Abstract
Gastric cancer (GC) is responsible for 9% of cancer deaths worldwide. Over 950,000 new cases are diagnosed each year, and about 90% of them are in advanced stage, requiring chemotherapy. In Europe there has been research based on pre- and postoperative chemotherapy treatment, using 5-fluorouracil, epirubicin, cisplatin, capecitabine, and docetaxel. Chemotherapy significantly impairs the quality of life of patients; however, the final effects are not always satisfactory. There is scientific evidence that gastric mucus tumors and signet ring cell carcinomas have a pattern of specific signatures, that distinguish them from other gastric cancer subtypes, and may be associated with a poor response to systematic treatment. Signet ring cell carcinoma is less chemosensitive than others, and the increase in the percentage of signet ring cells correlates with resistance to chemotherapy. Perioperative chemotherapy in advanced signet ring cell carcinomas is an independent factor of poor prognosis and survival, which is explained by the toxicity of neoadjuvant treatment. Therefore, curative surgical resection enhanced by standardized lymphadenectomy remains the recommended gold standard in GC therapy. According to presented studies, early detection and aggressive treatments for this subtype of GC is a reasonable approach. This review paper is mostly addressed to physicians who are interested in updating to the state of the art concerning different subtypes of gastric carcinoma.
Introduction
Gastric cancer (GC) is the fifth most frequently diagnosed cancerCitation1 and the fourth cause of cancer death worldwide, with a median overall survival of ≤12 months for advanced stage.Citation2 It is perceived as a one of the major public health problems.Citation3 It is a heterogeneous disease with different genetic and molecular alterations.Citation4 The average age of morbidity for patients with GC is more than 50 years of age, and it is very rare in the younger population.Citation5–Citation8 Less than 10% of patients are below 45 years of age (early-onset gastric carcinoma).Citation9 In the recent years, a decrease in the overall incidence has been observed. However, recent studies have shown that the incidence of signet ring cell carcinoma (SRCC) subtype has been constantly increasing.Citation10–Citation13 This tendency toward higher percentage of SRCC cases of gastric adenocarcinoma can largely be explained by changes in the pathological classifications of cancers.Citation14
Nowadays, the concept of multistage carcinogenesis is widely accepted and confirmed in many reports. It is a multistage process specified by different types of mutations and epigenetic alterations in the bunch of multiple genes, which finally lead to development of the malignancy. Recognition patterns of GC, including cell cycle regulators, factors that regulate apoptosis, microsatellite instability, multidrug resistance proteins, factors that influence cell membrane properties, module of HER2 expression, and agents that impact on the progression of GC and peritoneal metastasis, are important signatures in the early diagnosis of this disease.Citation15,Citation16
Prevention of GC remains a major priority. Additionally, patients displaying high risk should be directed for early detection and chemoprevention. Surgical resection enhanced by standardized lymphadenectomy is still the gold standard in GC therapy.Citation17
In the recent decades, different pathohistological classification systems have been established for GC. However, there is still a question as to which classification should be followed to make patient-specific decisions, regarding diagnosis and treatment.Citation18 The most common classification of gastric carcinoma is the Lauren’s classification, which was established in 1965. It differentiates intestinal and diffuse types of GC, which show distinguishing features like morphology, genetics, clinical characteristics, progression pattern, and epidemiology.Citation19
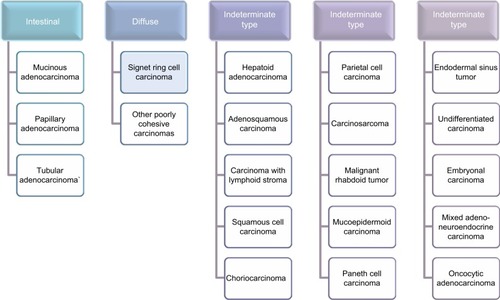
Diffuse-type GC is created by poorly cohesive single cells with no gland formation and is often referred to as SRCC, because of the frequent presence of signet ring cells. Intesti nal-type GC contains glandular or tubular components with various degrees of differentiation and is associated with intestinal metaplasia of the gastric mucosa and with the presence of Helicobacter pylori bacterium.Citation20,Citation21 Later, the indeterminate type was added to describe an uncommon histology.Citation19,Citation22 It is important that signet ring cell adenocarcinomas are always classified, by definition, as “undifferentiated type” or “diffuse type”; however, not all GCs classified as “undifferentiated” or “diffuse” type are signet ring cell cancers.
The present review summarizes the data about the different methods available for the treatment of SRCC of stomach based on current medical knowledge and research conducted in this field.
Classification of the gastric SRCC
SRCC was initially included in the “unclassified” type of GC for diagnostics and research purpose; however, since the publication of a paper in 1990, the WHO classified the signet ring cell adenocarcinoma as one of the specific types of GC, which is shown in . According to WHO’s classification, SRCC has been described as a weakly cohesive type of carcinoma, mostly encompassing tumor cells with leading cytoplasmic mucin and a crescent-shaped nucleus quirkily located.Citation23,Citation24 The WHO classification published in 2010 seems to present the most detailed pathohistological diversification, as it includes all types of gastric tumors, even those of very low frequency.Citation25 Histological and microscopic characteristics reveal the presence of signet ring cells in over 50% of the tumor in SRCC.Citation25–Citation28 There are no interactions between round-shaped cells. They contain a number of large vacuoles filled with mucins, which are secreted from the cells.Citation29 As a result, it could play a role in carcinogenesis. SRCC shows a specific process of oncogenesis, which differentiates it from other types of GC. The two main pathologic processes at the cellular level involve the loss of cell–cell adhesion molecules and accumulation of mucin in large vacuoles.Citation14 Some SRCCs have mutations in E-cadherin which is encoded by the CDH1 gene. Its role in carcinogenesis and epithelial–mesenchymal transition (EMT) has been widely studied in many types of cancer,Citation30,Citation31 but in SRCC E-cadherin is thought to be involved earlier in tumor initiation.Citation32 Other adherence molecules could be also involved in some cases, such as somatic mutations of b-catenin/APC genes or dysregulation of the Wnt/b-catenin pathway.Citation33 Moreover, expression of CDH1 and other adherence molecules could be downregulated upstream among various signaling pathways. However, the mechanisms and pathways underlying mucin secretion and accumulation in cells are not well recognized.
Considering the epidemiology of SRCC, it is more frequent in women than non-SRCC, occurring among younger patients of age ranging from 55 to 61 years, 7 years before the occurrence of non-SRCC.Citation11,Citation34 SRCC also differs in the clinical features from non-SRCC. It is more frequent in the middle stomach and is associated with more advanced stages (mostly displaying as stage 4, T3/T4, and N2 cancers). However, according to some reports, SRCC is more frequent in early gastric cancer (EGC) than in advanced stage, which relates to peritoneal carcinomatosis.Citation34,Citation35
It is reported that the biological behavior of SRCC differs from other cell types. However, the controversial clinical outcomes of the SRCC are uncertain and depend on whether they are diagnosed at an early or advanced stage. Most studies have described a worse prognosis for this type of GC compared to other subtypes, while others reported no significant differences.Citation26,Citation36,Citation37,Citation39–Citation41 Some studies reported favorable 5-year survival rates for SRCC compared to other cell types.Citation10,Citation42–Citation45 Moreover, in studies comparing SRCC with other cell types in EGC, it was observed that SRCC had better prognosis with lower lymph node metastasis, for which endoscopic resection is suggested as the treatment.Citation44 However, these results are not confirmed, and it is advisable to treat patients by gastrectomy with lymph node dissection.Citation36,Citation38,Citation46 Reports of the clinicopathological characteristics and the prognosis of SRCC of the stomach are conflicting and appear to depend on the stage of the cancer at the time of diagnosis.
Differential prognostic implications of SRCC
The prognosis of SRCC in all the available studies has been reported as equivalent or better than for other gastric adeno-carcinomas in the early stage. The largest published study by Ha et al analyzed retrospectively 1,520 patients who underwent a curative gastrectomy, with early SRCC vs early undifferentiated carcinoma (mucinous, poorly differentiated adenocarcinoma) and early differentiated carcinoma (well or moderately differentiated tubular adenocarcinoma, papillary adenocarcinoma). The analysis showed that patients with SRCC had a better survival rate than patients with other gastric adenocarcinomas. The lower rate of lymph node metastasis and better prognosis indicate the endoscopic mucosal resection (EMR) as the way of treatment for patients with early SRCC, limited to the mucosa, with size less than 2 cm and with no lymphatic involvement.Citation44
The later studies have presented similar prognosis of SRCC after gastrectomy. Kwon et al retrospectively studied 769 patients with gastric carcinoma, who underwent gastrectomy from 1999 to 2009. Within a selected group, 326 patients (42.4%) displayed EGC and 443 patients (57.6%) had advanced gastric cancer (AGC). Fifty-one patients (15.6 %) had SRCC in EGC; there were important differences in age, sex, macroscopic type, location, and size between SRCC and the differentiated histological type. Fifty-seven patients (12.9 %) displayed SRCC in AGC. Age, location, sex, macroscopic type, size, perineural invasion, N stage, and hepatic metastasis were visibly different between SRCC and the differentiated histological type. The overall survival rate was different between SRCC and other cell types (P<0.001). Among all the study cases, age and TNM stage were important factors for predicting survival.Citation34 Patients with SRCC displayed similar clinicopathological characteristics with undifferentiated histology. The survival of patients with SRCC reflected a better prognosis in patients with undifferentiated gastric carcinoma.
A similar retrospective review was undertaken by Kunisaki et al including1,450 patients, among whom 174 had SRCC (early, 120; advanced, 54). It was found that patients with early SRCC had a significantly better survival rate than those with non-SRCC, with no difference in the extent or number of lymph node metastases.Citation43 The study of Jiang et al identified 211 patients with SRCC from a population of 2,315 patients with GC who were cured by gastrectomy. There was significant difference in the survival rate between SRCC and non-SRCC patients in the early stage.Citation42 The same conclusion (P=0.0104) was made by Hyung et al in a study comprising patients with EGC who had undergone gastrectomy (263 patients with SRCC), and by Otsuji et al (P<0.05) in a study with a cohort of 1,498 patients (among 154 patients diagnosed with SRCC, 94 were in early stage).Citation47,Citation48
A study performed by Zhang et al among a group of 218 patients with SRCC and 1,221 patients with non-SRCC showed that the overall 5-year survival was 44.9% and 36.0% for patients with SRCC and non-SRCC (P=0.013), respectively. Multivariate analysis indicated that lymph node metastasis and curative resection were significant factors affecting the survival rate.Citation36
Only two studies have shown that the prognosis of patients with SRCC was similar to those with non-SRCC of the stomach.Citation45,Citation49 One was conducted by Kim et al who compared the overall 5-year survival of all the patients with SRCC carcinoma (60.2%) with patients with non-SRCC (48.9%) (P<0.01), among a total of 2,358 patients (8.7% with SRCC).Citation45 The second comparison made by Gronnier et al concluded that the 5-year overall survival benefit in SRCC patients (85% vs 76%, respectively; P=0.035) was not significantly different from non-SRCC patients. A better overall survival was observed in this study, which may be related to the younger age of SRCC patients.Citation49
Some studies have indicated endoscopic treatment, including EMR or endoscopic submucosal dissection (ESR), as an alternative way to gastrectomy. In the early cases of GC, the risk of lymph node metastasis is expected to be very low, perioperative outcomes are better, and long-term results are comparable.Citation14 Kang et al evaluated the histological type, invasion depth, lymphovascular invasion, and lymph node metastasis. They concluded that endoscopic resection may be an optional treatment for small mucosal gastric SRCCs, but should be performed only under strict indications.Citation50
In the next study performed by Gotoda et al, patients displayed well to moderately well-differentiated tumor, of size less than 3 cm and devoid of submucosal invasion, as well as patients with a well-differentiated, restricted, and nonulcerated submucosal lesion (T1sm1), of size less than 3 cm and showing no chance of lymph node metastasis.Citation51 The indications for endoscopic resection are the subject of additional research and various guidelines have been established to define them. They are based on two well-known prognostic factors for survival – limitation to the mucosa and invasion to lymph nodes. Therefore, it is highly essential to qualify every case of SRCC for appropriate surgery and consequently with extended lymphadenectomy, and also to add postoperative chemotherapy when indicated.
Conversely, the prognosis of SRCC is commonly defined as poor in AGC. Most studies showed significantly worse 5-year survival rate among patients with SRCC than with non-SRCC.Citation38,Citation39 However, other small studies did not indicate a significantly worse prognosis for SRCC.Citation42,Citation43,Citation45,Citation48,Citation52,Citation53 In a retrospective study, performed by Kim et al, 3,702 cases of GC, who underwent operation between 1981 and 1991, were investigated to compare the clinicopathological features of signet ring cell GC with other cell types. Among them, 450 patients (12.2%) displayed signet ring cell GC. SRCC in early stage was less invasive and showed decreased lymph node metastasis, whereas advanced stages of signet ring cell GCs are characterized as highly invasive with higher level of lymph node metastasis in comparison to other cell types. In AGCs, the prognosis of patients displaying the signet ring cell type was significantly worse than for the other types. This might be related to the characteristics of AGCs with signet ring cell type, which are associated with a large tumor size, extensive lymph node metastasis, and a deeper invasion, than other histological types. In conclusion, this group suggested that signet ring cell GC may display unique biological behavior.Citation39
Similar results were achieved by Kunisaki et al, Otsuji et al, and Chon et al.Citation43,Citation48,Citation54 The survival of stage-matched intestinal-type SRCC tumors in a cohort of Western patients was the subject of study by Bamboat et al. In a prospective review, a group of 569 patients was divided into three histological subgroups based on the Lauren’s classification. The risk of death from GC was the lowest for stage I SRCC and the highest for stage III SRCC.Citation10 Moreover, Kwon et al indicated factors for predicting the prognosis, which encompassed age and TNM stage.Citation34 Li et al stated that surgical curability, besides TNM stage, is an independent factor affecting survival. Therefore, curative surgical operation with extended lymph node dissection is recommended in advanced stage of SRCC.Citation38
The retrospective analysis undertaken by Jiang et al compared 211 patients with SRCC and 2,104 patients with non-SRCC, showing that in AGC, there was no significant difference in survival rate between these types of GCs and that the signet ring cell histology was not an independent predictive factor.Citation42
The largest study carried out by Taghavi et al, including more than 10,000 cases of SRCC and non-SRCC, showed that the signet ring cell histology was not a prognostic factor for the tumor stage in AGC but was associated with more aggressive tumors.Citation11 However, in this research there are some confounding factors, which were unknown, eg, type of resection and perioperative treatment. Despite being the largest analysis, according to Pernot et al, this review does not close the debate, because patients with SRCC at a more advanced stage did not have a worse prognosis.Citation14
In conclusion, the prognosis of advanced-stage SRCC is controversial. However, most of the reports present a worse prognosis, suggesting a more aggressive SRCC phenotype and lower R0 resection rate,Citation55 which may be explained by a poorer prognosis. Nevertheless, there are single reports which showed that the presence of the signet ring cell histology is not an independent predictor of prognosis.
Genomic alterations of gastric SRCC
To investigate the significance of the different gene alterations among patients with SRCCs of the stomach, some studies have been investigated to reveal the molecular characteristics of this subtype of GC ().
In a study conducted by Muta et al,Citation56 the significance of E-cadherin gene alterations was examined among twenty-two SRCCs of the stomach: 12 advanced cancers and ten intramucosal, using the PCR single-strand conformation polymorphism method, on exons 5–9 and the adjacent 30–40 bp intron sequences of the E-cadherin gene. The obtained results indicated that in two of the ten intramucosal cancers, mobility shifts were notable; also, two of the 12 advanced cancers displayed aberrations in the E-cadherin gene, mostly in the intramucosal lesions. This group showed that E-cadherin gene mutations are a mutational event in the development of SRCC of the stomach. Additionally, direct sequencing confirmed the distribution of mutations in the E-cadherin gene, including branch point sequence in the intron, which is responsible for RNA splicing. The group suggested that branch point mutations play a significant role in the functional modifications of E-cadherin in SRCC of the stomach.
In the study performed by Wei et al,Citation57 the speculation of the prognostic and targeted therapy value in gastric SRCC by exploring the mutation profile of ERBB3 was investigated. Samples from 92 patients with advanced gastric SRCC were collected for analysis, where ERBB3 mutation was screened using next-generation sequencing and ERBB2 expression was tested by immunohistochemistry. The study demonstrated that 15.2% of gastric SRCC patients displayed ERBB3 mutation, providing a potential subgroup of gastric SRCC patients for targeted treatment of ERBB pathway. However, no difference of overall survival was observed, mainly because of the relatively small sample size and low ERBB2-positive rate in SRCC patients.
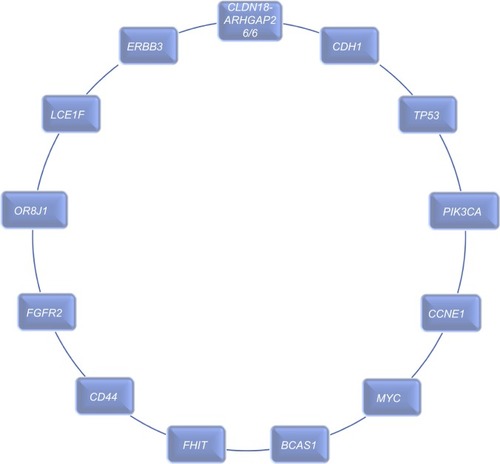
The first whole genome screening on high-content signet ring cell (HSRCC) tumors was investigated by Shu et al.Citation58 HSRCC belongs to diffuse type of GC, has relatively low mutation rate, increased occurrence of TP53 alterations,Citation59 foci deletion in FHIT, amplifications of multiple oncogenes like CCNE1, FGFR2, CD44, as well as enriched mutations in cell adhesion-related genes.Citation60 In this study, higher frequency of amplification in MYC and BCAS1 genes was described, and decreased mutation level in RHOA and ARID1A genes, which in fact support the outcome for genetic differences between HSRCC and other subtypes of diffuse GC. Importantly, increased level of GC-specific fusions, CLDN18–ARH-GAP26/6, has been detected in HSRCC.
Shu et al revealed a total of 949 genes, which had at least one somatic non-silent single nucleotide variant or small insertion–deletion mutation in coding region. Six significantly mutated genes were displayed: TP53 (25%), CDH1 (15.6%), PIK3CA (12.5%), ERBB2 (6.3%), LCE1F (6.3%), and OR8J1 (6.3%), but not the well-described SMGs altered genes in diffuse subtype of GC, such as SMAD4, ARID1A, and RHOA, which may highlight possible various genomic characteristics of SRCC from other diffuse types of GC. Despite low alteration rate in RHOA, various mutations were detected in its regulatory factors, like RhoGAPs (GTPase-activating protein, including ARHGAP1, ARH-GAP5, and ARHGAP26) or RhoGEFs (GDP/GTP-exchange factor, including ARHGEF2, ARHGEF5, ARHGEF33, and ARHGEF40). Additionally, protein–protein interaction (PPI) network analyses revealed 107 cell adhesion-related mutant genes, showing the significant role of cell adhesion pathway in SRCC tumorigenesis.
Treatment strategies considering the clinical features of SRCC
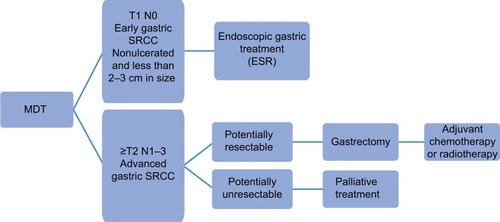
Due to large discrepancies in the reports published so far about the treatment of SRCC, further clinical trials in this area are needed, especially in Western countries. Currently, there is no higher-level evidence, specifically in SRCC patients, and therefore all recommendations are rather weak. Proposed management algorithm of patients with SRCC is shown in . Patients with very EGC (less than 3 cm in size, without ulceration, and with no metastasis to lymph nodes and to the peritoneum), might be redirected for EMR and ESR.Citation44 Additionally, early SRCC of the stomach generally has the same clinical characteristics as depressed-type of early gastric cancer; therefore, it is impossible to achieve R0 resection in most cases via EMR. It is also important not to follow only endoscopic resection in these cases, because at the period of presentation, patients are already at an advanced stage of the disease and need a total gastrectomy. When patients are also positive for CDH1 mutations, then a preventive radical total gastrectomy with extensive lymphadenectomy is suggested.
Figure 3 Proposed management algorithm for patients with SRCC.
Abbreviation: SRCC, signet ring cell carcinoma; MDT, multidisciplinary team; ESR, endoscopic submucosal dissection.
It is very difficult to establish the guidelines for the nonsurgical treatment of GC. Various options exist, which are adopted by different continents, such as perioperative chemotherapy, adjuvant chemotherapy, or adjuvant chemoradiotherapy. Unfortunately, there is still no final perception on the sensitivity of gastric SRCC toward chemotherapeutic drugs.
In the advanced stage of gastric SRCC, occurrence of signet ring cells is in itself a sign of weak prognosis. This is mainly provoked by metastasis to lymph nodes and to the peritoneum at the time of diagnosis.Citation61 Unfortunately, radical resection and aggressive chemotherapy are not sufficient to avoid the recurrence, which is observed in almost half of the patients. For SRCC of stomach with peritoneal metastasis, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) might be applied as a treatment strategy, which allow for a better response rate.
Among patients with SRCC of the stomach, chemoresistance is often encountered; therefore, targeted molecular therapy is a novel approach to prevent cancer metastasis. Targeting EMT might allow for selectively approaching tumor cells with gained motility. Mutations in Snail, Slug, and Twist, and activation of the phosphatidylinositol 3 kinase (PI3K)/AKT axis, Wnt/b-catenin signaling pathway, and transforming growth factor β have been reported in the pathogenesis of gastric SRCC. Therefore, they constitute important targetes for chemical inhibitors and small interfering RNA (siRNA) therapy, which can improve the prognosis of advanced SRCC patients.Citation62,Citation63
The details of the studies considering endoscopic treatment and gastrectomy, including study characteristics, treatment type, and treatment effects, are presented in . There is no consensus on the type of surgery to be performed, since total or subtotal gastrectomy can be performed in advanced gastric SRCC. However, radical gastrectomy with lymph node dissection is the gold standard for treatment of GC, and the current literature favors minimally invasive surgery.Citation64 Complete gastrectomy may be done among patients presenting with weakly differentiated histological subtype, such as when situated in the angularis section of the stomach.Citation65
Table 1 Characteristics of different studies, indicating the observed treatment effects in SRCC patients
Adjuvant chemotherapy
There are no specific studies which assessed the popular hypothesis that SRCC is less chemosensitive than non-SRCC. A retrospective study by Messager et al including 924 cases of resected SRCC was investigated to compare the survival of patients with SRCC treated with and without perioperative chemotherapy (based mainly on a fluorouracil-platinum doublet or triplet regimen). Perioperative chemotherapy provides no survival benefit in patients with gastric SRCC.Citation69 Other authors have found perioperative chemotherapy to be an independent predictor of poor survival (higher postoperative morbidity and negative oncologic outcomes), connected with toxicity of neoadjuvant treatment.Citation70 Conversely, another retrospective study performed by Heger et al showed that the response to neoadjuvant chemotherapy is rare in gastric SRCC, and that it is, however, associated with better outcome.Citation55
The choice of drugs for treatment of advanced gastric SRCC is empirical. 5-Fluorouracil or platinum, which are most often used in the perioperative setting, are the common options. Rougier et al showed that combination of 5-fluorouracil and cisplatinum is the most effective way in terms of tumor response in AGC with metastasis.Citation71 However, the aim of the Hultman’s et al’s investigation was to show ex vivo sensitivity of GC tumor cells among the analyzed patients, as well to compare it to popular cytotoxins and lately discovered drugs used in targeted therapies. The investigation revealed that SRCC and diffuse-type cases displayed significantly higher sensitivity to different drugs, like docetaxel, doxorubicin, and mitomycin C, in comparison to intestinal-type GC.Citation72 Another study indicated that docetaxel-based regimen is favorable to provide longer survival and lower risk of recurrence and death.Citation73 In addition, Kim et al indicated the potential benefit of taxane-based chemotherapy, but the benefit was limited to a reduced number of patients.Citation74 Another study revealed that the most effective treatment with an acceptable toxicity profile involves combining docetaxel, fluorouracil, and leucovorin/oxaliplatin. It may allow curative resection in initially unresectable patients.Citation75,Citation76
On the other hand, the retrospective analysis carried out by Cunningham et al showed no significant difference between survival and tumor location, time, period of treatment, or administration of adjuvant therapy, among patients who underwent surgery.Citation77 In a retrospective study, Lu et al analyzed a cohort of 2,199 consecutive patients with GC. First-line chemotherapy was not found to be associated with better survival. It also seemed that the three-drug regimen had statistically non-significant detrimental effects. In addition, the results had not been affected by the surgery status of patients, because the stratified analyses showed that there was no much difference in the results between the patients receiving surgery and those who did not undergo operation.Citation78
According to Lemoine et al’s study, patients with advanced gastric SRCC seemed to benefit less from chemotherapy. Objective response to chemotherapy rate was significantly lower in SRCC patients (5.3% vs 28.1%, P=0.0004). Progression-free survival was not significantly different between SRCC and non-SRCC patients (median =3.8 vs 4.9 months, P=0.07). Overall survival was significantly shorter in SRCC patients (median =5.6 vs 9.4 months, P<0.008). However, in multivariate analysis, SRCC was not an independent prognostic factor for overall survival.Citation79
Another way of administering chemotherapy is by HIPEC. In gastric SRCC, CRS and HIPEC should be restricted to highly selective patients. Königsrainer et al tried to evaluate the treatment protocol for SRCC. There are very few scientific reports about the management of SRCC with peritoneal metastases. That study compared the systemic chemotherapy consisting of 5-fluorouracil, folic acid, docetaxel, and oxaliplatin with CRS and HIPEC, applying cisplatin. Following complete cytoreduction and HIPEC, the progression-free survival was 6.2 months. However, complete CRS could only be achieved in 72% of patients. In summary, CRS and HIPEC cannot be recommended for patients with SRCC and peritoneal metastases in general.Citation80
Conclusion
Signet ring cells constitute an intermediate stage of squamous and adenocarcinoma cell or a glandular or mucin-secreting component in a squamous cell carcinoma. The prognosis of SRCC in all mentioned studies has been described as equivalent as or better than for other gastric adenocarcinomas in the early stage. Conversely, the prognosis of SRCC in AGC is rather poor, and most studies showed significantly worse 5-year survival rate among patients with SRCC than with non-SRCC.
SRCC of the stomach is considered to be less chemosensitive than non-SRCC type. The reason may be covered by a specific sensitivity profile, displaying greater sensitivity to taxane-based chemotherapy. The old combination of chemotherapy consists of epirubicin, cisplatin, and fluorouracil, which may not be as effective in SRCC. Additionally, the benefit of chemotherapy is also controversial in a perioperative setting.
Deep understanding of the molecular changes associated with SRCC of the stomach is needed to guide surgical and medical therapy. The first whole genome screening on HSRCC was investigated by Shu et al.Citation1 This study provides a broad overview of the clinical and genomic features of SRCC, informing the importance of frequent alterations in chemotherapy response among SRCC patients.
SRCC is a rare form of adenocarcinoma that predominantly affects the stomach, and a lot of investigations need to be carried out to improve the prognosis and treatment aspects of this disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- ZhangXYZhangPYGastric cancer: somatic Genetics as a guide to therapyJ Med Genet201754530531227609016
- KimBSOhSTYookJHKimBSSignet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancerSurgery201415561030103524792508
- GigekCOCalcagnoDQRasmussenLTGenetic variants in gastric cancer: risks and clinical implicationsExp Mol Pathol2017103110111128736214
- TakatsuYHikiNNunobeSClinicopathological features of gastric cancer in young patientsGastric Cancer201619247247825752270
- KunisakiCAkiyamaHNomuraMClinicopathological features of gastric carcinoma in younger and middle-aged patients: a comparative studyJ Gastrointest Surg20061071023103216843873
- LlanosOButteJMCrovariFDuarteIGuzmanSSurvival of young patients after gastrectomy for gastric cancerWorld J Surg2006301172016369709
- KongXWangJLChenHMFangJYComparison of the clinico-pathological characteristics of young and elderly patients with gastric carcinoma: a meta-analysisJ Surg Oncol2012106334635222331676
- MilneANSitarzRCarvalhoRCarneiroFOfferhausGJEarly onset gastric cancer: on the road to unraveling gastric carcinogenesisCurr Mol Med200771152817311530
- BamboatZMTangLHVinuelaEStage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinomaAnn Surg Oncol20142151678168524394986
- TaghaviSJayarajanSNDaveyAWillisAIPrognostic significance of signet ring gastric cancerJ Clin Oncol201230283493349822927530
- HensonDEDittusCYounesMNguyenHAlbores-SaavedraJDifferential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell typeArch Pathol Lab Med2004128776577015214826
- PengJXiaoPLiaoBYeJHeYAnalysis of clinicopathological features of 1879 cases of gastric cancer in Southern China: a single center experienceZhonghua Wai Ke Za Zhi2014523168170 Chinese24785452
- PernotSVoronTPerkinsGLagorce-PagesCBergerATaiebJSignet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challengeWorld J Gastroenterol20152140114281143826523107
- MachlowskaJMaciejewskiRSitarzRThe pattern of signatures in gastric cancer prognosisInt J Mol Sci2018196E165829867026
- SkieruchaMMilneANOfferhausGJAPolkowskiWPMaciejewskiRSitarzRMolecular alterations in gastric cancer with special reference to the early-onset subtypeWorld J Gastroenterol20162282460247426937134
- SitarzRSkieruchaMMielkoJOfferhausGJAMaciejewskiRPolkowskiWPGastric cancer: epidemiology, prevention, classification, and treatmentCanc Manag Res201810239248
- BerlthFBollschweilerEDrebberUHoelscherAHMoenigSPathohistological classification systems in gastric cancer: diagnostic relevance and prognostic valueWorld J Gastroenterol201420195679568424914328
- LaurenPThe two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classificationActa Pathol Microbiol Scand196564314914320675
- VogelaarIPvan der PostRSvan KriekenJHJUnraveling genetic predisposition to familial or early onset gastric cancer using germline whole-exome sequencingEur J Hum Genet201725111246125228875981
- BosmanFTCarneiroFHrubanRHTheise Nd: WHO Classification of Tumours of the Digestive System4th edLyon, FranceIARC2010
- LeocataPVenturaLGiuntaMGastric carcinoma: a histopatho-logical study of 705 casesAnn Ital Chir19986933313379835105
- LauwersGCarneiroFGrahamDCuradoMFranceschiSClassification of tumours of the digestive system4th edLyonIARC Press20104858
- Fenoglio-PreserCMunozNCarneiorFGastric carcinomaHamiltonSRAaltonenLAWorld Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive SystemLyonIARC Press20003839
- FléjouJ-FClassification OMS 2010 des tumeurs digestives: la quatrième edition [WHO Classification of digestive tumors: the fourth edition]Ann Pathol2011315 SupplS27S31 French22054452
- HuBEl HajjNSittlerSLammertNBarnesRMeloni-EhrigAGastric cancer: classification, histology and application of molecular pathologyJ Gastrointest Oncol20123325126122943016
- SarbiaMBeckerKFHöflerHPathology of upper gastrointestinal malignanciesSemin Oncol200431446547515297939
- WernerMBeckerKFKellerGHöflerHGastric adenocarcinoma: pathomorphology and molecular pathologyJ Cancer Res Clin Oncol2001127420721611315254
- FukuiYMechanisms behind signet ring cell carcinoma formationBiochem Biophys Res Commun201445041231123325019985
- VleminckxKVakaetLMareelMFiersWvan RoyFGenetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor roleCell19916611071192070412
- CavallaroUChristoforiGCell adhesion and signalling by cadherins and Ig-CAMs in cancerNat Rev Cancer20044211813214964308
- HumarBBlairVCharltonAMoreHMartinIGuilfordPE-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and manCanc Res200969520502056
- ChiurilloMARole of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature reviewWorld J Exp Med2015528410225992323
- KwonKJShimKNSongEMClinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomachGastric Canc20141714353
- HonoréCGoéréDMessagerMRisk factors of peritoneal recurrence in esogastric signet ring cell adenocarcinoma: results of a multicentre retrospective studyEur J Surg Oncol201339323524123313257
- ZhangMZhuGZhangHGaoHXueYClinicopathologic features of gastric carcinoma with signet ring cell histologyJ Gastrointest Surg201014460160620033340
- PiessenGMessagerMLeteurtreEJean-PierreTMarietteCSignet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentationAnn Surg2009250687888719855261
- LiCKimSLaiJFAdvanced gastric carcinoma with signet ring cell histologyOncology2007721–2646818004078
- KimJPKimSCYangHKPrognostic significance of signet ring cell carcinoma of the stomachSurg Oncol1994342212277834113
- RibeiroMMSarmentoJASobrinho SimõesMABastosJPrognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinomaCancer19814747807847226025
- HassHGSmithUJägerCSignet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): single-center experience of 160 casesOnkologie2011341268268622156447
- JiangCGWangZNSunZLiuFNYuMXuHMClinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional studyJ Surg Oncol2011103770070321308685
- KunisakiCShimadaHNomuraMMatsudaGOtsukaYAkiyamaHTherapeutic strategy for signet ring cell carcinoma of the stomachBr J Surg200491101319132415376179
- HaTKAnJYYounHKNohJHSohnTSKimSIndication for endoscopic mucosal resection in early signet ring cell gastric cancerAnn Surg Oncol200815250851318071825
- KimDYParkYKJooJKClinicopathological characteristics of signet ring cell carcinoma of the stomachANZ J Surg200474121060106415574148
- LeeJHChoiIJKookMCNamBHKimYWRyuKWRisk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histologyBr J Surg201097573273620235088
- HyungWJNohSHLeeJHEarly gastric carcinoma with signet ring cell histologyCancer2002941788311815962
- OtsujiEYamaguchiTSawaiKTakahashiTCharacterization of signet ring cell carcinoma of the stomachJ Surg Oncol19986742162209579367
- GronnierCMessagerMRobbWBIs the negative prognostic impact of signet ring cell histology maintained in early gastric adeno-carcinoma?Surgery201315451093109924075273
- KangSHKimJSMoonHSSignet ring cell carcinoma of early gastric cancer, is endoscopic treatment really risky?Medicine20179633e753228816940
- GotodaTYanagisawaASasakoMIncidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centersGastric Canc200034219225
- YokotaTKuniiYTeshimaSSignet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological typesTohoku J Exp Med1998186212113010223615
- MaeharaYSakaguchiYMoriguchiSSignet ring cell carcinoma of the stomachCancer1992697164516501312889
- ChonHJHyungWJKimCDifferential prognostic implications of gastric signet ring cell carcinomaAnn Surg2017265594695327232252
- HegerUBlankSWiechaCIs preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach?Ann Surg Oncol20142151739174824419755
- MutaHNoguchiMKanaiYOchiaiANawataHHirohashiSE-cadherin gene mutations in signet ring cell carcinoma of the stomachJpn J Canc Res1996878843848
- WeiJXuBJinSYuLLiuBErbB pathway alterations in advanced gastric signet-ring cell carcinoma (SRCC) and the implications for targeted therapyJ Clin Oncol20173515_supple15586
- ShuYZhangWHouQPrognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancerNat Commun201891244729961079
- Cancer Genome Atlas Research NetworkComprehensive molecular characterization of gastric adenocarcinomaNature2014513751720220925079317
- WongSSKimK-MTingJCGenomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencingNat Commun201451547725407104
- RoukosDHCurrent status and future perspectives in gastric cancer managementCancer Treat Rev200026424325510913380
- ZerlinMJuliusMAKitajewskiJWnt/Frizzled signaling in angiogenesisAngiogenesis2008111636918253847
- ShintoOYashiroMKawajiriHInhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cellsBr J Cancer2010102584485120145621
- SantoroREttorreGMSantoroESubtotal gastrectomy for gastric cancerWorld J Gastroenterol20142038136671368025320505
- MorgagniPGarceaDMarrelliDResection line involvement after gastric cancer surgery: clinical outcome in nonsurgically retreated patientsWorld J Surg200832122661266718825453
- PhalanusitthephaCGrimesKLIkedaHEndoscopic features of early-stage signet-ring-cell carcinoma of the stomachWorld J Gastro-intest Endosc201577741746
- KimMNKimHKShimCNTumour size is related to the curability of signet ring cell early gastric cancer with endoscopic sub-mucosal dissection: a retrospective single centre studyDig Liver Dis2014461089890224973115
- BozzettiFMarubiniEBonfantiGMiceliRPianoCGennariLSubtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian Trial. Italian Gastrointestinal Tumor Study GroupAnn Surg1999230217017810450730
- MessagerMLefevreJHPichot-DelahayeVThe impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative studyAnn Surg2011254568469322005144
- RobbWBMessagerMGronnierCHigh-grade toxicity to neoadjuvant treatment for upper gastrointestinal carcinomas: what is the impact on perioperative and oncologic outcomes?Ann Surg Oncol201522113632363925676845
- RougierPDucreuxMMahjoubiMEfficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysisEur J Cancer199430A9126312697999410
- HultmanBMahtemeHSundbomMLjungmanMLarssonRNygrenPBenchmarking of gastric cancer sensitivity to anti-cancer drugs ex vivo as a basis for drug selection in systemic and intraperitoneal therapyJ Exp Clin Canc Res201433110
- ChenLShiYYuanJEvaluation of docetaxel- and oxaliplatin-based adjuvant chemotherapy in postgastrectomy gastric cancer patients reveals obvious survival benefits in docetaxel-treated mixed signet ring cell carcinoma patientsMed Oncol201431915925119501
- KimSFiteniFPaget-BaillySThe impact of taxane-based preoperative chemotherapy in gastroesophageal signet ring cell adeno-carcinomasJ Hematol Oncol201585225976888
- PernotSMitryESamalinEBiweekly docetaxel, fluorouracil, leucovorin, oxaliplatin (TEF) as first-line treatment for advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: safety and efficacy in a multicenter cohortGastric Canc2014172341347
- PernotSDubreuilOTougeronDDocetaxel, 5FU, oxaliplatin (TEFOX) in 1st line treatment of signet ring cell and/or poorly differentiated gastric adenocarcinoma: a retrospective study of AGEOJ Clin Oncol201533 Suppl AbstrE15048
- CunninghamSCKamangarFKimMPSurvival after gastric adenocarcinoma resection: eighteen-year experience at a single institutionJ Gastrointest Surg2005971872515862270
- LuMYangZFengQThe characteristics and prognostic value of signet ring cell histology in gastric cancer: a retrospective cohort study of 2199 consecutive patientsMedicine20169527e405227399088
- LemoineNAdenisABoucheOSignet ring cells and efficacy of first-line chemotherapy in advanced gastric or Oesogastric junction adenocarcinomaAnticancer Res201636105543555027798928
- KönigsrainerIHorvathPStrullerFKönigsrainerABeckertSBeckertinitialSInitial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastasesJ Gastric Cancer201414211712225061539