Abstract
Background
The aim of this study was to develop and validate reliable nomograms to predict individual overall survival (OS) and cancer-specific survival (CSS) for patients with primary tracheal tumors and further estimate the role of postoperative radiotherapy (PORT) for these entities.
Patients and methods
A total of 405 eligible patients diagnosed between 1988 and 2015 were selected from the Surveillance, Epidemiology, and End Results database. All of them were randomly divided into training (n=303) and validation (n=102) sets. For the purpose of establishing nomograms, the Akaike information criterion was employed to select significant prognostic factors in multivariate Cox regression models. Both internal and external validations of the nomograms were evaluated by Harrell’s concordance index (C-index) and calibration plots. Propensity score matching (PSM) method was performed to reduce the influence of selection bias between the PORT group and the non-PORT group.
Results
Two nomograms shared common variables including age at diagnosis, histology, N and M stages, tumor size, and treatment types, while gender was only incorporated in the CSS nomogram. The C-indices of OS and CSS nomograms were 0.817 and 0.813, displaying considerable predictive accuracy. The calibration curves indicated consistency between the nomograms and the actual observations. When the nomograms were applied to the validation set, the results remained reconcilable. Moreover, the nomograms showed superiority over the Bhattacharyya’s staging system with regard to the C-indices. After PSM, PORT was not associated with significantly better OS or CSS. Only squamous cell carcinoma (SCC) patients in the PORT group had improved OS compared to non-PORT group.
Conclusion
The first two nomograms for predicting survival in patients with primary tracheal tumors were proposed in the present study. PORT seems to improve the prognosis of SCC patients, which needs further exploration.
Introduction
Primary tracheal tumor, a rare disease, represents 0.5% of all malignancies.Citation1 Several reports have estimated an incidence of ~0.9 new cases per 1,000,000 persons per year.Citation2,Citation3 Major histological subtypes include squamous cell carcinoma (SCC) and adenoid cystic carcinoma (ACC).Citation4 SCC was reported to be associated with men and smokers,Citation5 while nonsmokers were more likely to suffer from ACC.Citation6 Patients with tracheal tumors generally present symptoms such as cough and dyspnea. However, these signs are nonspecific, and most patients are misdiagnosed until advanced disease is detected through radiography or bronchoscopy.Citation7 Although radical surgical resection with negative margins has been suggested to be the optimal option for primary tracheal tumors, the resection rate is remarkably heterogeneous, and the five-year overall survival (OS) among these patients varies from 47% to 79% based on previous studies.Citation8–Citation10 However, treatment failure attributed to local recurrence and distant metastasis is not rare. According to several retrospective reports, patients with tracheal tumors appear to benefit from adjuvant therapy,Citation11,Citation12 but the roles of postoperative radiotherapy (PORT) and chemotherapy remain controversial.Citation13–Citation15 Moreover, considering the notably low incidence, there is no widely accepted staging system with sufficient predictive prognostic value for these neoplasms. Thus, the evaluation of tracheal tumors is neither accurate nor convenient for clinicians.
The current literature has shown the precise predictive value of nomograms in the management of other tumors, and they have been broadly implemented in clinical practice.Citation16–Citation18 In this study, we aimed to build a nomogram for patients diagnosed with primary tracheal tumors by using the data extracted from the Surveillance, Epidemiology, and End Results (SEER) database and further investigate the prognostic value of PORT by applying a propensity score matching (PSM) method.
Materials and methods
Selection of study cohort
We conducted this retrospective study using data retrieved from the SEER database (1973–2015), supported by the National Cancer Institute, which provides information regarding cancer patients in 18 registries of the US coverinĝ28% of the US population.Citation19 We identified the primary tracheal tumors according to the primary site code C33.9, and the search was limited to the period from 1988 to 2015 (information on tumor size, nodal status, metastatic sites, and extent of disease were not collected in the database until 1988). Only patients who met the following criteria were included: 1) age above 18 years; 2) trachea as the first primary site; 3) survival time ≥1 month; and 4) cases with inadequate tumor data (tumor extension, lymph node status, and metastasis). Those with a death certificate or autopsy were excluded. A total of 405 eligible cases were identified and constituted the primary cohort of our study. To develop and validate the nomograms, the primary cohort was randomized into a training cohort (n=303) and validation cohort (n=102).
Definition of tumor data
Considering that there is no American Joint Committee on Cancer (AJCC) TNM staging system for tracheal tumors, we utilized the tumor features from the SEER database and categorized the patients according to the following classification: 1) Extension status: Extension 1 (E1), invasive tumor confined to trachea; E2, involving adjacent connective tissues (arch of aorta, azygos vein, right brachiocephalic vein, carotid sheath, common carotid artery, jugular arch, phrenic nerves, pretracheal fascia, recurrent laryngeal nerve, subclavian artery, and vagus nerve) or adjacent organs/structures (cricoid cartilage, esophagus, pleura, not otherwise specified: parietal and visceral; right and left main bronchi, sternum, thymus, thyroid gland, and vertebral column); E3, further contiguous extension; 2) Lymph node status: N0, no regional lymph node involved; N1, regional lymph nodes involved (mediastinal, paratracheal, and pretracheal); and 3) Metastatic status: M0, no metastatic site involved; M1, distant lymph node(s) only, organ metastasis other than distant lymph node(s), or distant metastasis plus distant lymph node(s). To properly estimate the prognostic value of tumor size in patients with tracheal tumor, the tumor size was stratified into two categories (cutoff point 3.9 cm) by using the X-tile program (version 3.6.1), which is a practical tool assisting marker cut point analysis according to the minimal P-value.
Data collection
Data extracted for each case included demographics (age at diagnosis, gender, race, marital status, and Contract Health Service Delivery Areas [CHSDA] region), tumor characteristics (as mentioned above), primary tumor treatment (surgery or radiation therapy), months of survival, cause-specific death classification, and vital status.
Statistical analysis
We employed Student’s t-test for continuous data. Categorical variables were evaluated using the chi-squared test or Fisher’s exact test. We used the OS (the interval from the date of initial anticancer treatment until the date of death or the last day of follow-up) and cancer-specific survival (CSS) (the time from the data of initial treatment until death from tracheal tumor) as the primary endpoints for comparison. Kaplan–Meier analysis and log-rank test were used for survival analysis. Univariate and multivariate survival analyses were conducted using the Cox proportional hazards model. Nomograms were constructed based on the significant prognostic factors of the multivariate Cox regression analyses. We used the backward step-down process based on Akaike information criterion (AIC) to finally recruit independent prognostic factors into the formulations of the nomograms. Both internal and external validations of the nomograms were conducted based on the training cohort and validation cohort. Calibration curves of our nomograms were employed to determine whether the predicted survival and observed survival were in agreement, and the curves were corroborated with 1,000 resamples conducted for validation. We further assessed the performances of nomograms and a staging system proposed by BhattacharyyaCitation20 by using the Harrell’s concordance index (C-index).Citation21 The C-index ranges from 0.5 to 1.0, and a larger C-index shows better accuracy for predicting the prognosis.Citation22 The comparison between the nomogram and the Bhattacharyya’s staging system was performed with the rcorrp.cens function in the Hmisc package in R.Citation23 Recognizing that selection bias may influence the comparison of survival outcomes between patients who did or did not receive PORT, similar with previous study,Citation24 a PSM analysis was conducted to reduce the potential differences among baseline characteristics between the two groups. The patients were matched on the basis of a greedy nearest neighbor algorithm with the caliper set at 0.1. The matching fixed ratio was 1:1. All statistical analyses were processed with R software version 3.4.2 (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org/), and a two-side P-value <0.05 was considered to be statistically significant.
Results
Patient baseline characteristics
A total of 405 patients were identified with primary tracheal tumors, and the demographics and characteristics of the training cohort (303 patients) and validation cohort (102 patients) are detailed in . The majority of patients in both sets were of white ethnicity, male, married, and from the Pacific Coast and East regions. The most common histological subtype was SCC. For both training and validation cohorts, most patients were classified in the E1, N0, and M0 stages and received either surgery or radiotherapy.
Table 1 Clinicopathological characteristics of primary tracheal patients in training and validation cohorts
summarizes the characteristics of the primary cohort across histological subtypes. SCC was primarily associated with elderly males, while ACC was more likely found in younger female patients. Compared to ACC and other histologies, patients with SCC also more likely experienced node metastasis and distant metastasis, and were more likely to have undergone radiotherapy. However, among patients who received both radiotherapy and surgery, ACC was the predominant subtype.
Table 2 Clinicopathological characteristics of primary tracheal patients in each histologic group
Univariate and multivariate Cox regression of the training cohort
For the training cohort, univariate analyses were performed to identify clinical variables that were significantly associated with OS and CSS. As shown in , age, race, gender, histological subtypes, treatment types, E, N, and M stages, and tumor size were significantly associated with OS. Regarding CSS, race lost its significance, while other variables continued to be significant parameters.
Table 3 Univariate analysis of clinical characteristics for OS and CSS in primary tracheal patients in the training cohort
In the multivariate analysis, all of the original variables were initially entered into the Cox regression model. To determine the independent prognostic factors in this cohort, which could be adopted into the nomograms, we used the least value of AIC to select variables consistent with previous study.Citation25 Age, marital status, histological subtypes, treatment, N stage, M stage, and tumor size were identified as prognostic factors for OS. Regarding CSS, marital status was excluded, while gender became significant indicator. The results of multivariate Cox regression are demonstrated in .
Table 4 Multivariate analysis of clinical characteristics for OS and CSS in primary tracheal patients in the training cohort
Construction of nomogram for OS and CSS
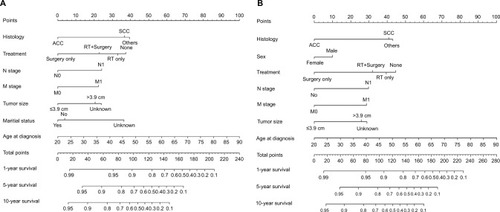
Nomograms for predicting 1- and 5-year OS () and CSS () were constructed by using the covariates mentioned above, and score details of each nomogram predictor are shown in . By adding the scores of all the selected variables, the probabilities of survival of the individual patients could be accurately determined.
Table 5 Points of prognostic factors in the nomograms
Validation of the nomograms
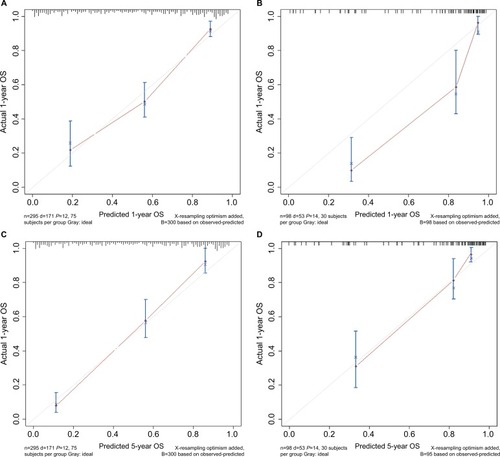
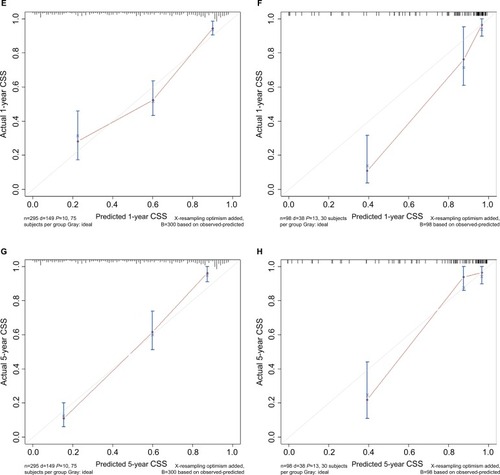
The C-indices of the nomograms to predict OS were 0.813 (95% CI: 0.764–0.862) and 0.838 (95% CI: 0.748–0.927) for the training and validation cohorts, respectively, exhibiting excellent consistency with actual OS. Similarly, when the training and validation cohorts were applied to the nomogram for CSS, the C-index values were also greater than 0.7 (0.817 and 0.863, respectively). As shown in , both internal and external calibration curves for 1- and 5-year OS and CSS suggested strong agreement between the prediction by nomogram and the actual outcomes in the training and validation cohorts.
Figure 2 Calibration curves of (A and C) 1- and 5-year OS and (E and G) CSS for training cohort, and (B and D) OS and (F and H) CSS for validation cohort.
We further compared the OS/CSS predictive ability between the nomogram and Bhattacharyya’s staging system. As shown in , the C-indices of the nomograms were significantly higher than those of the Bhattacharyya’s staging system for both OS and CSS (C-index: OS 0.810 vs. 0.660, P<0.001; CSS 0.820 vs. 0.700, P<0.001).
Table 6 Comparison of nomograms with Bhattacharyya’s staging system
Value of PORT for tracheal patients
For the purpose of evaluating the prognostic value of PORT comprehensively, a PSM analysis was conducted between patients who received surgical resection, either with or without PORT, based on age, race, gender, CHSDA region, histological subtypes, E, N, and M stages, tumor size, and marital status. Ultimately, 76 patients with PORT and 76 patients without PORT were enrolled in the matching cohort (). In the matching cohort, there was no significant difference regarding clinical characteristics, and neither OS nor CSS were significantly different between the two groups. In the subgroup analysis, PORT was not associated with superior outcome for ACC (figures not shown). Patients with SCC who received PORT had significantly better prognoses compared with those who did not, though only for OS (5-year OS: 61.5% vs 23.0%, P<0.05; ).
Discussion
Due to the extremely low incidence of primary tracheal tumors, neither an AJCC staging system nor a reliable guideline for the management of these uncommon neoplasms has been established. Previously published investigations were primarily composed of case reports, reviews, and single-institutional experience.Citation1,Citation3,Citation7,Citation26 No large-scale study regarding the prognostic factors of tracheal tumors has been reported. As the graphical depictions of statistical models, nomograms have been used to predict individual survival outcomes among patients with other diseases.Citation27–Citation29 Because a multitude of advantages have been suggested, prognostic nomograms are widely accepted in clinical practice. By using the SEER dataset, we build two novel, user-friendly, and accurate nomograms for predicting the overall and disease-specific survival for patients with primary tracheal tumors. To the best of our knowledge, this study is the first to develop clinicopathological prediction models based on a large series of patients for this unique condition. Both internal and external validations of our nomograms showed effective discrimination.
The absence of a universally adopted staging system made it difficult for clinicians to properly assess tracheal neoplasms. Until the present, only three studies stated their proposed guidelines for the staging of tracheal malignancy. One staging system, suggested by Licht et al, was based on limited number of patients and thus lacked sufficient prognostic value.Citation7 In 2006, Macchiarini et alCitation1 proposed a more elaborate system, which references the experience of head and neck malignancies. However, the lack of external validation has restricted the usage of this staging model. Only one staging system, proposed by BhattacharyyaCitation20 has been validated and modified by another study.Citation3 Therefore, we compared our models with Bhattacharyya’s staging system and found the discrimination performance of our nomograms superior to Bhattacharyya’s classification. Moreover, the previous staging system ignored patients with nonregional lymph node or distant organ metastases and utilized data from patients diagnosed in the last century. In the present study, we selected several accessible parameters that reflect tumor characteristics, as well as patient status, to build comprehensive nomograms for tracheal neoplasm patients.
Our study identified several clinicopathological characteristics as important prognostic factors for OS and CSS among patients with tracheal tumors. As expected, the effect of age at diagnosis was highly significant for both OS and CSS. Prior studies focused on other malignancies also suggested that elderly patients always had worse survival than younger ones.Citation30,Citation31 Younger patients are believed to tolerate more aggressive treatment modalities than older patients. This tolerance could explain the differences of survival outcomes between these two groups. In ACCs, early detection has also been reported to associate with increased resectability rates and even improved survival.Citation6 Another interesting finding was that females had better CSS than males. We noted that females were more likely to suffer from ACCs, while SCCs were more common among males. The survival difference among males compared to females could partly be explained by the different tumor biology behavior between ACCs and SCCs. Wang et alCitation32 reported that consultation rates were significantly different between men and women, with men being more likely to endure symptoms for a long period and thus develop advanced disease with a less favorable prognosis, which could also be one explanation for the present study. Moreover, married patients had superior OS than their unmarried competitors. One explanation for this could be the difference in health-care seeking behavior between married and unmarried patients.Citation33,Citation34
In our analysis, histological type was an important issue for both OS and CSS, and was included in our nomograms. Patients with SCCs at diagnosis had inferior outcomes than those with ACCs, with 5- and 10-year OS rates for ACCs of 79.0% and 62.3%, respectively, compared to 25.8% and 15.6% for SCCs, respectively (figures not shown). Our finding was consistent with those of previous studies. Gaissert et alCitation9 compared the survival outcomes between 101 patients with ACCs and 90 patients with SCCs and found that the prognosis of ACCs was significantly superior to SCCs (5-year OS 52% vs. 39%, P<0.001). Another study based on oriental populationsCitation35 also suggested an approximately twofold 5-year survival rate disparity between these two subtypes. In our study, SCCs were more likely to present with local extension, as well as lymph node metastasis, than ACCs at diagnosis. Our study, together with published reports, might indicate different genetic alterations and tumor biology in different histological subgroups; however, further studies are warranted to evaluate this issue.
The presence of lymph node metastasis was found to be a poor prognostic indicator in our study, which has also been supported by a previous epidemiological study.Citation20 For distant metastasis, our study innovatively classified nonregional node metastasis and distant organ metastasis as M1, which was consequently incorporated into the nomograms via the AIC-based selections. According to a prior study reviewing their 40 years of experience with treatment of ACC and SCC, Gaissert et al observed distant metastasis in 10% of diagnoses. Because the information of site-specific metastases was not collected until 2010, we further analyzed the metastatic patterns of tracheal tumor patients by querying the SEER database from 2010 through 2015. Among 125 eligible patients, the most frequent metastatic site was lung (16 patients, 12.8%) followed by liver (7 patients, 5.6%). The incidences of bone and brain metastases were 3.2% and 1.6%, respectively. In 1996, Maziak et alCitation8 evaluated the proportion of metachronous metastases in 17 patients, which was in keeping with our findings. However, our study recruited a larger cohort and demonstrated the patterns of site-specific metastasis in primary tracheal malignancies for the first time. It is not a surprise to find that the tumor extension was not an independent prognostic indicator for OS and CSS in our study. In fact, the size and location of the primary tumor have been reported to be more important than the extension of tumors.Citation36,Citation37 Another serial studyCitation9 also suggested that more advanced tumor extension might not correlate with higher mortality risk. Referring to the prognostic value of tumor size, our study selected 3.9 cm as the cutoff point, which was similar with Macchiarini’s study.Citation1 Simultaneously, the AIC-based analyses demonstrated that those with tumor size <3.9 cm were associated with better prognosis.
Complete surgical resection with negative margins (R0 resection) has always been considered to be the mainstay strategy for tracheal tumors.Citation6,Citation38 Our study revealed that patients who underwent surgery had better survival outcomes compared to nonsurgery patients. However, due to the nature of the SEER database, details of surgical margins were lacking. Further large-scale studies examining the value of R0 resection compared to R1, and even R2, resection is needed. The role of PORT remains controversial. Few reportsCitation12,Citation39 suggested that PORT seems to be effective for R1/R2 resected disease. However, these two studies are limited by small sample size and were less likely to provide sufficient information to determine the prognostic value of PORT. Xie et alCitation40 conducted a population-based study to investigate this issue but did not separate PORT from neoadjuvant radiotherapy. Honings et alCitation10,Citation14 even drew a contradictory conclusion. To adjust for potential selection bias, we used the PSM method and evaluated the survival benefit from PORT in patients who received surgery: the favorable impact of PORT was only found for SCC patients. However, this should be taken cautiously due to the fact that the sample size was relatively limited and was from subgroup analysis. Previous studies have suggested lower radiosensitivity for ACC while neutron radiotherapy seems to improve the local-regional control for this condition.Citation41 In contrast, Schraube et alCitation42 and Jeremic et alCitation11 noted significantly improved survival among SCC patients who underwent radiotherapy. Conversely, Yang et alCitation6 suggested that ACC patients with R1 resection showed improved disease free survival of PORT but not in terms of OS. Our study, together with previous studies, suggested that the exact role of PORT in influencing survival remains unclear.
Several inevitable limitations in our study should be considered. First, as mentioned above, information on tumor location, surgical margins, techniques, prescription dose as well as fraction times of radiotherapy could not be ascertained from SEER. Second, prior studies evaluating the role of chemotherapy in the treatment of tracheal tumors have generated mixed conclusions. However, we could not examine this issue due to the lack of chemotherapy information in the SEER database. Finally, the database also lacks recurrence, performance status, and laboratory and pathological parameters, which could be used to build a more comprehensive nomogram.
Despite the abovementioned limitations, as well as the rarity of primary tracheal malignancies, our study created and validated the first two nomograms based on the combination of both anatomic and non-anatomic parameters, including clinical and epidemiological features, by using a large population-based approach. PORT seems to improve the prognosis of SCC patients, which warrants further investigation.
Acknowledgments
This study was based on the publicly available SEER database, and permission was received from the National Cancer Institute to access the database for the purposes of research (the private SEER ID 13499-Nov2016). As this research did not include interaction with humans or use personal identifying information, informed consent was not required. We thank the American Journal Experts (https://www.aje.com/) for editing this manuscript.
Supplementary material
Figure S1 OS for SCC tracheal patients who underwent PORT or not in the matched cohort.
Abbreviations: OS, overall survival; PORT, postoperative radiotherapy; SCC, squamous cell carcinoma.
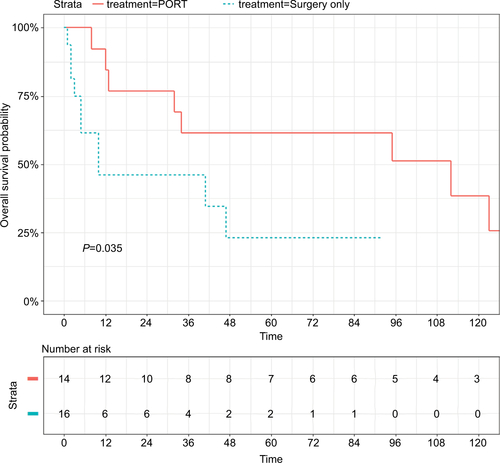
Table S1 Patient characteristics according to the administration of PORT before and after PSM
Disclosure
The authors report no conflicts of interest in this work.
References
- MacchiariniPSchirrenJMuleyTMoykopfIVPrimary tracheal tumoursLancet Oncol200671839116389188
- NouraeiSMMiddletonSENouraeiSAManagement and prognosis of primary tracheal cancer: a national analysisLaryngoscope2014124114515023868448
- WebbBDWalshGLRobertsDBSturgisEMPrimary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experienceJ Am Coll Surg2006202223724616427548
- AhnYChangHLimYSPrimary tracheal tumors: review of 37 casesJ Thorac Oncol20094563563819357541
- HoningsJGaissertHAVerhagenAFUndertreatment of tracheal carcinoma: multidisciplinary audit of epidemiologic dataAnn Surg Oncol200916224625319037701
- YangHYaoFTantaiJZhaoYTanQZhaoHResected tracheal adenoid cystic carcinoma: improvements in outcome at a single institutionAnn Thorac Surg2016101129430026431923
- LichtPBFriisSPetterssonGTracheal cancer in Denmark: a nationwide studyEur J Cardiothorac Surg200119333934511251276
- MaziakDEToddTRKeshavjeeSHWintonTLvan NostrandPPearsonFGAdenoid cystic carcinoma of the airway: thirty-two-year experienceJ Thorac Cardiovasc Surg19961126152215328975844
- GaissertHAGrilloHCShadmehrMBLong-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carinaAnn Thorac Surg20047861889189715560996
- HoningsJvan DijckJAVerhagenAFvan der HeijdenHFMarresHAIncidence and treatment of tracheal cancer: a nationwide study in the NetherlandsAnn Surg Oncol200714296897617139460
- JeremicBShibamotoYAcimovicLMilisavljevicSRadiotherapy for primary squamous cell carcinoma of the tracheaRadiother Oncol19964121351389004356
- CalzadaAPMillerMLaiCKElashoffDAAbemayorESt JohnMAAdenoid cystic carcinoma of the airway: a 30-year review at one institutionAm J Otolaryngol201233222623121907453
- AllenAMRabinMSReillyJJMentzerSJUnresectable adenoid cystic carcinoma of the trachea treated with chemoradiationJ Clin Oncol200725345521552318048830
- HoningsJGaissertHAWeinbergACPrognostic value of pathologic characteristics and resection margins in tracheal adenoid cystic carcinomaEur J Cardiothorac Surg20103761438144420356756
- UrdanetaAIYuJBWilsonLDPopulation based cancer registry analysis of primary tracheal carcinomaAm J Clin Oncol2011341323720087156
- ValentiniVvan StiphoutRGLammeringGNomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trialsJ Clin Oncol201129233163317221747092
- KarakiewiczPIBrigantiAChunFKMulti-institutional validation of a new renal cancer-specific survival nomogramJ Clin Oncol200725111316132217416852
- SongWZhuZGWuQA nomogram to predict overall survival for biliary tract cancerCancer Manag Res2018101535154129942155
- Surveillance, Epidemiology and End Results Program, About the SEER Program2018 Available from: https://seer.cancer.gov/about/overview.html, 2018Accessed May, 5, 2018
- BhattacharyyaNContemporary staging and prognosis for primary tracheal malignancies: a population-based analysisOtolaryngol Head Neck Surg2004131563964215523440
- HarrellFELeeKLMarkDBMultivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errorsStat Med19961543613878668867
- HarrellFERegression Modeling StrategiesNew York, NYSpringer New York2001
- FrankEHR Package version 3.9-2 Available from: https://cran.r-project.org/package=HmiscAccessed February 1, 2017
- WenJChenJChenDEvaluation of the prognostic value of surgery and postoperative radiotherapy for patients with thymic neuroendocrine tumors: a propensity-matched study based on the SEER databaseThorac Cancer2018111
- ZhangZYLuoQFYinXWDaiZLBasnetSGeHYNomograms to predict survival after colorectal cancer resection without preoperative therapyBMC Cancer201616165827553083
- ThotathilZSAgarwalJPShrivastavaSKDinshawKAPrimary malignant tumors of the trachea – the Tata Memorial Hospital experienceMed Princ Pract2004132697314755137
- KawaiKIshiharaSYamaguchiHNomogram prediction of metachronous colorectal neoplasms in patients with colorectal cancerAnn Surg2015261592693225185472
- PilottoSSperdutiILeuzziGPrognostic Model for Resected Squamous Cell Lung Cancer: External Multicenter Validation and Propensity Score Analysis exploring the Impact of Adjuvant and Neo-adjuvant TreatmentJ Thorac Oncol201813456857529269009
- FakhryCZhangQNguyen-TânPFDevelopment and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancerJ Clin Oncol201735364057406528777690
- QuaresmaMColemanMPRachetB40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based studyLancet201538599741206121825479696
- SkyrudKDBrayFMøllerBA comparison of relative and cause-specific survival by cancer site, age and time since diagnosisInt J Cancer2014135119620324302538
- WangYHuntKNazarethIFreemantleNPetersenIDo men consult less than women? An analysis of routinely collected UK general practice dataBMJ Open201338e003320e003327
- WuWFangDShiDBianXLiLEffects of marital status on survival of hepatocellular carcinoma by race/ethnicity and genderCancer Manag Res201810233229379317
- GomezSLHurleySCancholaAJEffects of marital status and economic resources on survival after cancer: a population-based studyCancer2016122101618162527065317
- ZhengjaiangLPingzhangTDechaoZReddy-KolanuGIlankovanVPrimary tracheal tumours: 21 years of experience at Peking Union Medical College, Beijing, ChinaJ Laryngol Otol200812211123518331654
- GelderCMHetzelMRPrimary tracheal tumours: a national surveyThorax19934876886928153914
- AllenMSMalignant tracheal tumorsMayo Clin Proc19936876806848394484
- D’CunhaJMaddausMASurgical treatment of tracheal and carinal tumorsChest Surg Clin N Am20031319511012698640
- OginoTOnoRShimizuWIkedaHAdenoid cystic carcinoma of the tracheobronchial system: the role of postoperative radiotherapyRadiat Med199513127297597201
- XieLFanMSheetsNCChenRCJiangGLMarksLBThe use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysisInt J Radiat Oncol Biol Phys201284246447022365629
- DouglasJGLaramoreGEAustin-SeymourMKohWStelzerKGriffinTWTreatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapyInt J Radiat Oncol Biol Phys200046355155710701733
- SchraubePLatzDWannenmacherMTreatment of primary squamous cell carcinoma of the trachea: the role of radiation therapyRadiother Oncol19943332542587716265