?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Globally, the prevalence and mortality rates of lung cancer have been escalated with the increasing trend of tobacco smoking. The toxicity and irresponsive nature of the available drugs for lung cancer treatment demands an alternative approach.
Methods
In this study, four known compounds namely, cirsimaritin (4′,5, -dihydroxy-6,7-di-methoxyflavone) (1), eupatorin (5,3′-dihydroxy-6,7,4′-trimethoxyflavone) (2), betulin (Lup-20 (29)-ene-3, 28-diol) (3), and β-amyrin acetate (12-Oleanen-3yl acetate) (4) have been isolated from the leaves extract of Quercus incana. Preliminary screening of these natural compounds (1–4) was performed against non-small cell lung carcinoma (NCI-H460) and normal mouse fibroblast (NIH-3T3) cell lines.
Results
The compounds were found to be antiproliferative against cancer cells with wide therapeutic index in comparison to the normal cells. Effects of betulin (3) on cell migration, invasion, apoptosis, and expression of important apoptosis- and metastasis-related markers were observed at different concentrations. The results showed significant dose-dependent induction of apoptosis after the treatment with betulin (3) followed by increased expression of the caspases family (ie, caspase-3, -6, and -9), proapoptotic genes (BAX and BAK), and inhibiting anti-apoptotic genes (BCL-2L1 and p53). Furthermore, wound healing and transwell invasion assays suggested that betulin (3) could also regulate metastasis by inhibiting MMP-2/-9. Osteopontin, a central regulator of apoptosis and metastasis was also inhibited in a dose-dependent manner.
Conclusion
The present findings suggest that betulin (3) can be an attractive chemotherapeutic target for treating resistant lung cancers.
Introduction
Lung cancer, being the most prevalent cancer worldwide, has been ranked third among the top 10 malignancies in Pakistan.Citation1 In 2012, a report confirmed that 9.8% of men were diagnosed with lung cancer per 100,000 cases in Pakistan.Citation2 Excessive smoking habits in young- and middle-aged men accounted for the high prevalence of lung cancer.Citation3 Primarily, lung cancer is divided into two types: small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLC represents 85% of the lung cancer cases occurring worldwide, whereas only 15% accounts for SCLC.Citation4 Current treatment regimen includes surgery coupled with the use of chemotherapeutic drugs. The commonly used chemotherapy drugs are methotrexate, bevacizumab, carboplatin, cisplatin, docetaxel, gefitinib, vinorelbine, and paclitaxel.Citation5 Despite the aforementioned facts, the rate of survival was limited only to 65% because of poor prognosis. High recurrence rate, metastasis potential, and chemoresistance pose a great challenge in treating lung cancer and are also associated with high mortality rates.Citation3
Metastasis and inhibition of apoptosis are responsible for cancer advancement, resistance against the drugs, and mortality.Citation6 Apoptosis is defined as programmed cell death that removes unwanted or mutated cells from the body. An equilibrium between proapoptotic and anti-apoptotic proteins is necessary for programmed cell apoptosis. Cysteine proteases (ie, caspases) and proteins of the BCL-2 family are the key regulators of this process.Citation7,Citation8 Typically, a cell follows either intrinsic pathway or extrinsic pathway in caspases-mediated apoptosis. The intrinsic pathway is mediated inside the cell in response to DNA damage, hypoxia, and/or oxidative stress. It involves mitochondrial disassembly followed by the release of cytochrome c in the cytoplasm. This cytochrome c with other proapoptotic proteins forms apoptosome thereby activating a family of effector caspases.Citation9 Extrinsic pathway, however, involves death receptors with adaptor proteins which in turn activate caspases proteins.Citation10 Once executioner caspases (ie, caspases-3 and caspase-6) are activated, breakdown of cytoskeleton and DNA fragmentation occurs, leading to the formation of apoptotic bodies.Citation11 The later events are marked as late phase of apoptosis.Citation12
Alternatively, metastasis is a complex process of tumor spread from the preliminary site to close or distant body parts. It involves invasion of tumor in the circulatory and lymphatic system followed by metastatic colonization in other organs. The family of matrix metalloproteinases (MMPs) plays important roles in the migration and invasion of in situ carcinoma.Citation13 MMPs are zinc proteases that belong to the matrixin family.Citation14 Under this category, gelatinases A and B are of prime importance because of their direct involvement in cancer progression. During tumorigenesis, MMP-2/-9 degrade the basement matrix through which tumor cells enter the blood and spread to other organs.Citation15 Considerably, lung cancer metastasizes to the adrenal gland, liver, brain, and bones.Citation16 With the evolution of resistant carcinoma and side effects of the present chemotherapeutic regimens, there is an immense need for new chemical entities with low side effects, high potency, and high selectivity toward molecular targets.
Quercus incana belongs to the genus Quercus (oak), the largest genus of family Fagaceae. Tropical and subtropical regions of Asia as well as temperate zones of Northern Hemisphere contain various species of genus Quercus.Citation17 The species of this genus are pharmacologically active and possess great medicinal value such as antioxidant, antifungal, antibacterial, and/or anticancer activities.Citation18,Citation19 This genus has great medicinal importance and was traditionally used in pharmacology for its astringent, hemostatic, and antiseptic properties.Citation20 The Q. incana has great medicinal uses; it may be used as astringent, diuretic, antidiarrheal and to treat asthma problems.Citation21 Bark and leaves of this plant are used as antipyretic, antirheumatic, and in wound healing. Acorns bark and leaves are best served for antidiabetic and anti-arthritic purposes.Citation22 Recently, triterpenes have been reported for their apoptotic and anti-cell migratory activities against breast cancer.Citation23 Thus, the present study was conducted to isolate and characterize some known flavonoids and triterpenes from the chloroform fraction of Q. incana leaves. Flavonoids and triterpenes have already been reported for their potent anticancer activity against various cancers.Citation24,Citation25 Therefore, this study was designed to evaluate the anticancer potential of isolated compounds against NSCLC (NCI-H460) cell line. Furthermore, the molecular mechanism of the most potent apoptotic and antimetastatic compounds was demonstrated.
Methods: experimental procedures
Collection of plant material (leaves of Q. incana)
During April to June 2011 plant leaves were collected within Abbottabad region and identified taxonomically as Q. incana from the botany department at the Post Graduate College Abbottabad. The sample was deposited at the college herbarium as voucher specimen (#2550).
Extraction and purification
The leaves of Q. incana were shade dried and ground to a coarse powder. The extraction and fractionation of Q. incana was described in our previous study.Citation19 The chloroform fraction was subjected to column chromatography to isolate the bioactive constituents.
Cell culture
The NSCLC (NCI-H460) and normal mouse fibroblast (NIH-3T3) cell lines were grown and passaged as mentioned earlier by us using RPMI medium.Citation46 Both cell lines were commercially purchased by cell culture biobank (PCMD, ICCBS) from American Type Culture Collection (ATCC). The biobank provided the cell lines to our research group for experimental purpose.
Cell viability assay
The efficacy of the isolated compound to inhibit metabolically active cells was determined by MTT assay. NCI-H460 cells at 10,000 cells/well density were seeded in a 96-well plate for 24 hours followed by treatment at different concentrations (10, 25, 50, 75, and 100 μM) of the compounds. After 48 hours of treatment the reduction in viability of cells using MTT dye was evaluated as mentioned earlier.46 Percent inhibition was calculated by using following equation:
By using software (EZ-Fit Enzyme Kinetics; Perrella Scientific Inc., Amherst, NH, USA) IC50 of the test compounds were calculated. Similarly, these compounds were tested for their cytotoxicity against normal NIH-3T3 cells.
Cell migration assay
1×106 NCI-H460 cells were seeded in each well of a 6-well plate and were grown to confluence. The next day a scratch wound was made using 200 µL micropipette tip. After washing with PBS, betulin at varying concentrations was added and incubated for 48 hours. Images of the wounded area, taken immediately after the treatment, were considered as 0 hour, whereas further images were taken at 24 and 48 hours using a phase-contrast microscope at 10× magnification.Citation47 Images were processed and percent closure was calculated using image J software.
Zymography
5×106/well NCI-H460 cells were seeded in a 6-well plate in serum free media with or without test compound. After 48 hours, media from each well was collected and centrifuged at 400 g for 5 minutes. 10 µL of the supernatants were subjected to 10% (w/v) SDS-PAGE in the presence of 0.4% (w/v) gelatin. Following the electrophoresis, gel was thrice washed with 2.5% (v/v) Triton X-100 (Wako Chemicals, Osaka, Japan) for 20 minutes. After washing, the gel was first equilibrated in activation buffer (50 mM Tris HCl pH 7.5, 0.1 mM NaCl, 10 mM CaCl2 and 1 mM ZnCl2) for 20 minutes followed by overnight incubation in the fresh buffer at 37°C. The next day, gel was stained with 0.2% (w/v) Coomassie Brilliant Blue R-250 (Biobasic, Markham, ON, Canada) in 7.5% glacial acetic acid and 5% methanol, followed by destaining in glacial acetic acid:methanol:water (1:3:6) till gelatinolytic bands appeared. The gel was scanned and gelatinolytic bands were quantified using FluorChem software (Bio-Rad, Hercules, CA, USA).
Transwell invasion assay
To evaluate the invasive potential of NCI-H460 cells, Tran-swell inserts (Millipore, Etobicoke, ON, Canada) coated with Geltrex (Life Technologies, Carlsbad, CA, USA) (9 mg/mL) were used. 40,000 cells with or without betulin were added to the wells and incubated for 48 hours in CO2 incubator at 37°C. 500 µL culture media was added outside the inserts as chemoattractant. Later, the matrix and media were carefully removed from the Transwell inserts using a cotton plug. The inserts were washed with PBS and invaded cells were fixed using 3.7% formaldehyde for 10 minutes at room temperature (TR). Inserts were again washed with PBS and stained with crystal violet for 20 minutes. Excess stain was removed by gentle PBS washing repeatedly. Inserts were then observed and photographed under inverted microscope (4× magnification).
YO-PRO-1™ apoptotic assay
NCI-H460 cells were seeded at a density of 5×105 in a 6-well plate. After 24 hours, cells were treated with different concentrations of the test compound followed by 48 hours incubation at 37°C under a humidified 5% CO2 incubation. Following the incubation, cells were harvested using 0.05% trypsin and the cell pellet was washed twice with PBS at 400× g for 5 minutes to remove all the media and trypsin from the cells. After washing, cells were re-suspended in 1 mL PBS and stained using YO-PRO-1™ and PI as supplied by the Vybrant™ Apoptosis Assay Kit #4 (Thermo Fisher Scientific) following the manufacturer protocol. Briefly, cells were incubated with 1 µL YO-PRO-1™ stock solution (component A) and 1 µL PI stock solution (Component B) for 30 minutes on ice. Later, cells were analyzed on FACSCalibur™ (BD, Franklin Lakes, NJ, USA). YO-PRO-1™ and PI were excited at 488 nm, and fluorescence was measured at 530 and 620 nm, respectively. A total of 10,000 events were acquired from each sample. The percentages of live, apoptotic and dead cells were determined using CellquestPro software (BD biosciences, San Jose, CA, USA).
DAPI staining
2.2 × 104 NCI-H460 cells per well were seeded in a 24-well plate and placed overnight at 37°C in 5% humidified CO2 incubator. The next day, following the washing with PBS, cells were treated with betulin. After the incubation of 48 hours, the media was removed, cells were fixed with 3.7% formaldehyde in PBS for 10 minutes followed by PBS washing and DAPI (1:1000) staining for 20 minutes at 37°C. After staining, cells were again washed with PBS and observed for nuclear changes under Nikon fluorescence microscope at 40× magnification. Images of random fields were taken and were counted for apoptotic cells.
JC-1 assay
The change in the mitochondrial membrane potential after the treatment with test compounds was analyzed by JC-1 (5′,6,6 ′ -tetrachloro-1,1′,3,3′ -tetraethylbenzimidazolylcarbocyanine iodide) dye using manufacturer (Thermo Fisher Scientific) protocol. Briefly, 5×105 NCI-H460 cells were seeded in a 6-well plate. The next day, cells were treated with or without the test compound at different concentrations. After 48 hours of incubation, cells were washed with PBS and harvested using 0.05% trypsin. Later the cell pellet was washed twice with PBS before incubating it with JC-1 dye for 30 minutes at 37°C in 5% CO2 incubator. After incubation, cells were pellet down through centrifugation at 400× g for 5 minutes to remove the dye and were resuspended in 500 µL PBS followed by analysis on flow cytometry on FACSCalibur™ (Becton Dickinson). JC-1 was excited at 520 nm, whereas fluorescence was measured at 590 nm. 10,000 events of each sample were recorded and the percentages of cell population in each quadrant were analyzed using CellquestPro software.
RT-PCR
RNA extraction of NCI-H460 cells after treatment was performed using TRIzol reagent (Thermo Fisher Scientific) following the standard protocol. Briefly, 1×106 cells/well were seeded in a 6-well plate. The next day, cells were treated with the test compound for 48 hours in a 5% CO2 incubator at 37°C. After the incubation, total RNA of the cells was extracted and absorbance ratio 260/280 was determined using Nanodrop-ND-2000 spectrophotometer (Thermo Fisher Scientific). cDNA from 1 µg of extracted RNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer instructions. Later, real-time PCR was performed in a 25 µL reaction mixture using 1 µL cDNA as a template and primers at given annealing temperatures (). 1% agarose (w/v) gel electrophoresis was performed to resolve the PCR products. Control of GAPDH was used as housekeeping gene.
Immunocytochemistry
To analyze the effects of betulin (3) on various protein markers, 20,000 NCI-H460 cells were seeded in a 24-well plate with or without betulin. After 48 hours treatment, media was discarded and cells were carefully and thoroughly washed with PBS. Then cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Again, wells were washed with PBS and 150 µL Triton X-100 was added to the wells for 10 minutes. Cells were incubated with blocking solution for 30 minutes in a humidified environment followed by addition of primary antibody (1:100 dilution in blocking solution) overnight at 4°C. The next day, cells were washed with PBS and respective secondary antibody (Thermo Fisher Scientific) was added to the wells for 1 hour. Finally, DAPI staining was done followed by observing expression of markers under fluorescent microscope at 10× magnification. The primary antibodies used against the markers include BCL-2L1 (Santa Cruz Biotechnology Inc., Dallas, TX, USA), Ki-67 (EMD Millipore, Billerica, MA, USA), caspase-3 (EMD Millipore), caspase-6 (EMD Millipore), caspase-8 (EMD Millipore), and osteopontin (Abcam, Cambridge, MA, USA).
Clonogenic assay
8,000 cells per well in a 6-well plate were seeded and treated with or without betulin the next day. After the treatment of 48 hours, cells were washed with PBS carefully and were allowed to grow in culture media for next 15 days in CO2 incubator at 37°C. The media was changed every third day to ensure the supply of optimal growth conditions to the cells. After incubation, cells were fixed with 3.7% formaldehyde and stained with 0.1% crystal violet. Excess stain was removed by repeated washing with PBS. The colonies of H460 cells were observed and photographed under inverted microscope (4× magnification).
Statistical analysis
Results of the all presented data are reported as means±SD and level of significance were analyzed by Student’s t-test. The values of P<0.05 between the control and treated groups were considered as significant.
Results
Isolation of compounds from Q. incana
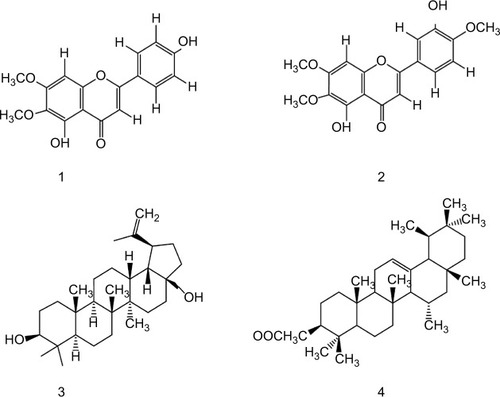
The chloroform fraction of leaves of Q. incana was fractionated in solvent of increasing polarity (ie, n-hexane, n-hexane-chloroform, chloroform, chloroform–methanol, and methanol) by column chromatography using silica gel column. On the basis of thin-layer chromatography profile, the eight fractions were combined to get five fractions. Fraction 3 was further fractionated to two subfractions, ie, C-1 and C-2, when eluted with n-hexane-chloroform (increasing polarity) and rechromatographed over silica gel. Compounds 1 (34 mg) and 2 (64 mg) were purified from subfraction C-2 when loaded on fresh silica gel with mesh size 230–400 µm and eluted with chloroform: n-hexane (9:1). Compound 3 (43 mg) was obtained from fraction 4, when eluted with methanol:chloroform (1:9). However, compound 4 (47 mg) was obtained after elution of fraction 2 in chloroform:n-hexane (7:3) (). The physical and spectroscopic characterization of the compounds is provided in the Supplementary materials.
Characterization of compounds
Compound 1
Pale yellow crystal; C17H14O6. IR (KBr) υmax1650 (C=O), 1599 (C=C), 1356 (C–O), 1252 (C–O), 650 (unsaturated alkene); EI-MS m/z: (rel. int.) 315 [M+1] + (38), 299 (45), 271 (100), 153 (15), HR-EI-MS: m/z 314.0790 (calculated 314.0896 for C17 H14 O6); 1H-NMR (DMSO-d6, 400 MHz): δ 3.74 (3H, s, –OCH3), 3.94 (3H, s, –OCH3), 7.94 (1H, d, J=8.8 Hz, H-2′,H-6′), 6.92 (1H, d, J=8.5 Hz, H-3′, H-5′), 10.41 (1H, 4′-OH), 12.95 (1H, 5-OH) 6.94, 6.82 (2×1, H, 2× s, H-8, H-3); 13C-NMR (DMSO-d6, 100 MHz): δ 182.0 (C-4), 164.0 (C-2), 161.4 (C -4′), 158.9 (C-7), 152.5 (C-9), 152.0 (C-5), 131.9 (C-6), 128.4 (C -6′), 128.4 (C-2′), 121.0 (C-1′), 115.9 (C-3′), 115.9 (C -5′), 105.1 (C-10), 102.5 (C-3), 91.0 (C-8), 60.0 (C -6-OCH3), 56.1 (C -7-OCH3). It was characterized as 4′,5, -dihydroxy-6,7-dimethoxyflavone or cirsimaritin.Citation26
Compound 2
Yellow crystal; C18H16O7. IR (KBr) umax3470 (OH), 1650 (C=O), 1599 (C=C), 1478 (R), 1365 (C–O). EI-MS m/z: (rel. int.) 344 [M] + (86), 329 (45), 326 (64), 301 (100).HR-EI-MS: m/z 344.0896 (calculated 344.0930 for C18 H16 O7). 1H-NMR (DMSO-d6, 400 MHz): δ 3.89 (3H, s, 6-OCH3), 3.97 (3H, s, OCH3-4′), 3.88 (3H, s, 7-OCH3), 6.55 (1H, s, H-3), 6.52 (1H, s, H-8), 7.44 (1H,s, H-2′), 10.40 (1H,s, 4′-OH), 12.90 (1H,s, 5-OH), 7.38 (1H, dd, J=8.2, 2.1, H-6′), 6.91 (1H, d, J=8.8 Hz, H-5′);13C-NMR (DMSO-d6, 100 MHz): δ 182.6 (C-4), 163.8 (C-2), 151.2 (C -4′), 158.7 (C-7), 152.1 (C-9), 156.0 (C-5), 132.0 (C-6), 119.1 (C -6′), 113.2 (C-2′), 123.7 (C-1′), 147.1 (C-3′), 111.7 (C -5′), 107.0 (C-10), 104.4 (C-3), 94.8 (C-8), 60.9 (C-6-OCH3), 55.9 (C-7-OCH3). It was characterized as 5,3′-dihydroxy-6,7,4′-trimethoxyflavone or eupatorin.Citation27
Compound 3
White amorphous powder; C30H50O2. IR (KBr) umax3448 (OH), 3055 (=C–H), 1638 (C=C). EI-MS m/z: (rel. int.) 442 (35), 412 (26), 344 (12), 288(5), 257 (8), 248 (30), 189 (100). HR-EI-MS: m/z 442.3811 (calculated 442.3843 for C30H50 O2). 1H-NMR (CDCl3, 300 MHz): δ 0.66 (1H, d, J=9 Hz, H-5), 0.88 (3H, s, H-24), 0.87 (3H, s, H-25), 0.95 (3H, s, H-23), 0.74 (3H, s, H-27), 1.02 (3H, s, H-26), 1.59 (1H, s, H-19), 1.68 (3H, br, s, H-28), 4.67, 4.56 (1H, dd, J=4.0, 12.3 Hz, H-30), 3.15 (1H, m, H-3), 1.19 (1H, dd, J=3.9,12.3 Hz, H-11).; 13C-NMR (CDCl3, 75MHz): δ 38.7 (C-1), 27.4 (C-2), 79.1 (C-3), 38.8 (C-4), 55.3 (C-5), 18.3 (C-6), 34.3 (C-7), 41.0 (C-8), 50.5 (C-9), 37.3 (C-10), 20.9 (C-11), 25.2 (C-12), 37.2 (C-13), 42.8 (C-14), 27.1 (C-15), 29.2 (C-16), 47.9 (C-17), 48.9 (C-18), 48.8 (C-19), 150.5 (C-20), 29.8 (C-21), 34.0 (C-22), 28.1 (C-23), 15.4 (C-24), 16.1 (C-25), 16.1 (C-26), 14.8 (C-27), 60.6 (C-28), 19.1 (C-29), 109.7 (C-30). It was characterized as Lup-20 (29)-ene-3, 28-diol or betulin.Citation28
Compound 4
White needles; C32H52O2. IR (KBr) umax1730 (C=O), 1640 (C=C). EI-MS m/z: (rel. int.) 468 [M]+ (27), 453 (10), 408 (20), 218 (100), 203 (10). HR-EI-MS: m/z 468.3951(calculated 468.3968 for C32H52O2).1H-NMR (CDCl3, 300 MHz): δ 0.77 (3H, s, H-28), 0.88 (3H, s, H-23), 0.92 (3H, s, H-24), 0.86 (3H, s, H-29), 0.87 (3H, s, H-30), 0.99 (3H, s, H-26), 1.01(3H, s, H-25), 1.04 (3H, s, H-27), 2.05 (3H, s, OAc), 4.51 (1H, dd, J=9.7 Hz, H-3), 5.13 (1H, t, J=3.6 Hz, H-12); 13C-NMR (CDCl3, 75 MHz): δ 38.5 (C-1), 23.3 (C-2), 80.8 (C-3), 37.5 (C-4), 55.2 (C-5), 18.3 (C-6), 33.0 (C-7), 39.8 (C-8), 47.8 (C-9), 36.9 (C-10), 22.9 (C-11), 124.3 (C-12), 136.5 (C-13), 42.6 (C-14), 27.9 (C-15), 26.7 (C-16), 33.5 (C-17), 59.1 (C-18), 39.4 (C-19), 39.7 (C-20), 31.6 (C-21), 41.5 (C-22), 28.2 (C-23), 15.4 (C-24), 14.9 (C-25), 16.5 (C-26), 17.2 (C-27), 28.5 (C-28), 23.3 (C-29), 21.2 (C-30), 21.4 (C-OMe), 170.4 (C-O). It was characterized as 12-Oleanen-3yl acetate or β-amyrin acetate.Citation29
Selective antiproliferative potential of isolated compounds (1–4) against NSCLC cells
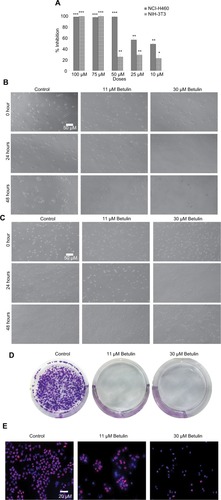
The effect of the compounds isolated from Q. incana on the viability of NSCLC cells (NCI-H460) was evaluated by MTT assay. All the four compounds ie, cirsimaritin (4′,5, -dihydroxy-6,7-dimethoxyflavone) (1), eupatorin (5, 3′-dihydroxy-6,7,4′-trimethoxyflavone) (2), betulin (Lup-20 (29)-ene-3, 28-diol) (3), and β-amyrin acetate (12-oleanen-3yl acetate) (4) were found to be antiproliferative against the cancer cells with low toxicity against normal fibroblast cells (NIH 3T3 cells; ). Among these, betulin (3) was found to be four times more active against cancer cells compared to normal 3t3 cells and was also found to be more potent (IC5011.5 µM) compared to cisplatin (19 µM; ). Furthermore, microscopic analysis revealed that after 24 hours of treatment with betulin, H460 cells started to undergo consequential morphological changes subsuming membrane blebbing and formation of apoptotic bodies (). In contrast, 3T3 cells regained their normal morphology and started to propagate after 48 hours of treatment with betulin at 11 and 30 µM concentrations (). The inhibition observed by betulin against NCI-H460 cells was dose and time dependent. In addition, betulin has completely eradicated cancer cells even after 15 days of incubation in culture media during colony formation assay compared to the control (). Immunocytochemical analysis further indicated the significant inhibition of the proliferative marker Ki-67 in the treated cells ().
Figure 2 (A) Cytotoxic effects of betulin from Quercus incana against NSCLC and normal cells at various concentrations. *P<0.05 was considered as significant. **P<0.01; ***P<0.001. Morphological changes in (B) NCI-H460 cells (C) NIH-3t3 cells after 24 and 48 hours of treatment with betulin, observed under phase contrast microscope at 10× magnification. (D) Effect of betulin on colony formation of H460 cells after 48 hours treatment of betulin. Cells were supplied with fresh culture media every third day and were incubated at 37°C in humidified 5% CO2 environment for 15 days after the treatment. (E) Immunocytochemical analysis of expression of proliferative marker Ki-67 after 48 hours of treatment with betulin, observed under fluorescent microscope at 20× magnification.
Abbreviation: NSCLC, nonsmall cell lung cancer.
Based on potent activity against cancer cells in comparison to 3T3 cells (), betulin (3) was further evaluated for its apoptotic and antimetastatic potential against lung cancer cells.
Table 1 IC50 values of the compounds from chloroform fraction of Quercus incana against NSCLC and normal cells
Betulin inhibits migration and invasion of lung carcinoma in vitro
Cell migration and invasion are the crucial steps in the process of metastasis.Citation30 The antimigratory effect of botulin (3) was established by using bidirectional wound healing assay. Cell migration was significantly inhibited toward the scratch compared with the untreated control at 24 and 48 hours treatment () in a dose-dependent manner. After 48 hours, betulin treated cells showed 17% migration compared to the control, resulting in 41% wound healing (). Moreover, the invasive ability of NCI-H460 cells was inhibited in a dose-dependent manner as shown by transwell membrane invasion assay ().
Figure 3 (A) Effects of betulin on the migration of NCI-H460 cells. Using phase contrast microscope at 10× magnification, scratch areas were photographed and area of the wound was calculated using Image J. (B) Percent closure of the wound after 24 and 48 hours of the treatment. In control well, the rate of migration of the cells toward the wound was increased to 41% after 48 hours in treated groups cells migration was significantly reduced. **P<0.01 vs control. *P<0.05; ***P<0.001. (C) Effects of betulin on invasion of NCI-H460 cells using transwell assay. Membrane was photographed under bright field at 4× magnification using inverted microscope. (D) Effects of betulin on expression levels of MMP-2 and -9 in NCI-H460 cells after 48 hours treatment as evaluated by RT-PCR. (E) Gelatinolytic activity of MMP-2/-9 in response to the treatment with different concentrations of betulin as analyzed by zymography.
Abbreviation: MMP, matrix metalloproteinases.
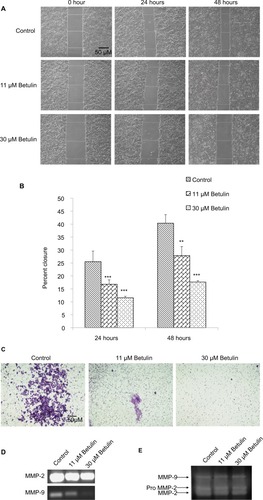
It has already been shown that MMP-2 and MMP-9 play major roles in extracellular matrix degradation.Citation31 The expression of MMP-2/-9 was investigated using RT-PCR and zymography. This result positively correlated with earlier data, with the increasing concentration of betulin (3) signifi-cant decrease in the gene expression and enzymatic activity of MMP-2/-9 was observed ().
Betulin induces cell death via mitochondrial-mediated apoptotic pathway
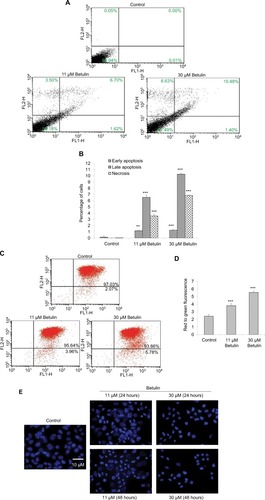
To evaluate whether cell death caused by betulin followed apoptotic dogma, various techniques were employed. Through fluorescence-activated cell sorting (FACS), it was analyzed that betulin treated H460 cells significantly underwent apoptosis in a dose-dependent manner compared to untreated control. It was observed that the majority of the treated cells lied in the late phase of apoptosis which elevated with increasing concentration ( and ). As apoptosis is related with the disruption of mitochondrial membrane potential (MMP, Δψm), JC-1 staining was performed to investigate the effects of betulin on MMP of treated H460 cells. By principle, high red to green fluorescence ratio is associated with polarized (or functional) mitochondria or vice versa. Therefore, the results revealed collapse of MMP in H460 cells posttreatment of betulin for 48 hours (). Furthermore, in agreement with the FACS results, after 48 hours treatment, highly condensed nucleus, apoptotic bodies and DNA fragmentation were clearly observed in H460 cells through DAPI staining. On the contrary, the nucleus of control cells remained intact ().
Figure 4 (A) Betulin (3) induced apoptosis in NSCLC cell line in a dose-dependent manner. Flow cytometric analysis of the apoptosis induced by the indicated concentrations of the compound. An increased number of cells were observed in treated groups compared to control. (B) Graphical representation of the cells in different phases of apoptosis after 48 hours of treatment. **P<0.01 compared to the untreated control. ***P<0.001. (C) Effects of betulin (3) on the mitochondrial membrane potential (Δψm) of the cells after 48 hours. Following the treatment with different concentrations, an increase in positive green fluorescent cells was observed, indicating loss of Δψm compared to the control. (D) Histogram shows the percentages of the cells with disrupted Δψm in treated groups. ***P<0.001 vs the untreated control. (E) Effects of betulin (3) on the nuclear morphology of NCI-H460 cells. Increase in DNA condensation and change in the shape of the nucleus of the treated groups have been observed in a dose and time-dependent manner compared to the control.
Abbreviation: NSCLC, nonsmall cell lung cancer.
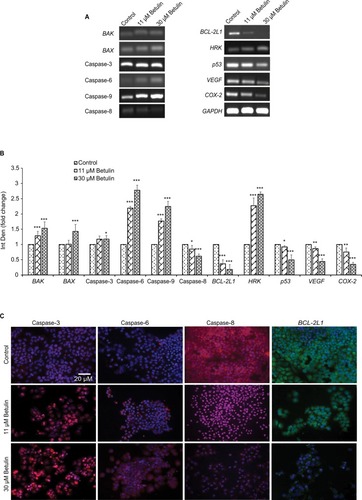
Gene expression analysis of markers responsible for apoptosis was also carried out to elucidate further the molecular mechanism underlying the apoptosis (). The results showed dose-dependent upregulation and downregulation of genes involved in apoptosis. The expression of p53 and BCL-2L1 genes were significantly downregulated, and are responsible for inhibiting apoptosis. However, the compound upregulated the expression of BAX and BAK genes responsible for the apoptosis. It also significantly upregulated the expression of caspases-3, -6, and -9; inducers of mitochondrial-mediated apoptosis cascade. However, the expression levels of caspases-6 and -9 were significantly higher in treated cells compared to caspase-3. Furthermore, immunofluorescence results confirmed significant upregulation of caspases-3 and -6 in treated cells compared to control cells (). Interestingly, substantial expression of caspase-8 was observed in control H460 cells, which decreased with the increase in betulin treatment. This supports the findings that betulin mediates mitochondrial-dependent apoptosis in H460 cells. In addition, the gene expressions of VEGF and COX-2, the most prominent cell proliferative and metastatic markers, were also found to be inhibited after 48 hours of treatment ().
Figure 5 (A) Analysis of effects of the compounds on the mRNA expression of cell survival and apoptotic genes. An upregulation was observed in genes of caspases family and proapoptotic genes ie, HRK, BAX and BAK, while anti-apoptotic BCL-2L1 and p53 genes and cell survival VEGF and COX-2 genes significantly downregulated. Interestingly, initiator caspase-8 of death receptor pathway is also inhibited after the treatment with betulin. GAPDH was used as reference gene. (B) Quantitative analysis of expression of apoptotic genes compared to control. *P<0.05 compared to control. **P<0.01; ***P<0.001. (C) Immunocytochemical analysis of major apoptotic markers after 48 hours treatment with betulin observed under fluorescent microscope at 20× magnification. The caspase-3 and caspase-6 are highly expressed in treated cells while caspase-8 is downregulated, suggesting activation of intrinsic pathway of apoptosis. Furthermore, anti-apoptotic BCL-2L1 show marked inhibition posttreatment.
Abbreviation: Int Den, integrated density.
Betulin induces antineoplastic effects via osteopontin regulation
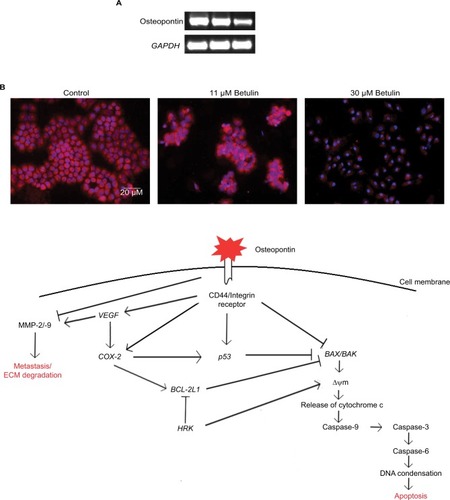
Osteopontin, a phosphorylated glycoprotein, has been widely implicated for cancer progression and metastasis.Citation32 Therefore, effects of betulin on the expression of osteopontin were determined. The results indicated that betulin positively inhibited osteopontin levels analyzed by RT-PCR and immunocytochemistry (). A significant downregulation was observed in a dose-dependent manner ().
Figure 6 (A) The gene expression analysis of osteopontin in response to the treatment with betulin after 48 hours as observed via RT-PCR. The results show pronounced downregulation of expression of the marker in comparison to GAPDH, the reference gene. (B) Proteomic analysis of osteopontin compared with the untreated control studied through immunofluorescence assay under fluorescent microscope at 20× magnification. The expression of osteopontin in the cells after the treatment. (C) Proposed mechanism of osteopontin as central regulator of apoptosis and metastasis in H460 cells.
Abbreviation: MMP, matrix metalloproteinases.
Betulin sensitizes cisplatin in inhibiting H460 cells
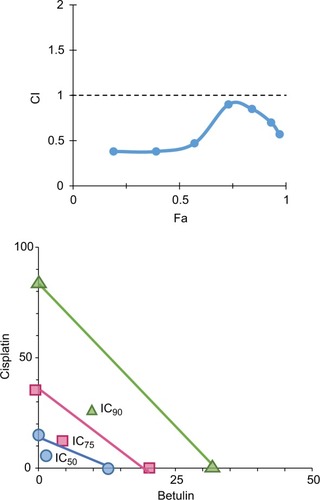
Based on “constant-ratio model”, the combination effects of betulin and cisplatin were studied using MTT assay. After 48 hours of treatment, the data were obtained and analyzed using the Chou–Talalay method. The combination index (CI) of 1/2th, 1/4th, 1/8th, and 1/16th IC50 showed strong sensitizes with fraction affected (Fa) values in the range 0.4–0.8 (data not shown). In , Fa–CI plot indicates CI at a particular effect produced by the drugs while isobologram represents that the combination doses of betulin and cisplatin were much lower than actual data points indicating IC50, IC75, and IC90 of individual drugs. As indicated, 11.5 µM betulin and 19 µM cisplatin induced 50% inhibition of H460 cells, whereas the combination of betulin with cisplatin elicited a similar response at less than 5 µM concentration.
Figure 7 Synergistic effects of betulin with standard drug cisplatin as represented by (A) Fa-CI plot and (B) classic isobologram showing data points of individual drugs vs actual data points of their combination at different inhibitory concentrations observed through MTT assay.
Abbreviations: Fa, fraction affected; CI, combination index.
Discussion
Globally, the mortality and morbidity rate of lung carcinoma is increasing despite of the advancement in the therapeutic strategies. Poor prognosis, resistance against available drugs and inefficacious treatment modalities necessitate the development of new chemotherapy drugs. Triterpenoids, a class of organic compounds, are well known for their multifunctional properties, including antimicrobial and anticancer activities against various cancer types.Citation33 In the present study, among the four triterpenes and flavonoids, isolated from the leaves of Q. incana, betulin (3) possessed the most potent antiproliferative, apoptotic and antimetastatic activities. Earlier studies report lower cytotoxic effects of betulin (3), isolated from other plant sources, against different cancer cell lines.Citation34–Citation36 In contrast, betulin (3) isolated from Q. incana was found highly and selectively active against the cancer cells at much lower doses (11.55 µM), even less than standard drug cisplatin (19 µM). The compound is also noncytotoxic to normal NIH-3T3 cells at higher doses ie 50 µM (). Therefore, it was subjected to further investigation at IC50 and IC80 concentrations (ie, 11 µM and 30 µM) for in-depth analysis of its apoptotic and antimetastatic potential.
Mutations often result in the uncontrolled cell growth that leads to tumor formation. In order to overcome tumor progression and problems related to chemoresistance, induction of apoptosis and inhibition of metastasis to other organs are important factors.Citation11 Betulin (3) induced significant apoptosis in H460 cells after 48 hours treatment (). Interestingly, apoptosis assay revealed that the majority of the cell population after treatment falls in the late apoptotic phases. These results further complement the findings of DAPI staining, in which the cancer cells, after 48 hours, showed events of late phase apoptosis ie, shrunken nuclei and loss of normal morphology compared to the nuclei of control cells (). Among two apoptotic pathways, betulin (3) initiates the mitochondrial pathway as evidenced by altered expression of apoptotic markers, which regulates intrinsic pathway (). RT-PCR and immunofluorescence assay revealed, betulin upregulated caspase-9 expression, while caspase-8 was significantly downregulated. Caspase-8 is considered as a caspases initiator in death receptor mediated apoptosis.Citation9 Therefore, the data suggests that betulin activates intrinsic pathway machinery thereby shutting down proteins of extrinsic pathway in order to induce apoptosis in H460 cells. Furthermore, betulin (3) shows potential antimetastatic activity by inhibiting the wound healing and invasion of cells through transwell inserts compared to the untreated controls. There was an approximate threefold reduction in the wound healing rates of treated cells compared to the untreated cells. Significant inhibition of the expression and enzymatic activity of MMP-2/-9 in a dose-dependent manner was also observed in post betulin treatment ().
In current era, various treatments and their modalities are being continuously introduced to minimize the cancer-related morbidity and mortality. Early diagnosis is an important factor that could help in early removal of cancer hence increasing the survival capabilities.Citation37 Biomarkers are the main players of early and easy detection of any abnormality being progressed in the body. Recently, osteopontin has received much attention because of its central functionality in tumor progression in various cancers.Citation38,Citation39 High levels of serum osteopontin is correlated with tumor advancement.Citation40,Citation41 This study demonstrates potential inhibition of the gene and protein expressions of osteopontin after treatment with betulin (3) at various concentrations. As reported, osteopontin has central roles in interlinking many regulatory and physiological pathways, regulating important markers of apoptosis, metastasis, angiogenesis, bone remodeling pathways downstream of it.Citation42–Citation44 Herein we describe its interplaying role between metastatic and apoptotic pathways of H460 cells (). Expression profile indicated that betulin has potential inhibitory effects on the markers of cell survival and proliferation, ie, VEGF, COX-2, and p53 proteins and metastatic MMP-2/-9.Citation43 Osteopontin functions as positive regulator for these proteins, mediating major processes of metastasis and tumor progression leading to cancer advancement. Moreover, betulin (3) further activates the crucial apoptotic markers, ie, BAK/BAX, HRK and caspases family proteins, while inhibiting BCL-2L1.Citation44 Reports suggest that osteopontin shuts off these proteins and indirectly activates anti-apoptotic BCL-2L1 via COX-2 pathway to inhibit the intrinsic pathway of apoptosis.Citation45
In conclusion, we report the apoptotic and antimetastatic activities of betulin (3), isolated from new plant source, ie, leaves of Q. incana. In addition, the compound followed the cascade of the intrinsic pathway for inducing apoptosis in cancer cells and also inhibited important markers of metastasis. Moreover, strong synergism at lower doses of betulin and cisplatin was also observed after 48 hours (). Therefore, the findings suggest that betulin (3) can be an attractive chemotherapeutic target for treating resistant lung cancers.
Author contributions
BZ and AA are both to be considered as first authors. AK and SAA conceived and designed the study. RS and UF performed isolation. BZ and AA performed anticancer activities. SAA also performed gel experiment. AAH and AK analyzed the data. AK and AA wrote the manuscript with input and comments from all co-authors. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to the International Center for Chemical and Biological Sciences, Hussain Ebrahim Jamal Research Institute of Chemistry, University of Karachi, Higher Education Commission (HEC) Pakistan, and COM-SATS Institute of Information Technology, Pakistan, for providing financial support for this work.
Supplementary materials
Figure S1 Effects on protein expression of (A) BCL-2L1, (B) caspase-3, (C) caspase-6, (D) caspase-8, (E) Ki-67, and (F) osteopontin after 48 h treatment with betulin at different concentrations.
Notes: DAPI staining indicates nuclei of cells, whereas FITC/TxR image shows expression of the respective marker under fluorescent microscope at 20× magnification.
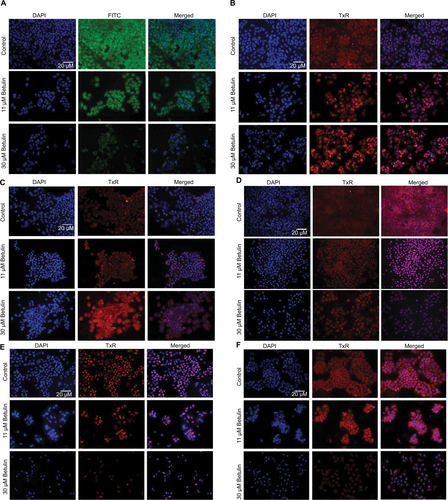
Table S1 Primer sequences of the genes used for RT-PCR.
References
- HanifFPerveenKJawedHN-(2-hydroxyphenyl)acetamide (NA-2) and Temozolomide synergistically induce apoptosis in human glioblastoma cell line U87Cancer Cell Int201414113314225663820
- AlharbiRHOX/PBX interaction as a therapeutic target in Acute Myeloid LeukemiaPhD thesisGuildfordUniversity of Surrey2015
- KrisnamurtiDGBLouisaMAnggraeniEWanandiSIDrug efflux transporters are overexpressed in short-term tamoxifen-induced MCF7 breast cancer cellsAdv Pharm Sci20162016616
- GuanHXieLLeithäuserFKLF4 is a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphomaBlood201011691469147820519630
- ChoiY-CYCYoonSByunYMicroRNA library screening identifies growth-suppressive microRNAs that regulate genes involved in cell cycle progression and apoptosisExp Cell Res2015339232033226485640
- SuoHSongJIA-LEJLZhouYInduction of apoptosis in HCT-116 colon cancer cells by polysaccharide of Larimichthys crocea Larimichthys crocea swim bladderOncol Lett20159297297825624917
- ZakerFAmirizadehNNasiriNGene expression and methylation pattern in HRK apoptotic gene in myelodysplastic syndromeInt J Mol Cell Med201652909927478805
- WangSTuJZhouCThe effect of Lfcin-B on non-small cell lung cancer H460 cells is mediated by inhibiting VEGF expression and inducing apoptosisArch Pharmcal Res2015382261271
- KimBMMaengKLeeK-HKHHongSHCombined treatment with the Cox-2 inhibitor niflumic acid and PPARγ ligand ciglitazone induces ER stress/caspase-8-mediated apoptosis in human lung cancer cellsCancer Lett2011300213414421067863
- BenusGFWierengaATde GorterDJInhibition of the transforming growth factor beta (TGFbeta) pathway by interleukin-1beta is mediated through TGFbeta-activated kinase 1 phosphorylation of SMAD3Mol Biol Cell20051683501351015917296
- ShiYFuXHuaYThe side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cellsPLoS One201273e3335822428030
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health RankingsHealth Profile: Pakistan. World Life Expectancy Available from: http://www.worldlifeexpectancycom/country-health-profile/pakistanAccessed January 3, 2019
- FarhanaBCancer Registration in PakistanLahorePulse International2014
- SiegelRNDJemalAEpidemiology of Lung CancerTexasElsevier Press2013
- JafriSHGlassJShiRZhangSPrinceMKleiner-HancockHThymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivoJ Exp Clin Cancer Res20102918720594324
- HerbstRSHeymachJVLippmanSMLung cancerN Engl J Med2008359131367138018815398
- HassanMWatariHAbualmaatyAOhbaYSakuragiNApoptosis and molecular targeting therapy in cancerBiomed Res Int201420142123
- FernaldKKurokawaMEvading apoptosis in cancerTrends Cell Biol2013231262063323958396
- HerrIDebatinKMCellular stress response and apoptosis in cancer therapyBlood20019892603261411675328
- IndranIRTufoGPervaizSBrennerCRecent advances in apoptosis, mitochondria and drug resistance in cancer cellsBiochim Biophys Acta20111807673574521453675
- KasibhatlaSTsengBWhy target apoptosis in cancer treatment?Mol Cancer Ther20032657358012813137
- LoweSWLinAWApoptosis in cancerCarcinogenesis200021348549510688869
- WongRSApoptosis in cancer: from pathogenesis to treatmentJ Exp Clin Cancer Res20113018721943236
- DeryuginaEIQuigleyJPMatrix metalloproteinases and tumor metastasisCancer Metastasis Rev200625193416680569
- Shuman MossLAJensen-TaubmanSStetler-StevensonWGMatrix metalloproteinases: changing roles in tumor progression and metastasisAm J Pathol201218161895189923063657
- LuPWeaverVMWerbZThe extracellular matrix: a dynamic niche in cancer progressionJ Cell Biol2012196439540622351925
- SchuchertMJLuketichJDSolitary sites of metastatic disease in non-small cell lung cancerCurr Treat Options Oncol200341657912525281
- NasirEAliSIFlora of West PakistanRawalpindBotany Department, Gordon College1976
- GeHMShenYZhuCHPenicidones A-C, three cytotoxic alkaloidal metabolites of an endophytic Penicillium spPhytochemistry200869257157617804027
- SarwarRFarooqUKhanAEvaluation of antioxidant, free radical scavenging, and antimicrobial activity of Quercus incana RoxbFront Pharmacol20156e013132127726635607
- LeporattiMLIvanchevaSPreliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and ItalyJ Ethnopharmacol2003872–312314212860298
- HaqFAhmadHAlamMTraditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), PakistanJ Med Plants Res2011513948
- ShinwariMIKhanMAFolk use of medicinal herbs of Margalla Hills National Park, IslamabadJ Ethnopharmacol2000691455610661883
- HsuRJHsuYCChenSPThe triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cellsBMC Complement Altern Med20151516525885960
- GillBSKumarSNavgeetTriterpenes in cancer: significance and their influenceMol Biol Rep201643988189627344437
- SaralammaVVGKimEHLeeHJRahaSLeeWSFlavonoids: a new generation molecule to stimulate programmed cell deaths in cancer cellsJ Biomed Transl Res2017183037
- MarkhamKRRevised structures for the flavones cirsitakaoside and cirsitakaogeninPhytochemistry1983221316317
- MoradkhaniSKobarfardFAyatollahiSAMPhytochemical investigations on chemical constituents of Achillea tenuifolia LamIranian J Pharm Res201413310491054
- LiYWuYQShiYPLupane triterpenoids from Salvia roborowskii MaximPharmazie2003581293793814703979
- JodehSBasalatNObaidAABouknanaDHammoutiBAdsorption of some organic phenolic compounds using activated carbon from cypress productsJ Chem Pharm Res201462713723
- van ZijlFKrupitzaGMikulitsWInitial steps of metastasis: cell invasion and endothelial transmigrationMutat Res20117281–2233421605699
- ValastyanSWeinbergRATumor metastasis: molecular insights and evolving paradigmsCell2011147227529222000009
- ShevdeLASamantRSRole of osteopontin in the pathophysiology of cancerMatrix Biol20143713114124657887
- PetronelliAPannitteriGTestaUTriterpenoids as new promising anticancer drugsAnticancer Drugs2009201088089219745720
- FernandesCPCorrêaALLoboJFTriterpene esters and biological activities from edible fruits of Manilkara subsericea (Mart.) Dubard, SapotaceaeBiomed Res Int2013201328081023509702
- KrólSKKiełbusMRivero-MüllerAStepulakAComprehensive review on betulin as a potent anticancer agentBiomed Res Int201520153111
- YadavVRPrasadSSungBKannappanRAggarwalBBTargeting inflammatory pathways by triterpenoids for prevention and treatment of cancerToxins20102102428246622069560
- ChanKKMatchettKBMcenhillPMProtein deregulation associated with breast cancer metastasisCytokine Growth Factor Rev201526441542326088937
- WangYYangJLiuHThe association between osteopontin and survival in non-small-cell lung cancer patients: a meta-analysis of 13 cohortsOnco Targets Ther20158351326648743
- FanXHeCJingWIntracellular osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesisCancer Res2015751869725398438
- RodriguesLRTeixeiraJASchmittFLPaulssonMLindmark-MänssonHThe role of osteopontin in tumor progression and metastasis in breast cancerCancer Epidemiol Biomarkers Prevent200716610871097
- ShiLWangXRole of osteopontin in lung cancer evolution and heterogeneitySemin Cell Dev Biol201764404727578008
- WeiRWongJPCKwokHFOsteopontin – a promising biomarker for cancer therapyJ Cancer20178122173218328819419
- SalehSThompsonDEMcconkeyJMurrayPMooreheadRAOsteopontin regulates proliferation, apoptosis, and migration of murine claudin-low mammary tumor cellsBMC Cancer201616135927282619
- ZhuQLuoXZhangJOsteopontin as a potential therapeutic target for ischemic strokeCurr Drug Deliv201714676677227848883
- HussainHAHarveyAJEvolution of breast cancer therapeutics: Breast tumour kinase’s role in breast cancer and hope for breast tumour kinase targeted therapyWorld J Clin Oncol20145329925114846
- FarooqUNazSZehraBIsolation and characterization of three new anti-proliferative Sesquiterpenes from Polygonum barbatum and their mechanism via apoptotic pathwayBMC Cancer201717169429061136
- ChangCMChangPYTuMGEpigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2Oncol Rep20122851799180722923287