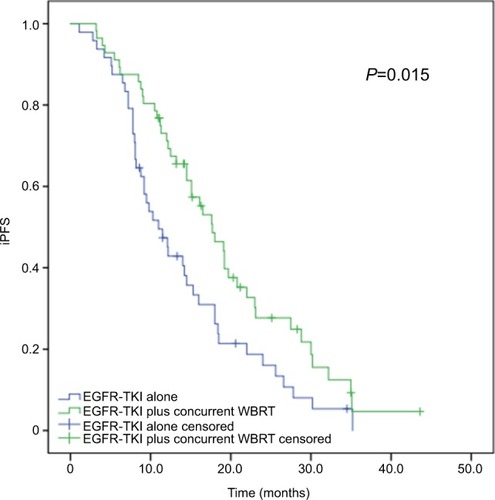
The subjects received follow-up for a median period of 23 months (range, 5–82 months). At the end of the follow-up period (June 30, 2017), 36 subjects remained alive and 66 cases were dead due to disease progression. Of all subjects, 86 cases (82.7%) had an intracranial progressive disease, with a median iPFS of 14.5 months (95% CI, 11.924–17.076 months). Intracranial progression developed in 76.8% (43/56) of the patients receiving EGFR-TKIs plus and WBRT, while intracranial progression occurred in 89.6% (43/48) of the cases given EGFR-TKIs alone. The median iPFS was 17.7 months (95% CI, 16.288–22.212 months) in the patients receiving concurrent EGFR-TKIs and WBRT, which was significantly longer than that of those given EGFR-TKIs alone (median iPFS, 11.0 months; 95% CI, 8.067–13.933 months; P=0.015), as shown in and .
Table 2 iPFS and OS according to subgroups
Figure 1 Kaplan–Meier curves for iPFS in advanced EGFR-mutant NSCLC patients.
Notes: The median iPFS is significantly longer in patients receiving concurrent EGFR-TKIs and WBRT than in those given EGFR-TKIs alone (17.7 vs 11.0 months, P=0.015).
Abbreviations: iPFS, intracranial progression-free survival; NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiotherapy.
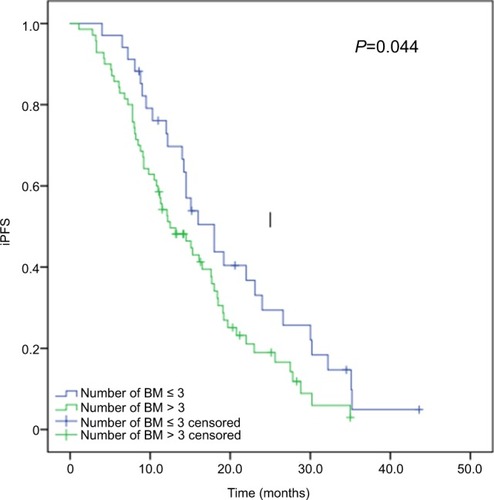
The 70 patients with more than three brain metastases had a median iPFS of 12.5 months (95% CI, 12.952–17.432 months), which was significantly shorter than that of 34 cases with three or less brain metastases (median iPFS, 18.0 months; 95% CI, 16.142–23.554 months; P=0.044), as shown in .
Figure 2 Kaplan–Meier curves for iPFS in advanced EGFR-mutant NSCLC patients with various number of brain metastases.
Note: Patients with more than three brain metastases had significantly shorter median iPFS than those with three or less brain metastases (12.5 vs 18.0 months, P=0.044).
Abbreviations: iPFS, intracranial progression-free survival; NSCLC, non-small-cell lung cancer.
Among the study subjects, there were 6 patients (5.8%) with complete response and 42 patients (40.4%) with partial response, yielding an ORR of 46.2%. In addition, there were 52 patients (50.0%) with stable disease. The ORR was comparable between the patients receiving concurrent EGFR-TKIs and WBRT and EGFR-TKIs alone (48.2% vs 54.2%, P=0.562; ). Cox multivariate regression analysis revealed that concurrent EGFR-TKIs and WBRT was an independent predictor of iPFS (P=0.004) and more than three brain metastases was a potential independent predictor unfavorably affecting iPFS in advanced EGFR-mutant NSCLC patients (P=0.017; ).
Table 3 Response to therapy in the two study cohorts
Table 4 Univariable and multivariable analyses of covariables associated with intracranial progression-free survival
Survival outcomes
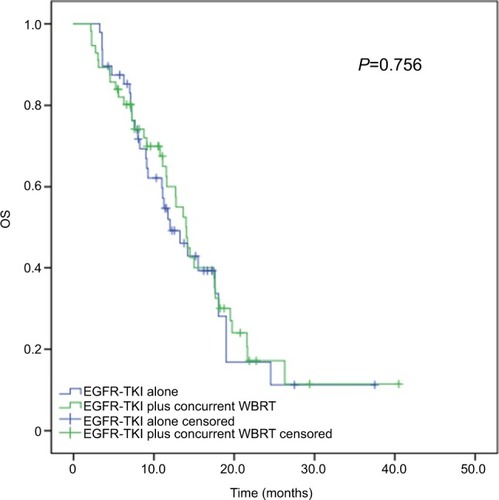
Thirty-six patients survived until the end of the followup period. Among all study subjects, the median OS was 27.3 months (95% CI, 23.109–31.491 months), and no significant difference was found in the median OS between the patients receiving concurrent EGFR-TKIs and WBRT (median OS, 28.1 months; 95% CI, 23.975–32.225 months) and EGFR-TKIs alone (median OS, 24.0 months; 95% CI, 17.428–30.572 months), with a P-value of 0.756. Among the 48 patients given EGFR-TKIs alone, there were 20 cases with salvage WBRT upon brain metastasis progression, and the median OS did not significantly differ in patients receiving concurrent EGFR-TKIs and WBRT (median OS, 28.1 months; 95% CI, 23.975–32.225 months), salvage WBRT (median OS, 36.1 months; 95% CI, 19.678–52.622 months), and EGFR-TKIs alone (median OS, 22.5 months; 95% CI, 14.990–30.010 months), with a P-value of 0.366 ().
Figure 3 Kaplan–Meier curves for OS in advanced EGFR-mutant NSCLC patients receiving various treatment regimens.
Note: The median OS did not significantly differ in patients receiving concurrent EGFR-TKIs and WBRT (median OS, 28.1 months; 95% CI, 23.975–32.225 months) and EGFR-TKIs alone (median OS, 24.0 months; 95% CI, 17.428–30.572 months), with the P-value being 0.756.
Abbreviations: NSCLC, non-small-cell lung cancer; OS, overall survival; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiotherapy.
Subgroup analyses
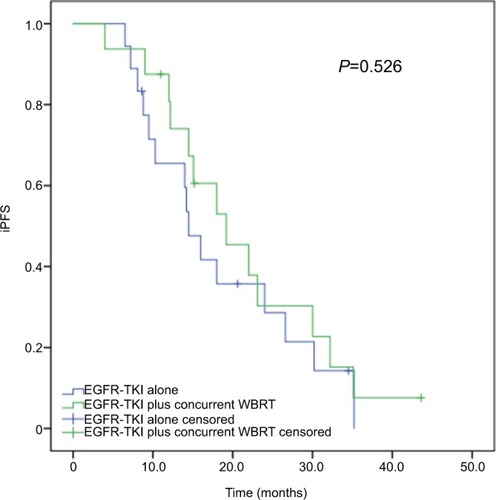
Subgroup analyses revealed that of the 70 patients with more than three brain metastases, the median iPFS was significantly longer in cases receiving concurrent EGFR-TKIs and WBRT (median iPFS, 17.6 months; 95% CI, 14.176–21.024 months) than in those given EGFR-TKIs alone (median iPFS, 9.2 months; 95% CI, 6.924–11.476 months; P=0.001), as shown in . Among the 34 patients with three or less brain metastases, however, no significant difference was detected in the median iPFS between the patients receiving concurrent EGFR-TKIs and WBRT and EGFR-TKIs alone (P=0.526; ).
Figure 4 Subgroup analysis of iPFS in advanced EGFR-mutant NSCLC patients with more than three brain metastases.
Note: The median iPFS was significantly longer in patients receiving concurrent EGFR-TKIs and WBRT than in those given EGFR-TKIs alone (17.6 vs 9.2 months, P=0.001).
Abbreviations: iPFS, intracranial progression-free survival; NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiotherapy.
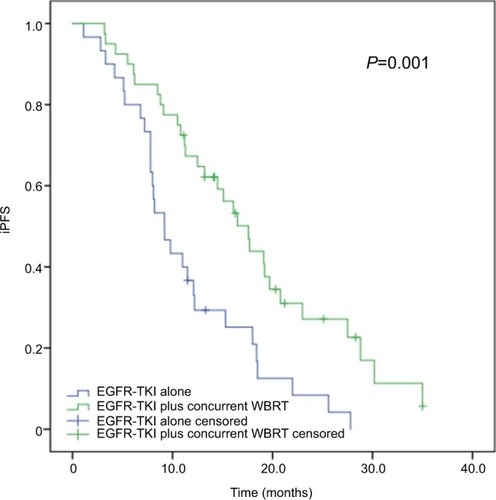
Figure 5 Subgroup analysis of iPFS in advanced EGFR-mutant NSCLC patients with three or less brain metastases.
Note: The median iPFS was comparable between the patients receiving concurrent EGFR-TKIs and WBRT and EGFR-TKIs alone (19.2 vs 14.5 months, P=0.526).
Abbreviations: iPFS, intracranial progression-free survival; NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiotherapy.
Toxicity
During the treatment period, the incidence of adverse events was 85.7% and 83.3% in the subjects receiving concurrent EGFR-TKIs and WBRT and EGFR-TKIs alone. In cases given EGFR-TKIs alone, the incidence of grade 3 and higher adverse events was 2.1%, and in the cases receiving concurrent EGFR-TKIs and WBRT, the incidence of grade 3 and higher adverse events was 8.9%, including a case with reduced drug doses due to the development of grade 3 diarrhea. The WBRT-related adverse events mainly included dizziness and headache, which were alleviated following dehydration treatment, as well as neurocognitive impairment (grade 1 and 2 in three cases and grade 3 in one case), as shown in .
Table 5 Treatment-related toxicities
Discussion
Currently, the treatment of advanced NSCLC with brain metastasis remains a great challenge,Citation8–Citation10 and EGFR-mutant NSCLC has been found to be more likely to present brain metastases.Citation24–Citation27 Prior to the introduction of EGFR-TKI, WBRT, surgical resection of the metastatic lesions, and SRS were the standard treatments of brain metastasis; however, radiotherapy and surgery cause non-ignorable brain damages, which may seriously affect patients’ survival and quality of life.Citation12–Citation16 EGFR-TKI is an emerging effective treatment option for EGFR-mutant lung cancer patients with brain metastasis.Citation33 Results from the CTONG-0803 Phase 2 clinical trial showed that the single-agent erlotinib at a daily dose of 150 mg was active and well tolerated as a second-line therapy in NSCLC patients with brain metastasis, which achieved 10.1 months iPFS.Citation34 Another multicenter, Phase 3, open-label, parallel, randomized controlled trial to compare the efficacy of icotinib vs whole-brain irradiation with or without chemotherapy revealed that icotinib treatment (median iPFS, 10.1 months) achieved significantly longer iPFS than WBI plus chemotherapy (median iPFS, 4.8 months) in patients with EGFR-mutant NSCLC and multiple brain metastases.Citation35 Since brain radiotherapy may cause brain injuries, the optimal timing of brain radiotherapy remains in dispute until now. In EGFR-mutant NSCLC patients with brain metastasis, is the first-line brain radiotherapy is required? Does brain radiotherapy serve as a salvage therapy upon intracranial tumor progression? These questions remain to be answered. Previous studies have shown that concurrent EGFR-TKI and brain radiotherapy (WBRT or SRS) may increase the intracra-nial disease control rate and extend the OS in EGFR-mutant NSCLC patients.Citation36–Citation38 In addition, a recent multi-institutional analysis demonstrated that SRS followed by EGFR-TKI resulted in the longest OS and allowed patients to avoid the potential neurocognitive sequelae of WBRT in EGFR-mutant NSCLC patients with brain metastases.Citation39
Our data showed that concurrent EGFR-TKIs and WBRT achieved significantly longer iPFS than EGFR-TKIs alone (median iPFS, 17.7 vs 11.0 months, P=0.015), which was similar to a previous study reporting that concurrent early brain radiotherapy with EGFR-TKI may improve intracranial disease control in EGFR-mutant NSCLC with brain metastasis.Citation38 Results from a Phase 2 clinical trial showed that erlotinib in combination with WBRT was well tolerated with a favorable objective RR in patients with brain metastases from NSCLC.Citation40 Due to the protection of the BBB, the drug concentration is not high in the central nervous system (CNS).Citation41 Therefore, optimal CNS penetration is a critical issue for patients with brain metastases.Citation42 Brain radiotherapy is found to destroy the BBB and increase the TKIs concentration in the cerebrospinal fluid.Citation43,Citation44 In addition, it has been shown that EGFR mutation is a radiosensitive NSCLC genotype and EGFR-TKI is supposed to be a radiosensitizer.Citation43,Citation45,Citation46 It is, therefore, hypothesized that EGFR-TKI and WBRT may have a synergistic effect, and the combination of EGFR-TKI and WBRT may result in better intracranial tumor control in NSCLC harboring EGFR mutations. Taking these findings together, it is considered that concurrent EGFR-TKIs with WBRT may achieve better intracranial tumor control in NSCLC harboring EGFR mutations.
In the current study, concurrent EGFR-TKIs with WBRT (median OS, 28.1 months) achieved extension of the OS relative to EGFR-TKIs alone (median OS, 24 months), although no significant difference was seen between these two regimens in terms of the median OS (P=0.756). Of the 48 patients initially treated with EGFR-TKI alone, there were 20 cases receiving salvage WBRT upon brain metastasis progression, and 58.3% of the cases lost the timing for WBRT due to physical intolerance and treatment abandonment. Our data showed no significant difference in the OS between the patients receiving concurrent EGFR-TKIs with WBRT and EGFR-TKIs alone. Follow-up revealed that the majority of the patients were well tolerant to WBRT, with only a case developing grade 3 neurocognitive impairment, who had a 4.5 OS. Further studies to examine the effect of WBRT-induced neurocognitive impairment on survival in EGFR-mutant NSCLC patients with brain metastasis are required. We, therefore, recommend concurrent EGFR-TKI with brain radiotherapy as an initial therapy for EGFR-mutant NSCLC patients with brain metastasis. A retrospective multi-institutional analysis showed that SRS followed by EGFR-TKI (46 months) achieved longer median OS than WBRT followed by EGFR-TKI (30 months) and EGFR-TKI followed by SRS or WBRT (25 months) at intracranial progression in EGFR-mutant NSCLC patients with brain metastases (P<0.001).Citation39 In addition, SRS was found to have less toxicity and avoided the potential neurocognitive sequelae from WBRT.Citation47,Citation48 EGFR-TKI plus brain radiotherapy, notably SRS, as an initial therapy, may improve the survival and avoid losing the timing for radiotherapy, which may be an optimal option for the treatment of EGFR-mutant NSCLC with brain metastasis. However, a prospective, randomized clinical trial is urgently needed to confirm the findings.
In this study, a Cox multivariate regression analysis revealed that the number of brain metastasis was an independent predictor of iPFS, and advanced EGFR-mutant NSCLC patients with three or less brain metastases at initial diagnosis affected iPFS favorably. Previous studies have demonstrated that ECOG performance status, EGFR mutation subtype, and absence of extracranial metastases are independent predictors of iPFS and OS in NSCLC patients with brain metastasis,Citation39,Citation49 which was not found in the present study. Our data report a single-institution experience and the study sample is small. Further large-scale, multicenter, retrospective studies to identify the factors affecting iPFS and OS in NSCLC patients with brain metastasis are warranted.
Currently, neurosurgery or radiosurgery is the standard option for the radical therapy of locally intracranial metastatic tumors, and SRS is accepted as the first choice for the local treatment of locally intracranial metastatic tumors.Citation50,Citation51 However, either surgery or radiotherapy is invasive and has side effects, while EGFR-TKI shows a high response and few adverse effects for intracranial metastatic tumors.Citation50,Citation51 To defer or avoid neurocognitive sequelae from WBRT, it is necessary to identify the patient population given EGFR-TKI alone as the initial therapy. Our subgroup analysis showed that concurrent EGFR-TKI and WBRT improved median iPFS compared with EGFR-TKI alone in patients with more than three brain metastases (P=0.001); however, no significant difference was observed between the two regimens in patients with three or less brain metastases (P=0.526). It is hypothesized that, in EGFR-mutant NSCLC with three or less brain metastases, EGFR-TKI alone may be an option as a first-line therapy; however, this requires further investigations to test such a hypothesis. Osimertinib, an oral, CNS-active, third-generation EGFR-TKI, was found to be effective in crossing the BBB and penetrating the CNS.Citation52 Data from the FLAURA study revealed that osimertinib achieved a significantly higher CNS objective RR (66%) than the first-generation EGFR-TKI and remarkably reduced the risk of intracranial progression in advanced NSCLC patients with EGFR-TKI-sensitizing mutation.Citation53 In Asian patients with previously untreated advanced NSCLC harboring exon 19 deletion (Ex19del)/L858R EGFR-TKI-sensitizing mutations, first-line osimertinib demonstrated a clinically meaningful improvement in PFS over the standard of care EGFR-TKI (gefitinib or erlotinib) (median PFS, 16.5 vs 11.0 months, HR =0.54, 95% CI, 0.41–0.72; P<0.0001).Citation54 However, more investigations are required to demonstrate the likelihood of osimertinib as an alternative of brain radiotherapy in EGFR-mutant, advanced NSCLC patients with brain metastases. In addition, prospective studies are needed to identify the patient population given EGFR-TKI alone in whom brain radiotherapy is not needed, in order to protect them against radiation-induced neurocognitive sequelae from WBRT.
Conclusion
The results of the present study demonstrate that concurrent EGFR-TKI and WBRT may improve intracranial disease control in advanced EGFR-mutant NSCLC with brain metastasis. Initial treatment with EGFR-TKI alone may cause a loss of timing for WBRT, and concurrent EGFR-TKI with WBRT may be an optimal option for EGFR-mutant NSCLC with brain metastasis at the initial diagnosis. However, EGFR-TKI alone may be an option as the first-line therapy in patients with three or less brain metastases, which may defer or avoid neurocognitive sequelae from WBRT. Further multi-institutional, prospective, randomized clinical trials to validate our findings and determine the optimal treatment for EGFR-mutant NSCLC with brain metastasis seem justified.
Data sharing statement
All data reported in this study are available upon request by contacting the corresponding author.
Acknowledgments
This study was supported by grants from the Fujian Provincial Medical Innovative Project (grant no. 2017-CX-7), Fujian Provincial Sci & Tech Innovative Joint Fund (grant no. 2017Y9080), and Fujian Provincial Sci & Tech Guiding Project (grant no. 2018Y0017).
Disclosure
The authors report no conflicts of interest in this work.
References
- HerbstRSHeymachJVLippmanSMLung cancerN Engl J Med2008359131367138018815398
- GouvinhasCDe MelloRAOliveiraDLung cancer: a brief review of epidemiology and screeningFuture Oncol201814656757529417838
- GoldstrawPBallDJettJRNon-small-cell lung cancerLancet201137898041727174021565398
- BerhouneMBanuEScotteFPrognonPOudardSBonanBTherapeutic strategy for treatment of metastatic non-small cell lung cancerAnn Pharmacother200842111640165218957625
- HerbstRSMorgenszternDBoshoffCThe biology and management of non-small cell lung cancerNature2018553768944645429364287
- RiihimäkiMHemminkiAFallahMMetastatic sites and survival in lung cancerLung Cancer2014861788425130083
- QuintLETummalaSBrissonLJDistribution of distant metastases from newly diagnosed non-small cell lung cancerAnn Thorac Surg19966212462508678651
- D’AntonioCPassaroAGoriBBone and brain metastasis in lung cancer: recent advances in therapeutic strategiesTher Adv Med Oncol20146310111424790650
- DaweDEGreenspoonJNEllisPMBrain metastases in non-small-cell lung cancerClin Lung Cancer201415424925724954227
- TaimurSEdelmanMJTreatment options for brain metastases in patients with non-small-cell lung cancerCurr Oncol Rep20035434234612781078
- WaqarSNSamsonPPRobinsonCGNon-small-cell lung cancer with brain metastasis at presentationClin Lung Cancer2018194e373e37929526531
- UlahannanDKhalifaJFaivre-FinnCLeeSMEmerging treatment paradigms for brain metastasis in non-small-cell lung cancer: an overview of the current landscape and challenges aheadAnn Oncol201728122923293129045549
- MulvennaPMThe management of brain metastases in patients with non-small cell lung cancer – is it time to go back to the drawing board?Clin Oncol (R Coll Radiol)201022536537320395118
- TsakonasGDe PetrisLEkmanSManagement of brain metastasized non-small cell lung cancer (NSCLC) – from local treatment to new systemic therapiesCancer Treat Rev20175412213128254730
- WonYKLeeJYKangYNStereotactic radiosurgery for brain metastasis in non-small cell lung cancerRadiat Oncol J201533320721626484304
- KhuntiaDBrownPLiJMehtaMPWhole-brain radiotherapy in the management of brain metastasisJ Clin Oncol20062481295130416525185
- ZimmermannSDziadziuszkoRPetersSIndications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastasesCancer Treat Rev201440671672224759599
- TabchiSKourieHRKlasterskyJConcurrent driver mutations/rearrangements in non-small-cell lung cancerCurr Opin Oncol201729211812228027105
- MokTSWuYLThongprasertSGefitinib or carboplatin–paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- BrownTBolandABagustAGefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancerHealth Technol Assess201014Suppl 2717921047494
- MaemondoMInoueAKobayashiKNorth-East Japan Study GroupGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MitsudomiTMoritaSYatabeYWest Japan Oncology GroupGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trialLancet Oncol201011212112820022809
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, CTONG-0802): a multicentre, open-label, randomised, Phase 3 studyLancet Oncol201112873574221783417
- HeonSYeapBYBrittGJDevelopment of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinibClin Cancer Res201016235873588221030498
- LeeYJChoiHJKimSKFrequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancerCancer201011651336134320066717
- BhattVRKediaSKessingerAGantiAKBrain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutationsJ Clin Oncol201331253162316423897953
- ShinDYNaIIKimCHParkSBaekHYangSHEGFR mutation and brain metastasis in pulmonary adenocarcinomasJ Thorac Oncol20149219519924419416
- HeonSYeapBYLindemanNIThe impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutationsClin Cancer Res201218164406441422733536
- IuchiTShingyojiMSakaidaTPhase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinomaLung Cancer201382228228724021541
- HanJYParkKKimSWFirst-SIGNAL: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lungJ Clin Oncol201230101122112822370314
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- ChenAPSetserAAnadkatMJGrading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0J Am Acad Dermatol20126751025103922502948
- SimoffMJLallyBSladeMGSymptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelinesChest20131435 Supple455Se497S23649452
- WuYLZhouCChengYErlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a Phase II study (CTONG-0803)Ann Oncol201324499399923129122
- YangJJZhouCHuangYIcotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (brain): a multicentre, Phase 3, open-label, parallel, randomised controlled trialLancet Respir Med20175970771628734822
- ChenYYangJLiXFirst-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinomaCancer Sci2016107121800180527627582
- MagnusonWJYeungJTGuillodPDGettingerSNYuJBChiangVLImpact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastasesInt J Radiat Oncol Biol Phys201695267367927034176
- LiuYDengLZhouXConcurrent brain radiotherapy and EGFR-TKI may improve intracranial metastases control in non-small cell lung cancer and have survival benefit in patients with low DS-GPA scoreOncotarget201786711130911131729340055
- MagnusonWJLester-CollNHWuAJManagement of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysisJ Clin Oncol201735101070107728113019
- WelshJWKomakiRAminiAPhase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancerJ Clin Oncol201331789590223341526
- BanksWAFrom blood–brain barrier to blood–brain interface: new opportunities for CNS drug deliveryNat Rev Drug Discov201615427529226794270
- UpadhyayRKDrug delivery systems, CNS protection, and the blood brain barrierBiomed Res Int2014201486926925136634
- KhalifaJAminiAPopatSGasparLEFaivre-FinnCInternational Association for the Study of Lung Cancer Advanced Radiation Technology CommitteeBrain metastases from NSCLC: radiation therapy in the era of targeted therapiesJ Thorac Oncol201611101627164327343440
- ZhangJYuJSunXMengXEpidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell lung cancerCancer Lett2014351161224861428
- JohungKLYaoXLiFA clinical model for identifying radio-sensitive tumor genotypes in non-small cell lung cancerClin Cancer Res201319195523553223897899
- SpanoJPFagardRSoriaJCRixeOKhayatDMilanoGEpidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectivesAnn Oncol200516218919415668269
- ChangELWefelJSHessKRNeurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trialLancet Oncol200910111037104419801201
- HabetsEJDirvenLWiggenraadRGNeurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective studyNeuro Oncol201618343544426385615
- YangWCXiaoFShihJYEpidermal growth factor receptor mutation predicts favorable outcomes in non-small cell lung cancer patients with brain metastases treated with stereotactic radiosurgeryRadiother Oncol2018126236837429111173
- LinXDeAngelisLMTreatment of brain metastasesJ Clin Oncol201533303475348426282648
- LinJJandialRNesbitABadieBChenMCurrent and emerging treatments for brain metastasesOncology (Williston Park)201529425025725952487
- MalapelleURicciutiBBaglivoSOsimertinibRecent Results Cancer Res201821125727630069773
- VansteenkisteJReungwetwattanaTNakagawaKCNS response to osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFR-TKI sensitising mutation (EGFRm)-positive advanced non-small cell lung cancer (NSCLC): data from the FLAURA studyAnn Oncol201728suppl_10mdx782
- ChoBCChewaskulyongBLeeKHOsimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subsetJ Thorac Oncol20191419910630240852
