Abstract
Background
Although the prognostic implications of negative lymph nodes (NLNs) has been reported for a variety of tumors, little information has been published about the NLNs in gallbladder cancer (GBC).
Patients and methods
In this study, clinicopathological characteristics and survival times of patients who had undergone surgery for GBC were collected from the Surveillance, Epidemiology, and End Results Program-registered TNM stage database and analyzed. Univariate and multivariate Cox proportional hazards models were used to identify the predictors of survival.
Results
It was found that a cutoff of one to two NLNs is optimal when assessing the association with survival, survival rates being consistently better with two or more NLNs than with fewer than two. This optimal cutoff value of 2 was identified as an independent prognostic factor by univariate and multivariate analyses (all P<0.001). Specifically, patients with two or more NLNs had better 5-year gallbladder cancer cause-specific survival than those with fewer than NLNs examined for stage I/II, stage III/IV, and all TNM stages (all P<0.001).
Conclusion
Our findings indicate that the number of NLNs is an independent prognostic factor after GBC surgery, and, together with the number of positive lymph nodes, this will provide better prognostic information than the number of positive lymph nodes alone.
Introduction
Gallbladder cancer (GBC) is the most frequent biliary tract malignancy worldwide. It is a rare but a fatal malignancy characterized by the absence of effective therapy and poor prognosis.Citation1 Surgery is the only definitively curative treatment, provides the only ray of hope for long-term survival.Citation2 However, even after operation, the survival was still poor.Citation3 The number of negative lymph nodes (NLNs) has been reported as a prognostic factor in various cancers.Citation4–Citation6 However, few studies have reported the prognostic implication of the number of NLNs in GBC. Because GBC is often diagnosed at an advanced stage, its cause-specific survival (GCSS) is usually poor.Citation7–Citation9 The aim of this study was to identify the prognostic significance of NLNs in patients who have undergone surgery for GBC.
Patients and methods
Patient selection from the Surveillance, Epidemiology, and End Results (SEER) database
The SEER Cancer Statistics Review, which comprises the most recent statistics on cancer incidence, mortality, survival, prevalence, and lifetime risk, is published annu ally by the Data Analysis and Interpretation Branch of the National Cancer Institute, USA. The current SEER database derives from 17 population-based cancer registries in the USA.Citation10 It contains no identifiers and is publicly available for studies of cancer-based epidemiology and lymph node (LN) staging of colorectal, gastric, esophageal, and other cancers.
Using the SEER-stat software (SEER*Stat 8.3.4), we searched for patients diagnosed as having a single primary GBC between 2004 and 2015.
SEER registry patients eligible for our study cohort included those with the following histologic type ICD-O-3: adenocarcinoma (8140, 8144, 8145), papillary adenocarcinoma (8260, 8261, 8263), mucinous adenocarcinoma (8480, 8481), adenocarcinoma with metaplasia (8574, 8576), papillary carcinoma (8050), duct carcinoma (8500), squamous cell carcinoma (8070, 8071, 8083), adenosquamous carcinoma (8560), signet ring (8490), small cell (8041, 8045), giant and spindle cell (8030, 8033, 8035), non-small cell carcinoma (8046), carcinoma not otherwise specified (8010, 8012, 8013), undifferentiated carcinoma (8020), cholangiocarcinoma (8160), neoplasm, malignant (8000), carcinosarcoma (8980), carcinoid tumor (8240, 8244, 8246), adenocarcinoma in adenomatous polyp (8210), solid carcinoma (8230), adenocarcinoma with mixed subtypes (8255), clear cell adenocarcinoma (8310), and mixed cell adenocarcinoma (8323). We excluded all other histologic types. Age was limited to ≥18 years. All study patients had histologically proven GBC. Other exclusion criteria were as follows: cause of death unknown, no surgery, unknown whether surgery had been performed, unknown TNM stage, unknown number of NLNs, and unknown tumor size.
Statistical analysis
Sex, age, race, years of diagnosis, histotype, seer historic stage A, grade, tumor size, TNM, T stage, total regional lymph nodes (TLNs), NLNs, positive lymph nodes (PLNs), histological grade, survival time, and GCSS were extracted from the SEER database. Patients within each American Joint Committee on Cancer substage were dichotomized based on the number of NLNs. The comparison of the rate of GBC death between the two groups for each substage was made using the Kaplan–Meier method. Multivariable Cox regression models were built for the analysis of risk factors for survival outcomes. The primary endpoint of this study was GCSS, which was calculated from the date of diagnosis to the date of cancer-specific death. Deaths due to GBC were treated as events and deaths from other causes were treated as censored observations. Statistical evaluation was conducted with SPSS 22.0 (IBM Corporation, Armonk, NY, USA). P-values <0.05 were considered statistically significant.
Results
Identification of cutoff points for optimal minimum number of NLNs for predicting GCSS in eligible patients from the SEER database
We identified 1,754 eligible patients in the SEER during the 12-year study period (between 2004 and 2015). The median follow-up period after resection is 15 months (range, 0–142.0 months). First, we treated NLN count as a continuous variable and used it as a single factor in univariate log-rank test (χ2=317.533, P<0.001), which identified it as a potentially valuable prognostic factor. Second, to evaluate the influence of different cutoff points on GCSS in patients with all TNM stages of disease, we further analyzed the prognostic effect of NLN counts from one to ten singly by determining the 5-year GCSS of patients with n (cutoff point) or more NLNs and less than n NLNs for each value for n from one to ten. Survival rates of patients with n or more NLNs were greatest when n ranged from one to two and the difference in survival was most significant (maximum of χ2 log-rank values) for n=1. Patients with less than one NLN had a 30.7% higher rate of deaths from GBC than those with one or more NLNs (8.9% vs 39.6%, χ2=255.347, P<0.001). Patients with two or more NLNs had the highest 5-year GCSS (40.9%). For all cutoffs greater than two NLNs, the survival rates were lower and gradually reduce (). The cutoff of two NLNs is probably the most accurate, at and above which the influence of NLNs count on survival is minimal.
Table 1 Univariate analysis for the influence of different cutoffs on gallbladder cancer cause-specific survival (GCCS)
Characteristics of eligible patients in the SEER database
The study cohort comprised 526 men and 1,228 women. Of these, 969 (55.25%) had fewer than two NLNs and 785 (44.75%) two or more NLNs. A greater proportion of patients in the group with two or more NLNs were women (68.9%), aged >60 years (66.0%), white (72.5%), diagnosed most recently (2012–2015; 47.1%), had adenocarcinomas (72.2%), had grade I/II disease (54.9%), had tumor diameters <3 cm (61.5%), and had TNM stage III/IV tumors (37.5%). Baseline patient and tumor characteristics are presented in . Age, year of diagnosis, histological type, grade, and TNM stage differed significantly between those with fewer than two NLN and those with two or more NLN (P<0.05).
Table 2 Baseline patient and tumor characteristics of patients with gallbladder cancer from the SEER database according to NLN status
Both the median number of NLNs and median number of PLNs were 1. As expected, patients with stage III/IV cancer had more PLNs (median of 1) than patients with stage I/II (median of 0). Conversely, patients with stage III/IV cancer had fewer NLNs (median of 1) than those with stage I/II cancer (median of 2). Patients with fewer than two NLNs had fewer TLNs examined (median of 1) than patients with two or more NLNs group (median of 5). The numbers of NLNs and TLNs were strongly correlated (r=0.929, P<0.001).
Effect of the number of NLNs on GCSS in eligible patients from the SEER database
According to univariate analysis, the following were significant risk factors for poor survival: year of diagnosis (P<0.001), age (P<0.001), histological type (P<0.001), TNM stage (P<0.001), SEER historic stage A (P<0.001), T stage (P<0.001), number of NLNs (P<0.001), number of PLNs (P<0.001), tumor size (P<0.001), and grade (P<0.001) (). According to multivariate analysis with Cox regression, age (P<0.001), histological type (P<0.001), TNM stage (P<0.001), SEER historic stage A (P<0.001), T stage (P<0.001), tumor size (P<0.001), grade (P<0.001), and number of NLNs (with optimal cutoff 2, P<0.001) were independent prognostic factors for GCSS. The number of NLNs was found to have a positive effect on survival (HR 0.654; 95% CI 0.572–0.746) ().
Table 3 Univariate and multivariate survival analyses for evaluating the influence on GCSS in data from the SEER database
Subgroup analysis to evaluate the effect of NLN counts according to TNM stage and N stage in eligible patients from the SEER database
After stratifying patients by TNM stage and N stage (except N2), the differences between GCSS for fewer than and two or more NLNs were statistically significant on both univariate and multivariate analyses (all P<0.05; ). Patients with all TNM stages and two or more NLNs had a 17.8% greater 5-year GCSS than those with two or fewer NLNs evaluated (40.9% vs 23.1%, P<0.001; ); patients with TNM I/II disease and two or more NLNs had a 19.1% greater 5-year GCSS than those with two or fewer NLNs evaluated (73.3% vs 54.2%, P<0.001; ); patients with TNM III/IV disease and two or more NLNs had a 6.3% greater 5-year GCSS than those with two or fewer NLNs evaluated (20.0% vs 13.7%, P<0.001; ); patients without a recorded TNM stage and two or more NLN had a 17.4% greater 5-year GCSS than those with two or fewer NLNs evaluated (38.1% vs 20.7%, P<0.001; ). Furthermore, for N1 stage, patients with two or more NLNs had 3.3% greater 5-year GCSS than those with two or fewer NLNs evaluated (17.3% vs 14.0%, P<0.001; ); and patients without an N stage and two or more NLNs had a 17.4% greater 5-year GCSS than those with two or fewer NLNs evaluated (38.1% vs 20.7%, P<0.001; ). Thus, according to these analyses, the number of NLNs (with an optimal cutoff of 2) is an independent positive predictor of GCSS on both univariate and multivariate analyses (P<0.05).
Table 4 Univariate and multivariate analyses of effect of NLN count on GCSS according to cancer stage
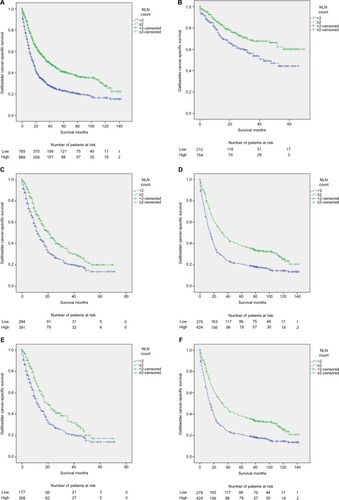
Figure 1 Survival curves in gallbladder cancer patients according to number of NLNs.
Notes: Log-rank tests according to number of NLNs (two or more vs fewer than two) for (A) all TNM stages: 40.9% vs 23.1%, respectively; χ2=85.325, P<0.001; (B) stage I/II: 73.3% vs 54.2%, respectively; χ2=14.236, P<0.001; (C) stage III/IV: 20.0% vs 13.7%, respectively; χ2=18.048, P=0.002; (D) TNM stage unavailable: 38.1% vs 20.7%, respectively; χ2=34.358, P<0.001; (E) stage N1: 17.3% vs 14.0%, respectively; χ2=11.306, P=0.001; and (F) N stage unavailable: 38.1% vs 20.7%, respectively; χ2=34.358, P<0.001. The absolute number of patients at risk is listed below the curve.
Abbreviation: NLNs, negative lymph nodes.
Discussion
GBC is the commonest malignant biliary neoplasm and the seventh most common gastrointestinal cancer. Operative resection remains the only curative therapy for GBC. NLN status has been confirmed as an independent prognosis factor for cancer-specific survival in colon,Citation4 gastric,Citation5esophageal,Citation11 cervical,Citation12 breast,Citation13 and rectal cancer.Citation14 However, little has been published about the number of NLNs as a potential prognostic factor for GCSS. In the present study, we showed that the number of NLNs is also a prognostic factor for GCSS.
Thus far, the mechanism by which NLNs affect prognosis of GCSS is unknown. An effect of lymph micrometastasis on survival has been shown for laryngeal,Citation15 gastric,Citation16 and lung cancer.Citation17 Yokoyama et alCitation18 reported a strong correlation between LN micrometastasis and indications of wide spread LN disease in patients with GBC. Furthermore, they demonstrated a significantly worse prognosis for patients with micrometastases.Citation18 Similarly, Sasak et al reported that survival is worse in patients with GBC who have micrometastases.Citation19 Because it is difficult to identify lymphatic micrometastasis intraoperatively, as many NLNs as possible should be resected to minimize the residual micrometastases and improve the prognosis. Jin et al have suggested that patients with GBC be managed at high volume centers with experience with complex patients because GBC treatment in high volume centers is associated with survival benefit.Citation20 Complete resection of the regional LNs may be crucial in the management of GBC.Citation21 The concept of the surgeon as a variable that affects outcomes is not new. Le Voyer et al indicated that surgeons with greater experience could better achieve complete resection. Patients from whom more nodes have been resected have potentially undergone more complete excision of their primary tumors and draining LNs. Complete excision of regional LNs means that more NLNs are examined. Likewise, the greater the number of NLNs identified, the more likely it is that complete resection has been achieved.Citation22
In our study, the optimal cutoff value for NLNs was 2 (all stages) after surgical resection of GBC. There was not a statistically significant difference in 5-year GCSS between patients with N2 stage disease and fewer than two NLNs (23 patients) vs two or more NLNs (17 patients), probably because of the small number of patients with N2 stage disease. In previous studies, the incidence of LN micrometastases has been lower in patients with GBC than in those with other cancers.Citation18,Citation23–Citation26 Others have validated NLN count as an independent prognostic factor by both univariate and multivariate analyses; however, the cutoff number of NLNs is reportedly much larger than what we found for GBC. For example, Lu et al concluded that 6 is the optimal cutoff value for NLN count as an independent prognostic factor for cervical CSS,Citation12 whereas Yang et al concluded it is 9 for breast CSS,Citation13 Li et al concluded it is 9 for rectal CSS,Citation14 and Li et al concluded it is 12 for gastric CSS.Citation27 Most GBCs are diagnosed at an advanced stage, about 70% of GBCs being diagnosed intra- or postoperatively. Patients with symptoms generally have advanced disease, which implies more LN metastases and fewer NLNs. Furthermore, because the gallbladder has no submucosal layer, organ invasion may develop more readily and earlier than in other cancers.Citation28
Tumor immune responses may be one of the reasons for the number of NLNs affecting prognosis. The benefits associated with a higher number of NLNs may simply reflect a host lymphocytic reaction to the tumor, as has been reported for colorectal cancer.Citation29 Nakakubo et al evaluated the extent of immune cell infiltration of tumors in 110 surgically resected gallbladders by immunohistochemistry and reported that large numbers of infiltrating dendritic cells (DCs) correlate with fewer metastases to LNs. Furthermore, they found DC numbers to be significantly correlated with duration of survival.Citation30
Although the current study is a large population-based study, it had some limitations. First, the use of chemotherapy and information on the quality of surgical care and pathological examination are not included in the SEER database. Second, use of the SEER database does not enable study of the mechanism for the association between the number of NLNs and GCSS. Third, the SEER GBC database does not provide data on adjuvant therapy, comorbidities, and recurrence rates.
Conclusion
Our study suggests that the number of NLNs examined is a prognostic factor after GBC surgery and provides more information about the prognosis than the number of positive LNs alone.
Acknowledgments
We thank Dr Trish Reynolds, MBBS, FRACP, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This work was supported by six kinds of talents of Jiangsu Province (No. WSW-087), the Scientific Research Subject of Jiangsu Province Health Department (No. H201661), and the Project of Invigorating Health Care through Science, Technology and Education: Jiangsu Provincial Medical Youth Talent (QNRC2016331).
Disclosure
The authors report no conflicts of interest in this work.
References
- MillerGJarnaginWRGallbladder carcinomaEur J Surg Oncol200834330631217964753
- GargPKPandeyDSharmaJThe surgical management of gallbladder cancerExpert Rev Gastroenterol Hepatol20159215516625155211
- BaiDSChenPQianJJJinSJJiangGQEffect of marital status on the survival of patients with gallbladder cancer treated with surgical resection: a population-based studyOncotarget2017816264042641328460434
- JohnsonPMPorterGARicciardiRBaxterNNIncreasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancerJ Clin Oncol200624223570357516877723
- DengJLiangHWangDEnhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodesAnn Surg Oncol20101741043105120039218
- ChenYZhangLTianJRenXHaoQCombining the negative lymph nodes count with the ratio of positive and removed lymph nodes can better predict the postoperative survival in cervical cancer patientsCancer Cell Int2013131623374254
- HundalRShafferEAGallbladder cancer: epidemiology and outcomeClin Epidemiol201469910924634588
- FongYJarnaginWBlumgartLHGallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative interventionAnn Surg2000232455756910998654
- DixonEVollmerCMSahajpalAAn aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American CenterAnn Surg2005241338539415729060
- National Cancer Institute [webpage on the Internet]Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence – SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969–2016 Counties National Cancer Institute DCCPS, Surveillance Research Program, released April 2018 based on the November 2017 submission Available from: www.seer.cancer.govAccessed December 4, 2018
- ZhuZChenHYuWNumber of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomyAnn Surg Oncol20142192857286324740827
- LuHGuoRYangHThe prognostic value of negative lymph node count for patients with cervical cancer after radical surgeryOncotarget2018922810281829416813
- YangJLongQLiHLvQTanQYangXThe value of positive lymph nodes ratio combined with negative lymph node count in prediction of breast cancer survivalJ Thorac Dis2017961531153728740666
- LiXLuHXuKWangHLiangXHuZNegative lymph node count is an independent prognostic factor for patients with rectal cancer who received preoperative radiotherapyBMC Cancer201717122728351352
- NegmHMoslehMFathyHHareedyAElbattawyACytokeratin immunohistochemically detected nodal micrometastases in N0 laryngeal cancer: impact on the overall occult metastasesEur Arch Otorhinolaryngol201327031085109222763427
- CaoLHuXZhangYHuangGAdverse prognosis of clustered-cell versus single-cell micrometastases in pN0 early gastric cancerJ Surg Oncol20111031535621031429
- YuYZhaoQHeXPWangZLiuXYZhangZPSignal transducer and activator of transcription 3 overexpression promotes lymph node micrometastasis in early-stage non-small cell lung cancerThorac Cancer20189551652229575778
- YokoyamaNShiraiYHatakeyamaKImmunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibodyCancer19998571465146910193935
- SasakiENaginoMEbataTImmunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with gallbladder carcinomaAnn Surg200624419910516794394
- JinLXPittSCHallBLPittHAAggressive surgical management of gallbladder cancer: at what cost?Surgery2013154226627323830031
- LinHTLiuGJWuDLouJYMetastasis of primary gallbladder carcinoma in lymph node and liverWorld J Gastroenterol200511574875115655837
- Le VoyerTESigurdsonERHanlonALColon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089J Clin Oncol200321152912291912885809
- IshidaKKatsuyamaTSugiyamaAKawasakiSImmunohistochemical evaluation of lymph node micrometastases from gastric carcinomasCancer1997796106910769070482
- GreensonJKIsenhartCERiceRMojzisikCHouchensDMartinEWIdentification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survivalCancer19947335635697507795
- SasakiMWatanabeHJassJRAjiokaYKobayashiMHatakeyamaKImmunoperoxidase staining for cytokeratins 8 and 18 is very sensitive for detection of occult node metastasis of colorectal cancer: a comparison with genetic analysis of K-rasHistopathology19983231992089568504
- SasakiMWatanabeHJassJROccult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in “node-negative” colorectal cancerJ Gastroenterol19973267587649430013
- LiXZhangWZhangXThe prognostic value of negative lymph node count for patients with gastric cancer who received preoperative radiotherapyOncotarget2017829469464695428159939
- HongEKKimKKLeeJNSurgical outcome and prognostic factors in patients with gallbladder carcinomaKorean J Hepatobiliary Pancreat Surg201418412913726155265
- GeorgeSPrimroseJTalbotRWessex Colorectal Cancer Audit Working GroupWill Rogers revisited: prospective observational study of survival of 3592 patients with colorectal cancer according to number of nodes examined by pathologistsBr J Cancer200695784184716969342
- NakakuboYMiyamotoMChoYClinical significance of immune cell infiltration within gallbladder cancerBr J Cancer20038991736174214583778
