Abstract
Purpose
The purpose of this study was to evaluate the prognostic significance of preoperative serum carcinoembryonic antigen (CEA) in primary mucinous ovarian carcinoma (MOC).
Patients and methods
We reviewed 57 patients with primary MOC, in whom preoperative serum CEA had been measured. All patients were treated at Peking Union Medical College Hospital between June 1996 and September 2016.
Results
Preoperative serum CEA was elevated (>5.0 ng/mL) in 10 patients (17.5%), with a median serum CEA of 9.6 ng/mL (5.4–111.7 ng/mL). CEA was significantly associated with preoperative serum cancer antigen 125 (P=0.002), surgical debulking status (P=0.015), and tumor stage (P=0.001). Univariate analysis showed that patients with elevated CEA had significantly worse overall survival (OS) than patients with normal CEA (5-year OS: 50.8% vs 91.9%, respectively; P=0.013), but there was no significant difference in progression-free survival between the two groups (P=0.307). Multivariate analysis further demonstrated that advanced stage was an independent prognostic predictor for OS (P=0.002, HR: 15.925, 95% CI: 2.745–92.404).
Conclusion
Elevated preoperative serum CEA was strongly correlated with advanced stage in primary MOC, which may indicate a poorer prognosis. Further investigation of the intrinsic relationship between CEA and primary MOC is now required.
Introduction
Mucinous ovarian carcinoma (MOC) is a relatively rare entity among the different histological subtypes of epithelial ovarian cancer (EOC). Primary MOC accounts for approximately 2%–3% of EOC cases.Citation1,Citation2 Although early-stage MOC presents with a good prognosis, the survival of patients with advanced-stage MOC is worse than that of other EOC subtypes, especially those afflicted by the serous subtype (5-year survival: 10.2%–25.7% vs 20.3%–33.6%, respectively).Citation3–Citation5 Therefore, the identification of prognostic factors may facilitate the decision-making process for MOC patients and thus improve their clinical outcomes.
Serum cancer antigen 125 (CA125) has been routinely used to monitor the response of patients with EOC to chemotherapy, disease progression, and recurrence.Citation6,Citation7 In MOC, however, the association between preoperative serum CA125 and survival is weak.Citation8 In fact, carcinoembryonic antigen (CEA) level is elevated more frequently than CA125 in patients with MOC,Citation9,Citation10 rendering CEA a particularly promising candidate for prognostic prediction. An increasing number of studies have suggested that pretreatment serum CEA level is an independent prognostic factor for colorectal cancer, breast cancer, non-small cell lung cancer, and gastric cancer.Citation11–Citation14 However, due to the rarity of this disease, the prognostic significance of preoperative CEA level in patients with MOC remains unknown. In this study, we aimed to evaluate the prognostic value of preoperative CEA elevation in patients with primary MOC.
Patients and methods
Study population
We reviewed data of all MOC patients treated at Peking Union Medical College Hospital (PUMCH) between 1996 and 2016. In our institute, review of digestive symptoms and fecal occult blood tests were performed routinely in patients with pelvic mass, to exclude the possibility of gastrointestinal tract origin. Gastrointestinal endoscopy was performed in suspected cases. Postoperatively, immunohistochemistry staining tests were further performed to distinguish cases of primary MOC from metastatic MOC. According to the 2014 WHO Classification of Tumors of Female Reproductive Organs,Citation15 primary MOC cases are often positive for cytokeratin 7 (CK7) and cytokeratin 20 (CK20; ~75% of cases), while colorectal and appendiceal tumors are usually negative for CK7 and positive for CK20. CK7 positivity and CK20 negativity favor the diagnosis of primary MOC (~25% of cases), pancreatobiliary cancer, and gastric cancer.Citation16 In addition, the immunostaining of paired box gene 8 (PAX8) and caudal-related homeobox transcription factor 2 (CDX2) can sometimes be useful. PAX8 is positive in ~50% of primary MOC cases but negative in patients with colorectal, appendiceal, pancreatobiliary, and gastric cancers.Citation15 CDX2 expression in primary ovarian mucinous tumors (40%) is lower than that in metastatic carcinomas of gastrointestinal tract origin (90%).Citation17 In the present study, we focused our attention on patients with primary MOC who had preoperative serum CEA values recorded in their medical records. Patients were excluded if they had metastatic MOC or mixed EOC subtypes.
A range of data were acquired from medical records, including age, preoperative serum CEA and CA125 values, types of surgery, surgical debulking status, laterality of disease, tumor size, stage, lymph node metastasis status, and adjuvant chemotherapy. All identifying patient data were anonymized. Optimal surgical debulking was defined as when the residual disease was ≤1 cm in size. Tumor size of ovarian or fallopian tube lesions was measured intraoperatively by gynecologists and recorded in the surgical note. Disease stage was reassessed according to the 2014 International Federation of Gynecology and Obstetrics (FIGO) staging system.Citation18 Following initial surgery, platinum-based chemotherapy was administered to patients in accordance with National Comprehensive Cancer Network guidelines.Citation19–Citation22
All patients were followed-up every 3 months for the first year after the completion of primary therapy, then every 6 months during the period between the second to the fifth year, and annually thereafter. At each follow-up visit, we reviewed symptoms, carried out a pelvic examination, measured serum tumor markers, and performed an ultrasound/computed tomography scan (if indicated). The last patient follow-up appointment was in October 2018.
Serum CEA analysis
Venous blood samples (3 mL per patient) were obtained within 2 weeks before the primary surgery for preoperative CEA measurement. Serum CEA levels were evaluated using an automatic electrochemistry luminescence immunoassay system (Cobas E601; Hoffman-La Roche Ltd., Basel, Switzerland). Normal CEA values should be ≤5.0 ng/mL.
Statistical analyses
All statistical analyses were performed using the IBM SPSS Statistics for Mac OS Package, Version 24.0 (IBM Corporation, Armonk, NY, USA). Categorical variables were compared by the Pearson chi-squared test or Fisher’s exact test. The Kaplan–Meier method was used to generate survival curves, and the log-rank test was used for univariate survival analysis. In order to identify independent prognostic factors, we performed multivariate analysis using the Cox proportional hazards model. Variables that were statistically significant in the univariate analysis were then included in the multivariate analysis. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of tumor recurrence, progression, or death. Overall survival (OS) was calculated from the date of diagnosis to the date of the last follow-up or death. A P-value <0.05 was considered to be statistically significant.
Ethics approval
The study was approved by the Ethics Committee of PUMCH, and written informed consent for this retrospective study was formally waived by the Ethics Committee. All procedures performed in this study were in accordance with the Declaration of Helsinki.
Results
Patient characteristics
A total of 57 consecutive primary MOC patients with available preoperative serum CEA values were included in our study. Patient characteristics are given in . The median age at the time of diagnosis was 30 years (range: 18–74 years). Preoperative serum CEA was elevated (>5.0 ng/mL) in 10 (17.5%) patients. The median preoperative serum CEA level in these patients was 9.6 ng/mL (range: 5.4–111.7 ng/mL). A total of 26 (46.4%) patients had elevated preoperative levels of serum CA125 (>35 U/mL).
Table 1 Clinicopathological characteristics of 57 patients with primary MOC
All patients underwent comprehensive staging surgery (CSS). A total of 35 (61.4%) patients received total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and appendectomy (including metastasectomy and multiple biopsies as required). In all, 22 (38.6%) patients underwent fertility-preserving CSS (including unilateral salpingo-oophorectomy, omentectomy, and appendectomy). Lymphadenectomy was performed in all cases, except for nine (16.1%) patients with grossly normal lymph nodes. Optimal surgical debulking was achieved in 53 (93.0%) patients. In all, 52 (91.2%) patients had unilateral disease. Five (8.8%) patients had bilateral tumors, raising suspicion for metastatic MOC. The results of gastrointestinal evaluation and immunohistochemical staining tests provided strong evidence that these five patients were all afflicted by primary MOC rather than metastatic MOC (). The mean tumor size was 15±8 cm (range: 2–40 cm). Forty-two, three, and 12 patients had stage I, II, and III diseases, respectively. All stage III patients had peritoneal metastasis, including two patients with retroperitoneal lymph node metastasis. All stage IC–III patients, and five stage IA patients, received adjuvant chemotherapy. The mean number of chemotherapy cycles was 4±2 cycles.
Table 2 Results of gastrointestinal evaluation and immunohistochemistry tests in five patients with bilateral tumors
During a median follow-up duration of 36 months (range: 2–144 months), seven (12.3%) patients experienced recurrence with a median PFS of 14 months (range: 5–43 months). Three patients were platinum resistant. The sites of recurrence included the perihepatic area (5/7), contra-lateral adnexal area (3/7), peritoneum (3/7), sigmoid (1/7), and hepatic flexure of the colon (1/7). Four stage I patients received repeat cytoreductive surgery and second-line platinum-based chemotherapy after recurrence. One of these patients died 3 months after recurrence because of respiratory and circulatory failure, and the remaining three patients were alive with no evidence of recurrence at the last follow-up visit. One patient with stage IIB and another with stage IIIC disease received only second-line chemotherapy after recurrence, and died of MOC 4 and 5 months after recurrence, respectively. One patient with stage IIC disease was lost to follow-up after recurrence. Six (10.5%) patients died with a median OS of 16 months (range: 3–45 months), including three recurrent patients and three patients with persistent disease. The 5-year PFS and OS rates for the entire cohort were 82.3% and 85.5%, respectively.
Preoperative serum CEA values and clinicopathological characteristics
The frequency of preoperative serum CEA elevation was compared in terms of preoperative serum CA125 (≤35 U/mL vs >35 U/mL), surgical debulking status (optimal vs suboptimal), laterality of disease (unilateral vs bilateral), tumor size (<10 cm vs ≥10 cm), and FIGO stage (early vs advanced). The results are given in . In brief, 34.6% (9/26) of patients with elevated levels of preoperative serum CA125 showed elevated CEA levels, as compared with 3.3% (1/30) of patients with normal CA125 levels (P=0.002). In addition, 75.0% (3/4) of patients with suboptimal debulking had elevated CEA levels, as compared with 13.2% (7/53) of patients with optimal debulking (P=0.015). Furthermore, 50.0% (6/12) of patients with advanced-stage disease had elevated CEA levels, as compared with 8.9% (4/45) of early-stage patients (P=0.001). There was no significant difference in the frequency of CEA elevation when comparing between patients with unilateral disease and those with bilateral disease (8/52 vs 2/5, respectively; P=0.208). In stage I patients, no significant difference was observed between patients with a tumor size <10 cm and those with a tumor size ≥10 cm (1/6 vs 3/36, respectively; P=0.474).
Table 3 Preoperative serum CEA values and clinicopathological characteristics in primary MOC
Preoperative serum CEA values and survival
Among the seven patients with recurrence, two patients (stage IC and stage IIIC, respectively) presented with elevated serum CEA levels both before primary surgery and at the time of recurrence. The remaining five patients (all with early stage disease) showed persistently normal serum CEA levels during the follow-up period.
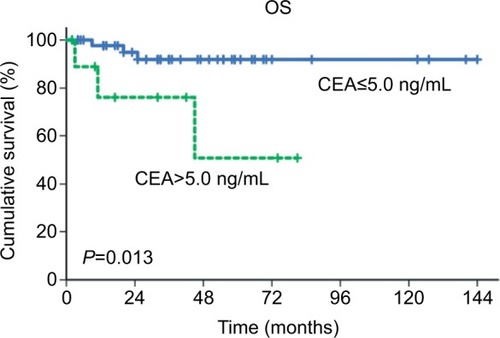
In univariate analysis, we analyzed preoperative serum CEA, preoperative serum CA125, fertility-preserving CSS, surgical debulking status, laterality of disease, tumor size, FIGO stage, and adjuvant chemotherapy (). No significant association was observed between any of these parameters and PFS. Elevated preoperative serum CEA (P=0.013), suboptimal debulking (P<0.001), and advanced stage (P<0.001) were significantly associated with a worse OS. The 5-year OS rate for patients with elevated CEA levels was 50.8%, while that for patients with normal CEA levels was 91.9% ().
Table 4 Univariate survival analysis of prognostic factors related to PFS and OSTable Footnotea
Figure 1 OS curves of patients with primary ovarian mucinous carcinoma according to preoperative serum CEA values (CEA≤5.0 ng/mL vs CEA>5.0 ng/mL).
Abbreviations: CEA, carcinoembryonic antigen; OS, overall survival.
In multivariate analysis of OS, a Cox proportional hazards model () was created which featured elevated preoperative serum CEA, suboptimal debulking, and advanced stage. In this model, advanced stage was the only independent prognostic factor for OS (HR: 15.925, 95% CI: 2.745–92.404, P=0.002).
Table 5 Multivariate survival analysis of OS by the Cox proportional hazards model
Discussion
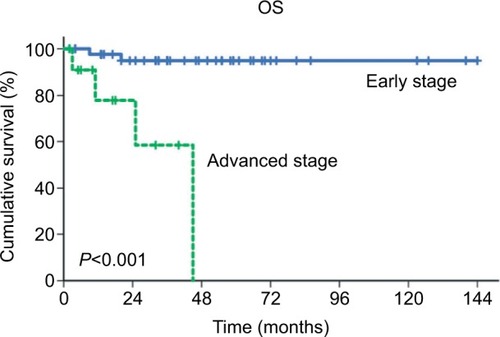
Prior to the present study, the prognostic value of preoperative serum CEA in patients with primary MOC remained unclear. Our analyses showed that patients with elevated CEA levels have significantly lower 5-year OS rates than patients with normal CEA levels (50.8% vs 91.9%, respectively; P=0.013), although the multivariate analysis showed that preoperative serum CEA was not an independent prognostic predictor. Instead, advanced stage was identified as an independent predictor of the risk of primary MOC-related death ().
Figure 2 OS curves of patients with primary ovarian mucinous carcinoma according to the FIGO stage (early stage vs advanced stage).
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; OS, overall survival.
Distinguishing between primary MOC and metastatic MOC is challenging. Metastatic ovarian involvement from other primary sites could be mistakenly reported as primary MOC, especially from colorectal sites.Citation23 In a previous study, Seidman et alCitation24 found that in bilateral tumors, 92%–95% of cases are metastatic. This finding has been validated by several other studies.Citation25,Citation26 In our present study, five (8.8%) patients had bilateral tumors, raising suspicion for metastatic MOC. In these patients, gastrointestinal evaluation and immunohistochemistry tests were performed to exclude metastatic MOC (). It is necessary to carefully differentiate primary MOC from metastatic disease in order to provide optimal treatment for patients.
CEA, first named by Dr Phil Gold and Dr. Samuel Freedman in 1965,Citation27 has long been recognized as a tumor marker, especially for mucin-containing tumors.Citation28 In MOC, the reported frequencies of preoperative serum CEA elevation are 41%–88%.Citation29,Citation30 Our current data suggest that only 17.5% of patients present with elevated preoperative serum CEA, which is much lower than that reported in previous studies. This phenomenon could be attributed to the inherent selection bias of retrospective studies. Primary MOC patients were excluded from our present study if preoperative measurement of CEA was not available. In fact, the proportion of advanced-stage patients in the current cohort was relatively lower than that in the excluded population (21.1% vs 27.9%, respectively; P=0.427). As a result, the frequency of preoperative serum CEA elevation could have been underestimated in this study.
Our results indicate that FIGO stage is an independent prognostic factor of OS in patients with primary MOC, which concurs with a previous study.Citation31 In our study, the proportion of patients with elevated CEA was significantly correlated with FIGO stage (early stage vs advanced stage: 8.9% vs 50.0%, respectively; P=0.001). On the basis of this, the impact of CEA on survival may be attributed to the correlation between CEA elevation and advanced stage, although elevated preoperative serum CEA (P=0.173) has no significant effect on OS in the multivariate analysis ().
Suboptimal cytoreductive surgery is associated with poor survival in patients with EOC.Citation32 Our data showed that patients with suboptimal debulking had a worse OS than patients with optimal debulking (P<0.001). In addition, suboptimal debulking was significantly correlated with CEA elevation (P=0.015). This may be explained by the finding that CEA elevation is strongly correlated with advanced-stage MOC cases, in which implantation metastases are more common and thus surgical debulking is more challenging.
Some studies report that elevated serum CEA correlates well with lymph node metastasis of gastric cancer,Citation14 breast cancer,Citation33 and squamous cell carcinoma of the uterine cervix.Citation34 In the present study, lymph node metastasis was found in 4.2% (2/48) of patients. These two patients both had elevated preoperative serum CEA values. One patient recurred with an even higher serum CEA than the preoperative level (48.6 ng/mL vs 24.0 ng/mL). The other patient had persistently elevated serum CEA and CA125 values, and was lost to follow-up after completing first-line chemotherapy. However, it is difficult to further investigate the association between CEA elevation and lymph node metastasis owing to the limited sample size of the current study.
There is no clear evidence that adjuvant chemotherapy is beneficial in early-stage MOC.Citation35 Compared with the serous subtype of EOC, advanced-stage MOC is associated with a poorer response rate to platinum-based chemotherapy and worse survival.Citation36 Since the phenotypic appearance and behavior of MOC exhibit some similarities to those of gastrointestinal cancers, gastrointestinal regimens are among the postoperative options for patients with MOC.Citation16,Citation22 In our cohort, three patients received gastrointestinal regimens, such as fluorouracil, oxaliplatin, capecitabine, and gemcitabine, combined with platinum regimens as first-line chemotherapy.
Limitations
There are some limitations of our study that need to be considered when interpreting our data. First, patients were excluded if preoperative CEA values were not available. This may have caused selection bias. In addition, we included only 57 patients with primary MOC due to its rarity. In the future, prospective multi-institute studies will be beneficial to minimize the effect of potential confounding factors.
Conclusion
Elevation of preoperative serum CEA was strongly correlated with advanced stage in patients with primary MOC and most likely indicated a poor prognosis. Further investigation of the intrinsic relationship between CEA and primary MOC is now required.
Acknowledgments
The authors wish to thank their colleagues in the Department of Pathology for reviewing the histopathological results and their colleagues in the Department of Medical Records for their assistance in retrieving medical charts.
Author contributions
WL, DC, and KS carried out conception and design of the study. WL contributed to data acquisition. WL and DC performed data analysis and interpretation. WL was involved in manuscript drafting. DC and KS helped in revising and editing the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- JangJYAYanaiharaNPujade-LauraineEUpdate on rare epithelial ovarian cancers: based on the Rare Ovarian Tumors Young Investigator ConferenceJ Gynecol Oncol2017284e5428541641
- MuellerJJLajerHMosgaardBJInternational study of primary mucinous ovarian carcinomas managed at tertiary medical centersInt J Gynecol Cancer201828591592429561302
- HessVA’HernRNasiriNMucinous epithelial ovarian cancer: a separate entity requiring specific treatmentJ Clin Oncol20042261040104415020606
- SchiavoneMBHerzogTJLewinSNNatural history and outcome of mucinous carcinoma of the ovaryAm J Obstet Gynecol20112055480.e1480.e821861962
- SimonsMEzendamNBultenJNagtegaalIMassugerLSurvival of patients with mucinous ovarian carcinoma and ovarian metastasesInt J Gynecol Cancer20152571208121525978291
- LedermannJARajaFAFotopoulouCNewly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201324Suppl 6vi24vi3224078660
- SteffensenKDWaldstrømMBrandslundIJakobsenAPrognostic impact of prechemotherapy serum levels of HER2, CA125, and HE4 in ovarian cancer patientsInt J Gynecol Cancer20112161040104721738039
- TangAKondalsamy-ChennakesavanSNganHPrognostic value of elevated preoperative serum CA125 in ovarian tumors of low malignant potential: a multinational collaborative study (ANZGOG0801)Gynecol Oncol20121261364022370601
- BastRCUrbanNShridharVEarly detection of ovarian cancer: promise and realityCancer Treat Res2002107619711775462
- HarrisonMLJamesonCGoreMEMucinous ovarian cancerInt J Gynecol Cancer200818220921417624989
- DuffyMJLamerzRHaglundCTumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines updateInt J Cancer2014134112513252223852704
- ParkBWOhJWKimJHPreoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomesAnn Oncol200819467568118037623
- OkadaMNishioWSakamotoTPrognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I diseaseAnn Thorac Surg200478121622115223432
- KochiMFujiiMKanamoriNEvaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancerGastric Cancer20003417718611984734
- KurmanRJCarcangjuMLHerringtonCSYoungRHWHO Classification of Tumours of Female Reproductive Organs4th edLyonIARC2014
- LedermannJALuveroDShaferAGynecologic Cancer Inter-Group (GCIG) consensus review for mucinous ovarian carcinomaInt J Gynecol Cancer2014249 Suppl 3S14S1925341574
- VangRGownAMWuLSImmunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7Mod Pathol200619111421142816980943
- PratJFIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneumInt J Gynaecol Obstet201412411524219974
- MorganRJCopelandLGershensonDNCCN Ovarian Cancer Practice Guidelines. The National Comprehensive Cancer NetworkOncology19961011 Suppl2933108953610
- MorganRJAlvarezRDArmstrongDKOvarian cancer, version 3.2012J Natl Compr Canc Netw201210111339134923138163
- MorganRJAlvarezRDArmstrongDKOvarian cancer, version 2.2013J Natl Compr Canc Netw201311101199120924142821
- MorganRJArmstrongDKAlvarezRDOvarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in OncologyJ Natl Compr Canc Netw20161491134116327587625
- ShimadaMKigawaJOhishiYClinicopathological characteristics of mucinous adenocarcinoma of the ovaryGynecol Oncol2009113333133419275957
- SeidmanJDKurmanRJRonnettBMPrimary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosisAm J Surg Pathol200327798599312826891
- KhunamornpongSSuprasertPPojchamarnwiputhSNa ChiangmaiWSettakornJSiriaunkgulSPrimary and metastatic mucinous adenocarcinomas of the ovary: Evaluation of the diagnostic approach using tumor size and lateralityGynecol Oncol2006101115215716300822
- YemelyanovaAVVangRJudsonKWuLSRonnettBMDistinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classificationAm J Surg Pathol200832112813818162780
- GoldPFreedmanSOSpecific carcinoembryonic antigens of the human digestive systemJ Exp Med196512234674814953873
- TsaiCCLinHHuangEYThe role of the preoperative serum carcinoembryonic antigen level in early-stage adenocarcinoma of the uterine cervixGynecol Oncol200494236336715297173
- KhooSKMackayEVCarcinoembryonic antigen (CEA) in ovarian cancer: factors influencing its incidence and changes which occur in response to cytotoxic drugsBr J Obstet Gynaecol19768310753759990213
- TholanderBTaubeALindgrenASjöbergOStendahlUTamsenLPretreatment serum levels of CA-125, carcinoembryonic antigen, tissue polypeptide antigen, and placental alkaline phosphatase in patients with ovarian carcinoma: influence of histological type, grade of differentiation, and clinical stage of diseaseGynecol Oncol199039126332227570
- MassadLSGaoFHagemannIPowellMClinical Outcomes among Women with Mucinous Adenocarcinoma of the OvaryGynecol Obstet Invest201681541141526583769
- NickAMColemanRLRamirezPTSoodAKA framework for a personalized surgical approach to ovarian cancerNat Rev Clin Oncol201512423924525707631
- WuSGHeZYRenHYUse of CEA and CA15-3 to predict axillary lymph node metastasis in patients with breast cancerJ Cancer201671374126722358
- HuangEYHuangYJChanchienCCPretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervixRadiat Oncol201271322289572
- TrimbosJBParmarMVergoteIInternational Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinomaJ Natl Cancer Inst200395210511212529343
- AlexandreJRay-CoquardISelleFMucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experienceAnn Oncol201021122377238120494964
