Abstract
Purpose
To compare the efficacy and safety of neoadjuvant chemotherapy (NACT) with gemcitabine (GEM) vs docetaxel plus cisplatin (CDDP) in locoregionally advanced nasopharyngeal carcinoma (NPC).
Methods
A total of 222 patients with locoregionally advanced NPC between February 2012 and May 2014 in our hospital who received NACT with GEM or docetaxel plus CDDP combined with concurrent chemoradiotherapy (CCRT) were retrospectively analyzed. Fifty-two patients treated with GEM plus CDDP (GP) combined with CCRT were matched with 52 patients who received docetaxel plus CDDP (TP) combined with CCRT.
Results
With a median follow-up time of 60 months (range, 14–72 months), the 5-year overall survival, progression-free survival (PFS), local relapse-free survival and distant metastasis-free survival (DMFS) rates were 78.8%, 66.0%, 81.0% and 75.9%, respectively, in the GP group and 79.4%, 60.5%, 79.6% and 73.6%, respectively, in the TP group. No statistically significant survival differences were found between the two groups. In multivariate analysis, T3–4 and N2–3 were prognostic factors for poor 5-year PFS and DMFS (all P-values <0.05). Patients in the TP group experienced less grade 3–4 thrombocytopenia but more grade 3–4 leucopenia and neutropenia than those in the GP group (all P-values <0.05). There were no significant differences between the two groups in other toxicities (all P-values >0.05).
Conclusion
NACT with GP or TP regimen achieved comparable clinical outcome with acceptable toxicities. Both regimens might be a treatment option for patients with locoregionally advanced NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a distinct type of head and neck cancer, which is rare in the west and endemic in South China and Southeast Asia.Citation1 It has a high incidence stretching from 20 to 30 per 100,000.Citation2 Because of the histological variation, epidemiology, specific anatomic location, and high sensitivity to irradiation, radiotherapy is the cornerstone of initial treatment of NPC.Citation3 For early-stage NPC patients, the 5-year overall survival (OS) rate of radiotherapy alone is about 84%– 90%.Citation4 However, many patients with NPC present with locoregionally advanced disease, and the outcome of them is unsatisfactory.Citation5 Based on the results of INT 0099 trail, concurrent chemoradiotherapy (CCRT) has become the basic treatment for locoregionally advanced NPC.Citation6 Therefore, the National Comprehensive Cancer Network guidelines has recommended platinum-based CCRT with or without adjuvant chemotherapy as the first-line treatment for NPC.Citation7
Nevertheless, CCRT may not be sufficient for certain high-risk subgroups, especially those with bulky tumors and/or extensive nodal disease who have higher potential for distant metastasis.Citation8,Citation9 Thus, it is necessary to add chemotherapy to CCRT. However, many patients suffered from severe toxicities during CCRT and could not tolerate the toxicities of adjuvant chemotherapy, which made the regimen of CCRT plus adjuvant chemotherapy undesirable. In recent years, neoadjuvant chemotherapy (NACT), delivered before CCRT, has become the initial choice of treatment for locally advanced NPC. As we know, NACT is beneficial for the rapid shrinkage of tumor size, which facilitates subsequent radiotherapy and early eradication of micrometastases, while not increasing toxicities during radiotherapy.
During the past two decades, many trials have investigated the role of NACT in NPC. Although some studies have achieved negative results,Citation10,Citation11 other studies have confirmed the survival benefits through adding NACT to radiotherapy.Citation12–Citation15 Under these circumstances, the efficacy of NACT remains uncertain and needs to be confirmed by more research. Although the regimen of 5-fluorouracil (5-FU) plus cisplatin (CDDP) has been widely used as the standard treatment for NPC, the main drawback of the regimen is inconvenience in administering continuous infusion 5-FU and development of common mucosal complications. Therefore, an exploration of effective therapies and regimens, which could improve outcome and decrease treatment-related toxicity in locoregionally advanced NPC is necessary.
Sun et alCitation16 conducted a multicenter, randomized controlled Phase III trial to compare the efficacy of docetaxel plus 5-FU and CDDP (TPF) NACT plus CCRT with that of CCRT alone in locoregionally advanced NPC. The results of this research showed that addition of TPF to CCRT significantly improved failure-free survival, OS, and distant failure-free survival. However, due to the treatment-related toxicities and patient refusal, less than one-third of patients had completed the 3 cycles of concurrent CDDP. A similar conclusion has been reached by Kong et al.Citation17 Recently, a Phase II trial by Wang et alCitation18 demonstrated that compared with TPF, docetaxel plus CDDP (TP) NACT resulted in similar survival outcomes but less severe toxicities.
Gemcitabine (GEM) is a pyrimidine analog, which inhibits DNA synthesis. A Phase III multi-center, randomized trail published in Lancet established GEM plus CDDP (GP) regimen as the standard first-line treatment option for patients with recurrent or metastatic NPC,Citation19 because GP regimen prolongs progression-free survival (PFS) for patients with recurrent or metastatic NPC. Other Phase II trials also indicated that GEM offers a satisfactory overall response rate and tolerable toxicities in patients with recurrent or metastatic NPC.Citation20–Citation22 Recently, some studies indicated that GP regimen can be used as NACT in locoregionally advanced NPC.Citation23–Citation26 Until now, the optimal NACT regimen for patients with locoregionally advanced NPC remains uncertain. Accordingly, we conducted this retrospective study to compare the efficacy and toxicity of GP and TP regimens as NACT for locoregionally advanced NPC patients.
Materials and methods
Patients
From February 2012 to May 2014, 222 pathologically confirmed locoregionally advanced NPC patients in Zhejiang Cancer Hospital who received NACT with GEM or TP combined with CCRT were retrospectively reviewed. Patients meeting the following enrollment criteria were recruited for the study: 1) pathologically confirmed NPC Stage III–IV B, 2) no distant metastasis, 3) no uncontrolled medical or psychiatric disease, 4) received GEM or TP as NACT, 5) without any previous malignancy or other concomitant malignant diseases, 6) have not received any antitumor treatment prior to admission, 7) Karnofsky Performance Status (KPS) Score ≥70. Due to the retrospective nature of the study, written informed consent was waived. This study was approved by the Research Ethics Committee of Zhejiang Cancer Hospital, which was performed in accordance with Declaration of Helsinki.
Clinical staging
The routine staging before treatment included complete history and physical examination, fiberoptic nasopharyngoscopy and biopsy, MRI of the head and neck region, chest CT, ultrasonography of the abdomen or CT, whole-body bone scan and complete blood cell count, comprehensive serum chemistry profile, and ECG. All patients were restaged according to the American Joint Committee on Cancer (AJCC) 2010 staging system.Citation27
Radiotherapy
Intensity-modulated radiation therapy (IMRT) was delivered to all the patients with 6 MV X-ray. Briefly, gross tumor volume of nasopharynx (GTVnx) included the primary tumor and positive retropharyngeal lymph nodes. Metastatic cervical lymph nodes were defined as gross tumor volume of involved cervical lymph nodes (GTVnd). The high-risk clinical target volume (CTV)1 included the GTVnx and GTVnd with a margin of 5–10 mm, entire nasopharynx, inferior two-thirds of the sphenoid sinus, the anterior third of the clivus, pterygoid fossae, posterior third of nasal cavity and maxillary sinuses, retropharyngeal nodes, parapharyngeal space, and the drainage of the upper neck. The low-risk CTV2 included CTV1 plus a margin of 3–5 mm, the lower neck and the supraclavicular lymphatic drainage region. The planning target volume (PTV) was defined as the area from 3 to 5 mm outside the CTV or GTV. The dose prescribed was as follows: 66–70 Gy to the PGTVnx and PGTVnd, 60 Gy to the PTV1, and 54 Gy to the PTV2. The total dose of the PGTVnx, PGTVnd, PTV1, and PTV2 were given in 30–33 fractions. All patients received one fraction daily for 5 days per week. Pinnacle version 7.6 planning system was used to design all the plans. Furthermore, maximum dose of each organ at risk was below its tolerance limit on the basis of the Radiation Therapy Oncology Group 0225 protocol.Citation28
Chemotherapy
In the GP group, the NACT regimen consisted of CDDP 25 mg/m2/day on days 1–3 and GEM 1,000 mg/m2/day on days 1 and 8 in a cycle of 21 days for 1–4 cycles. In the TP group, the NACT regimen consisted of CDDP 25 mg/m2/day on days 1–3 and docetaxel 75 mg/m2/day on day 1 in a cycle of 21 days for 1–4 cycles. Additionally, patients in both groups underwent IMRT concurrent with CDDP at a dose of 80–100 mg/m2 divided into 3 days in a cycle of 21 days for 1–2 cycles.
Follow-up
Treatment-induced toxicities were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. The follow-up time was calculated from the first day of treatment to the last follow-up or death. All patients were assessed every week during treatment and were regularly followed up after completion of the treatment once every 3 months during the first 2 years, once every 6 months from 3 to 5 years, and then once every year thereafter. The date of the final follow-up was in March 2018, and the median follow-up period was 60 months (ranging from 14 to 72 months). Patients who did not meet follow-up requirements more than twice were excluded. Our follow-up assessments consisted of evaluation of patient history, physical examination, fiberoptic nasopharyngoscopy, MRI examination for head and neck, chest CT, and ultrasonography of the abdomen or CT. Additionally, whole-body bone scan was performed when patient complained about pain in bone.
Statistical analyses
Propensity score matching (PSM)Citation29 was computed by logistic regression for each patient using the following covariates: age, gender, KPS, T category, N category, overall stage, NACT cycle, and WHO histological classification. Characteristics of patients were compared using chi-squared test or Fisher’s exact test. The Kaplan–Meier method was used to calculate the OS rate, local relapse-free survival (LRFS) rate, distant metastasis-free survival (DMFS) rate, and PFS rate. Multivariate analysis was estimated using the Cox proportional hazards model. Two-sided P-values <0.05 were considered to be statistically significant. The program Statistical Package for Social Sciences version 22 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Patient characteristics
From February 2012 to May 2014, we had identified 222 patients with locoregionally advanced NPC receiving either NACT with TP or GP regimen. For the whole cohort, the male (n=164) to female (n=58) ratio was 2.8:1, and the median age was 49 (range 17–74) years old. After matching by PSM, 52 pairs were selected from the original 222 patients and the baseline characteristics are summarized in . There were no significant differences between the two groups among the following variables: age (<50 vs ≥50), sex, histology, T stage, N stage, AJCC stage, KPS score, and NACT cycle (all P-values >0.05).
Table 1 Patient and disease characteristics
Efficacy
We evaluated the tumor response 3 months after the completion of radiotherapy. According to the RECIST criteria, responses were classified as complete response (CR), partial response, stable disease, or progressive disease. The tumor responses of the two groups are summarized in . In the TP group, the CR rate was 82.7% for the primary tumor and 78.8% for the metastatic nodes. But, in the GP group, the CR rate was 88.5% for the primary tumor and 84.6% for the metastatic nodes. There was no significant difference in CR rate either in the response of primary tumor or metastatic nodes between the two groups (all P-value >0.05).
Table 2 Tumor response 3 months after chemoradiotherapy in TP or GP group
Acute toxicity
Acute toxicities related to the two groups are summarized in . Patients in the TP group experienced less grade 3–4 thrombocytopenia but more grade 3–4 leucopenia and neutropenia than those in the GP group (11.5% vs 30.8%, P=0.017, 57.7% vs 25%, P=0.001% and 75% vs 34.6%, P<0.001, respectively). There were no significant differences between the two groups in anemia and non-hematologic toxicities, with all P-value >0.05. No treatment-related deaths were observed in either group.
Table 3 Frequency of acute toxicities from the two groups
Treatment outcomes
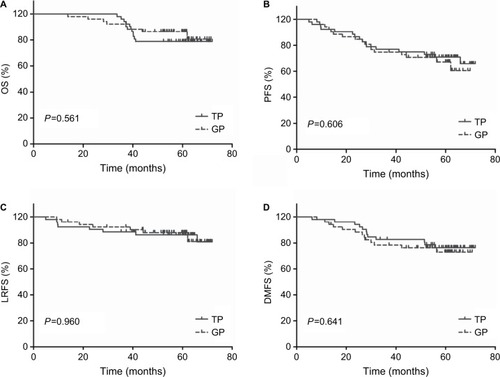
The median duration of follow-up was 60 months (range, 14–72 months). As shown in and , -year OS, PFS, LRFS, and DMFS rates did not differ significantly between the TP and the GP groups (OS: 78.8% vs 79.4%, P=0.561, PFS: 66% vs 60.5%, P=0.606, LRFS: 81% vs 79.6%, P=0.960, and DMFS: 75.9% vs 73.6%, P=0.641).
Table 4 Effect of prognostic factors on survival in univariate analysis
Figure 1 Kaplan–Meier survival curves for neoadjuvant chemotherapy with gemcitabine vs docetaxel plus cisplatin followed by concurrent chemoradiotherapy in the 52 pairs of patients with locally advanced nasopharyngeal carcinoma.
Notes: (A) OS. (B) PFS. (C) LRFS. (D) DMFS. P-values were calculated using the log-rank test.
Abbreviations: DMFS, distant metastasis-free survival; GP, gemcitabine plus cisplatin; LRFS, local relapse-free survival; OS, overall survival; PFS, progression-free survival; TP, docetaxel plus CDDP.
Prognostic factors
We used univariate and multivariable analysis to evaluate the factors which affected the survival of patients. The outcomes listed in showed that 5-year PFS and DMFS of patients with N0-1 stage were superior to those of N2-3 stage (PFS: 89.5% vs 57.2%, P=0.029, DMFS: 94.7% vs 69.8%, P=0.038), and patients with clinical stage IV were inferior to those patients with stage III (OS: 56.4% vs 85.2%, P=0.049, PFS: 41.8% vs 69.3%, P=0.003, DMFS: 44.8% vs 82%, P<0.001). Based on the results of previously reported studies and the univariate analysis, we evaluated several potential prognostic factors including sex, age, regimen, T stage and N stage. Multivariate analysis revealed T3-4 and N2-3 were prognostic factors for poor 5-year PFS and DMFS ().
Table 5 Impact of prognostic factors on treatment results by multivariate analysis
Discussion
Since the publication of INT 0099 trail, CCRT with or without adjuvant chemotherapy has become the fundamental treatment for locoregionally advanced NPC patients.Citation6 After that, the importance and value of CCRT has been proved repeatedly.Citation30–Citation34 Compared with 2D-CRT, IMRT improved the local control rate of patients with NPC, whereas it failed to improve distant metastasis control.Citation9,Citation35 Therefore, more aggressive systemic therapies are necessary to improve distant metastasis control in locoregionally advanced NPC patients.
Reducing the rate of distant metastasis, adding neoadjuvant or adjuvant chemotherapy is possible. As patients with bulky primary tumor and/or extensive nodal disease exhibited high rate of distance metastasis,Citation36 NACT followed by CCRT could be more efficacious than CCRT followed by adjuvant chemotherapy in reducing the incidence of distant metastasis and improving OS. Furthermore, due to the severe toxicities of CCRT, many patients could not tolerate the toxicities of adjuvant chemotherapy. Thus, compared with adjuvant chemotherapy, NACT was more suitable for locoregionally advanced NPC.
By now, no standard NACT regimen for locoregionally advanced NPC was established. This may be due to the lack of the most efficacious regimen. Lee et alCitation36 demonstrated that induction chemotherapy using CDDP and 5-FU could significantly reduce tumor bulk and improve tumor control. However, Hareyama et alCitation37 obtained opposite results. In this research, the use of CDDP and 5-FU chemotherapy prior to radiotherapy in patients with NPC did not show a significant improvement in DFS or OS. Furthermore, compared with other regimens, CDDP and 5-FU regimen have more adverse effects, including mucositis, severe gastrointestinal toxicity, and inconvenience of administering continuous infusion.
In an open-label, multicenter, randomized controlled Phase III trial at 10 institutions in China, Sun et alCitation16 found that NACT with TPF regimen followed by CCRT significantly improved locoregional failure-free survival, distant failure-free survival, and OS in locoregionally advanced NPC. Kong et alCitation17 and Ou et alCitation38 obtained similar results. Whereas, in the study performed by Sun et al,Citation16 due to the treatment toxicities and patient refusal, only 30% of patients in the TPF group completed 3 cycles of concurrent chemotherapy. Moreover, Wang et alCitation18 demonstrated that compared with TPF, NACT with TP regimen could obtain similar survival outcomes without severe toxicities. Hui et alCitation12 found that, compared with CCRT alone, NACT with TP regimen followed by CCRT significantly enhanced both 3-year PFS and OS rate by 28.7% and 26.4%, respectively, in locoregionally advanced NPC. A meta-analysis conducted by Tian et alCitation39 showed that taxane-containing NACT might be more efficient for short-term local control than non-taxane-containing regimens in locally advanced NPC. Unfortunately, the contradictory finding by Zhang et alCitation40 made the benefit controversial.
GEM is a pyrimidine analog, which inhibits DNA synthesis and has enhanced antitumor activities. A Phase III multi-center, randomized trial published in Lancet found that GP regimen could improve PFS for patients with recurrent or metastatic NPC.Citation19 The results from this research established GP regimen as the standard first-line treatment option for patients with recurrent or metastatic NPC. Furthermore, other studies obtained similar results.Citation20–Citation22 GP regimen has been widely used and investigated in recurrent or metastatic NPC and could prolong PFS with tolerable adverse effects; however, whether it is useful in locoregionally advanced NPC remain undetermined. Of late, many studies indicated that GP regimen administered as NACT achieved favorable clinical outcomes without serve toxicities.Citation23–Citation25 However, which regimen is the optimal NACT remains unclear.
Zheng et alCitation26 retrospectively reported that GP regimen benefited OS and had a trend toward better DMFS. GP might be superior to TP and PF regimens in the treatment of locoregionally advanced NPC. However, the numbers of patients receiving GP NACT in this study were limited, which involved only 13 patients in GP group. A retrospective research performed by Zhao et alCitation41 also suggested that GP had a trend toward improved OS than TP regime and GP/TP regime had better DFS and PFS than PF regime in non-endemic locoregionally advanced NPC patients. Our study enrolled a total of 222 patients, identified 52 patients with GP regimen NACT and 52 patients with TP regimen NACT for analysis using PSM method. This design was mimicking randomized control trial.Citation42 In our research, we observed similar clinical outcomes as previously reported. The 5-year OS, PFS, LRFS, and DMFS in the GP group were 79.4%, 60.5%, 79.6%, and 73.6%, compared with 78.8%, 66%, 81%, and 75.9%, respectively, in the TP group. There was no significant difference between the two groups.
The most common side effect in our research was hematological toxicities. Compared with TP group, the GP group has a higher incidence of grade 3–4 thrombocytopenia (30.8% vs 11.5%, P=0.017). However, it was reversible and transient interleukin-11 or thrombopoietin was administered to patients with >Grade 2 thrombocytopenia. For patients with Grade 4 thrombocytopenia and high risk of bleeding, allogeneic platelet transfusions might be suitable. Grade 3–4 leucopenia and neutropenia were more frequent in TP group (57.7% vs 25.0%, P=0.001; 75.0% vs 34.6%, P<0.001). The side effects were treated by the adoption of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Thus, in our current research, the most commonly seen hematological toxicities can be identified, prevented, and corrected. There were no treatment-related deaths in both groups. Non-hematological toxicities, such as nausea, vomiting, and dermatitis were mild and had no significant differences between the two groups. Patients seemed to be tolerant of both regimens. No severe complication was found and patients had a satisfactory dependence.
In this study, we investigated GP and TP regimen using PSM analysis
This largely resolved the drawback of divergent confounders and selection bias associated with the retrospective analysis of observational data. However, as our study is a retrospective study, it has certain limitations. First, the number of patients was relatively small, which may affect the conclusion of the research. Additionally, our research was carried out from a single institution in an endemic area. Whether the findings are also suitable for patients from other institutions and are applicable in the non-endemic area needs to be further investigated.
Conclusion
In summary, our research demonstrated that NACT with GP or TP regimen achieved comparable clinical outcome with acceptable toxicities. Therefore, NACT with GP or TP regimen might be an effective and safe choice for patients with locally advanced NPC. The results of this research need to be confirmed by well-designed, long-term, large-scale prospective clinical research.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (numbers 81502647 and 81502646) and the Zhejiang Medical and Health Science and Technology Project (numbers 2016146960 and 2018260661).
Disclosure
The authors report no conflicts of interest in this work.
References
- CaoSMSimonsMJQianCNThe prevalence and prevention of nasopharyngeal carcinoma in ChinaChin J Cancer201130211411921272443
- ChanATNasopharyngeal carcinomaAnn Oncol201021Suppl 7vii308vii31220943634
- ChanATTeoPMJohnsonPJNasopharyngeal carcinomaAnn Oncol20021371007101512176778
- LeeAWSzeWMAuJSTreatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experienceInt J Radiat Oncol Biol Phys20056141107111615752890
- SanguinetiGGearaFBGardenASCarcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional controlInt J Radiat Oncol Biol Phys19973759859969169804
- Al-SarrafMLeBlancMGiriPGChemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099J Clin Oncol1998164131013179552031
- ShahBAQureshiMMJalisiSAnalysis of decision making at a multidisciplinary head and neck tumor board incorporating evidence-based National Cancer Comprehensive Network (NCCN) guidelinesPract Radiat Oncol20166424825426777060
- LaiSZLiWFChenLHow does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients?Int J Radiat Oncol Biol Phys201180366166820643517
- SunXSuSChenCLong-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicitiesRadiother Oncol2014110339840324231245
- ChuaDTMaJShamJSLong-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trialsJ Clin Oncol20052361118112415657403
- FountzilasGCiuleanuEBobosMInduction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluationAnn Oncol201223242743521525406
- HuiEPMaBBLeungSFRandomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinomaJ Clin Oncol200927224224919064973
- XieFYQiSNHuWHSnQWhHComparison of efficacy of docetaxel combined cisplatin (TP regimen) and cisplatin combined 5-fluorouracil (PF regimen) on locally advanced nasopharyngeal carcinomaAi Zheng2007268880884 Chinese17697552
- AiroldiMGabrielePGabrieleAMInduction chemotherapy with carboplatin and taxol followed by radiotherapy and concurrent weekly carboplatin + taxol in locally advanced nasopharyngeal carcinomaCancer Chemother Pharmacol20116751027103420644931
- GoldenDWRudraSWittMEOutcomes of induction chemotherapy followed by concurrent chemoradiation for nasopharyngeal carcinomaOral Oncol201349327728223102863
- SunYLiWFChenNYInduction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicen-tre, randomised controlled trialLancet Oncol201617111509152027686945
- KongLZhangYHuCGuoYLuJJEffects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trialsCancer2017123122258226728192641
- WangFZJiangCEWangLAddition of 5-fluorouracil to first-line induction chemotherapy with docetaxel and cisplatin before concurrent chemoradiotherapy does not improve survival in locoregionally advanced nasopharyngeal carcinomaOncotarget2017853911509116129207632
- ZhangLHuangYHongSGemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trialLancet2016388100541883189227567279
- NganRKYiuHHLauWHCombination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II studyAnn Oncol20021381252125812181249
- MaBBTannockIFPondGRChemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinomaCancer200295122516252312467065
- WangJLiJHongXRetrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinomaOral Oncol200844546447017826303
- HeXOuDYingHExperience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinomaEur Arch Otorhinolaryngol201226931027103321706324
- JamshedAHussainRIqbalHGemcitabine and cisplatin followed by chemo-radiation for advanced nasopharyngeal carcinomaAsian Pac J Cancer Prev201415289990424568516
- WangFZSunQQJiangCEGemcitabine/cisplatin induction chemotherapy before concurrent chemotherapy and intensity-modulated radiotherapy improves outcomes for locoregionally advanced nasopharyngeal carcinomaOncotarget2017857967989680829228572
- ZhengWQiuSHuangLPanJIs Gemcitabine and cisplatin induction chemotherapy superior in locoregionally advanced nasopharyngeal carcinoma?Pak J Med Sci201531478178626430402
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- LeeNHarrisJGardenASIntensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225J Clin Oncol200927223684369019564532
- AustinPCThe relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studiesMed Decis Making200929666167719684288
- ChanATTeoPMNganRKConcurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trialJ Clin Oncol20022082038204411956263
- LeeAWTungSYChuaDTRandomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst2010102151188119820634482
- LinJCJanJSHsuCYPhase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survivalJ Clin Oncol200321463163712586799
- ChenYLiuMZLiangSBPreliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of chinaInt J Radiat Oncol Biol Phys20087151356136418472356
- WeeJTanEHTaiBCRandomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic varietyJ Clin Oncol200523276730673816170180
- LinSPanJHanLNasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective seriesInt J Radiat Oncol Biol Phys20097541071107819362784
- LeeAWLauKYHungWMPotential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinomaRadiother Oncol200887220421018329742
- HareyamaMSakataKShiratoHA prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinomaCancer20029482217222312001120
- OuXZhouXShiQTreatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boostOncotarget2015635383813839726485757
- TianRYeHXZhangBGUse of taxane-containing induction chemotherapy in combination with concurrent chemoradiotherapy in Chinese patients with locally advanced nasopharyngeal carcinoma: a meta-analysisOnco Targets Ther201583255326326604792
- ZhangLNGaoYHLanXWEffect of taxanes-based induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: a large scale propensity-matched studyOral Oncol2015511095095626209065
- ZhaoLXuMJiangWInduction chemotherapy for the treatment of non-endemic locally advanced nasopharyngeal carcinomaOncotarget2017846763677428036270
- StürmerTJoshiMGlynnRJAvornJRothmanKJSchneeweissSA review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methodsJ Clin Epidemiol200659543744716632131
