Abstract
Radiotherapy (RT) is a major treatment for malignant tumors. The latest data show that >70% of patients with malignant tumors need RT at different periods. Skin changes can be experienced by up to 95% of patients who underwent RT. Inflammation and oxidative stress (OS) have been shown to be generally associated with radiation-induced skin reactions (RISRs). Inflammatory response and OS interact and promote each other during RISRs. Severe skin reactions often have a great impact on the progress of RT. The treatment of RISRs is particularly critical because advanced RT technology can also lead to skin reactions. RISRs are classified into acute and chronic reactions. The treatment methods for acute RISRs include steroid treatment, creams, ointments, and hydrocolloid dressings, depending on the reaction grading. Chronic RISRs includes chronic ulcerations, telangiectasias, and fibrosis of the skin, and advanced treatments such as mesenchymal stem cells, hyperbaric oxygen therapy, superoxide dismutase, and low-intensity laser therapy can be considered. Here, we review and summarize the important mechanisms that cause RISRs as well as the standard and advanced treatments for RISRs.
Introduction
Radiotherapy (RT) is the primary treatment for many cancers, and it can damage the healthy tissues in both short and long term. The latest data show that >70% of patients with malignant tumors need RT. Radiation-induced skin reaction (RISR) is one of the main adverse effects. Acute RISRs may have severe sequelae, affecting the quality of life and the progress of cancer treatment. The main factors affecting patients’ quality of life include pain and discomfort caused by RISRs.Citation1 Therefore, it is crucial to alleviate or even eradicate the radiation-induced adverse events.Citation2
RISRs are often assessed as acute and chronic and classified on a scale of 1–4 on the basis of the Common Terminology Criteria for Adverse Events v3.0. Grade 1 changes include dry desquamation with generalized erythema. Grade 2 changes include brisk erythema or patchy moist desquamation. When the cumulative radiation dose reaches 40 Gy or higher, moist desquamation occurs at the folds of the skin.Citation3 Grade 3 changes include extensive moist desquamation outside of the skin folds. Grade 4 changes include ulcers, bleeding, and skin necrosis.Citation4 The chronic radiation-induced reactions include chronic ulcerations and wounds, fibrosis, telangiectasias, secondary skin cancers, and radiation-induced keratoses.Citation5 Chronic RISRs are true late-stage reactions that take months to years to develop after exposure to ionizing radiation (IR).Citation4 These chronic effects are more dependent on the type, area, volume, fraction size, and schedule of radiation rather than total radiation dose.Citation6
Many factors increasing the risk of acute RISRs have been identified. The severity of the reactions is related to both internal and external factors. External factors include the total radiation dose, fractioned delivery schedules, volume of irradiated tissue, and the internal radiosensitivity of the involved tissue.Citation7 Genetics influences the development of acute RISRs, particularly conditions resulting from mutations in DNA repair mechanisms. Ataxia telangiectasia is closely related to the mutation of ATM gene.Citation8 Patients with the disease are more likely to develop serious complications after RT because they cannot repair their DNA. Many clinical trials have attempted to study the relationship between radiosensitivity and radiation-associated complications.Citation9–Citation11
The mechanisms associated with RISRs include inflammatory response and oxidative stress (OS). Inflammatory response and OS interact and promote each other.Citation12,Citation13 After radiation-induced cell damage, cells die in various forms, especially mitotic death, leading to inflammation and chronic OS. In chronic phases, inflammation and OS can lead to changes in various cytokines, cell cycle changes, and DNA damage, sustaining the cascade leading to late reactions. Treatment is based on the severity of acute RISRs. Treatments are incorporated into wound care management to maintain a moist environment to speed up recovery. Permanence, gradualness, and irreversibility are a unique subset of the adverse effects of RT in chronic RISRs; fibrosis of the skin and soft tissue may progress from months to years after the treatment.Citation14 Chronic RISRs have a significant impact on the quality of life because of the irreversibility of the damage.Citation15
In this review, we summarize the important mechanisms that cause RISRs as well as some standard treatments and advanced innovative treatments for acute and chronic RISRs.
Mechanism of RISRs
Inflammatory response
Inflammatory response has been shown to be generally associated with RISRs.Citation12 In the initial period of RT, there is an immediate generation of an inflammatory response. The early inflammatory response to radiation is mainly caused by pro-inflammatory cytokines (IL-1, IL-3, IL-5, IL-6, and tumor necrosis factor [TNF]-a), chemokines (eotaxin and IL-8), receptor tyrosine kinase, and adhesions molecules (intercellular adhesion molecule 1 [ICAM-1], E-selectin, and vascular cell adhesion protein). These factors can create a local inflammatory response of eosinophils and neutrophils, leading to self-perpetuating tissue damage and loss of protective barriersCitation16 (). Janko et al ascertained that IL-1 had an important role in the development of RISRs. They found that mice that lack either IL-1 or the IL-1 receptor developed less inflammation and less severe pathological changes in their skin, especially at later time points. This study provided a potential therapeutic targeting of IL-1 for the remission of RISRs. The production of IL-1 in skin is mainly regulated by monocytes, macrophages, fibroblasts, keratinocytes, and many other immune mediators.Citation17 In the acute phase, all resident cells, including keratinocytes, fibroblasts, and endothelial cells, respond to IR by the activation of the early response genes and proteins, which include a lot of growth factors, chemokines, and cytokines. These various growth factors then attract inflammatory cells that participate in the second phase of RISRs.
Figure 1 Mechanisms associated with RISRs: inflammation and oxidative stress.
Abbreviations: COX-2, cyclooxygenase 2; ICAM-1, intercellular adhesion molecule 1; iNOS, inducible nitric oxide synthase; NF-kB, nuclear factor kB; NLRP3, nucleotide-binding domain, leucine-rich repeat-containing family, pyrin domain-containing 3; NO, nitric oxide; RISR, radiation-induced skin reaction; TNF, tumor necrosis factor; VCAM, vascular cell adhesion protein.
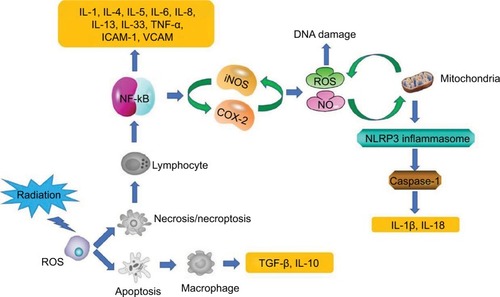
Nucleotide-binding domain, leucine-rich repeat-containing family, pyrin domain-containing 3 (NLRP3) inflamma-some upregulation at the expression or activation level has been reported to play an important role in radiation damage including RISRs in recent years.Citation18–Citation20 NLRP3 inflammasome is a multi-protein complex that activates caspase-1, which leads to the maturation of the pro-inflammatory cytokines IL-1β and IL-18.Citation21The study by Allam et alCitation22 showed that radiation-induced mitochondrial apoptosis can lead to the release of oxidative mitochondrial DNA into the cytoplasm and bound it to NLRP3 inflammasome in the cytosol, which causes activation of the NLRP3 inflammasome ().
The development of radiation-induced fibrosis is also mediated by inflammation, which begins immediately after RT and continues for months to years.Citation14 TNF-a, IL-6, and IL-1 are involved in the inflammatory response, while TGF-β and platelet-derived growth factor regulate fibroblast activity and promote the production of extracellular matrix proteins.Citation23 Fibroblasts are the key cells in the development of late radiation-induced fibrotic changes.Citation24 Permanently atypical fibroblasts can cause skin atrophy, contraction, and fibrosis.Citation25 The TGF-β is a regulatory protein that controls wound healing, proliferation, and differentiation of multiple cell types and synthesis of extracellular matrix proteins in the normal tissue inflammatory response.Citation26 Its main function on connective tissues in vivo is to promote growth. The proliferation of endothelial cell is also stimulated, but the growth of epithelial cell is inhibited. Mice lacking a downstream mediator of TGF-β, Smad3, demonstrated reduced tissue damage and fibrosis after irradiation and accelerated healing.Citation27 Although TGF-β is known as a central player in the fibrotic process, other cytokines and growth factors such as insulin-like growth factor 1 and connective tissue growth factors (CTGFs) are involved in this process in the skinCitation24 ().
Inhibition of pro-inflammatory cytokines such as MCP-1 and cyclooxygenase 2 (COX-2) can improve skin tolerance to RT.Citation28 Some studies have also reported COX-2 to be an important gene mediating the subsequent inflammation.Citation29 The study by Cheki et alCitation30 showed that inhibition of COX-2 by celecoxib can reduce inflammation of the dermis, MCP-1 mRNA expression, and RISRs.
Oxidative stress
Chronic OS participates in the development of several diseases, including late RISRs. The redox system plays a key role in the early and late effects of RISRs.Citation31,Citation32
Because 80% of the tissues and cells are made of water, much of the radiation damage from exposure to low linear energy transfer radiation (X rays, γ rays, and fast electrons) is due to radiolysis of water leading to the generation of ROS and reactive nitrogen species (RNS).Citation33 During normal cell function, ROS and RNS are important mediators for some cellular processes such as immune response, cell signaling, microbial defenses, differentiation, cell adhesion, and apop-tosis.Citation34 The ROS and RNS are the major sources of normal tissue damage after exposure to IR. The production of ROS undergoes molecular changes, damaging DNA, lipids, and proteins and activating early-response transcription factors and signal transduction pathways.Citation35 As a result, it causes damage to the skin tissue ().
Free radicals generated by IR upregulate the COXs, NOS, LOXs, and NADPH oxidase. Their effected changes in mitochondrial functions are markedly noticeable. Each of these enzymes is expressed differently in multiple cells, tissues, and organs in a specific way. NADPH oxidase is a membrane-bound oxidoreductase that transfers an electron from NADPH to the oxygen molecule. Several subtypes of these enzymes have been found in the cellsCitation31; NOX1–5, DUOX1, and DUOX2 are the most important subtypes of NADPH oxidase enzymes involved in respiratory burst after exposure to radiation.Citation36 These enzymes are highly stable and continuously produce ROS following exposure. Each of these enzymes is activated in a specific type of cellular tissue. In addition, enzymes can be stimulated by a large number of stressors and stimuli, as well as different types of cytokines and growth factors. A large number of inflammatory cytokines, chemokines, and hormones, such as IL-1, TNF-a, TGF-β, interferon (IFN)-γ, and angiotensin II are a part of the NOX system activation.Citation37
Nitric oxide (NO) has many targets within cells. Under stress conditions, such as inflammation, inducible nitric oxide synthase (iNOS) is the main source of NO and plays a key role in the process of OS and carcinogenesis. NO is produced by macrophages via iNOS enzyme in response to inflammatory stimulation. It is highly reactive and interacts with the mitochondria-derived superoxide to form more reactive peroxynitrite. However, high level of NO competes with O2 in the electron transport chain, which may inhibit respiration in the mitochondria. This effect may lead to an increase in superoxide generation, which amplifies the OS.Citation38 The study by Ohta et alCitation39 suggested that increased level of NO has a direct relation with radiation dose. In addition, they observed elevated levels of NO in the early hours after exposure ().
Inflammation plays a key role in redox activation. Direct exposure of normal cells to IR or ROS leads to nuclear and mitochondrial DNA damages, which may result in cell death through apoptosis, mitotic catastrophe, and necrosis.Citation40 Cell apoptosis can trigger the release of anti-inflammatory cytokines such as IL-10 and TGF-β, while necrosis may cause the release of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, IL-13, IL-33, and TNF-a and other inflammatory mediators.Citation41 Chronic inflammation can persist long after exposure without the immune system suppressing these reactions. This is associated with chronic oxidative damage, which causes genomic instability and damage to the normal function of the skin tissueCitation42 ().
Understanding the mechanisms of chronic oxidative damage and injury of affected cells, tissues, and organs after exposure to IR may contribute to the development of treatment and management strategies of the complications associated with RT.Citation31
Genomic etiology
DNA damage and response to IR are critical for understanding potential complications in normal tissues. With the first dose of RT, there is immediate tissue damage, production of short-lived free radicals, and irreversible breaks in cellular DNA.Citation43 IR induces single-strand and double-strand DNA breaks (DSBs). Most single-strand breaks can be repaired, while DSBs result in cell death. Exposure to high doses of IR can produce massive DNA damage in cells. Accumulation of unrepaired DNA damage can lead to the induction of deletion, mutations, chromosome aberrations, or cell death.Citation44
DNA repair system plays a key role in normal tissue tolerance to RT. There is a predictable increase in regulation of DNA repair genes after radiation exposure. Inflammation and OS may inhibit DNA damage repair. There is evidence that chronic inflammation lead to suppression of DNA repair response and mutations in tumor suppressor genes and oncogenes.Citation45 NO produced by immune cells including macrophages and neutrophils can inhibit DNA repair and alter the expression of certain genes.Citation46
Understanding radiation-induced DNA damage and DNA repair system may contribute to the development of adjuncts to RT.
Therapy of RISRs
Topical corticosteroids
Acute RISRs is an inflammatory reaction and is usually treated with topical corticosteroids. Topical corticosteroids have therapeutic effects because of their anti-inflammation, immunosuppression, anti-proliferation, and vasoconstriction characteristics.Citation47 The anti-inflammatory effects of corticosteroids are often achieved through vasoconstriction, reduced capillary permeability, and inhibition of leukocyte proliferation and migration, but the mechanism is not completely understood.Citation48 The inflammatory process in acute radiation is partially controlled by pro-inflammatory cytokines; IL-6 is considered as one of the key mediators of radiation-induced inflammation.Citation49,Citation50 Beetz et alCitation51 reported an upregulation of IL-6 expression in irradiated human epithelial cell lines, which could be inhibited by corticosteroids. Topical corticosteroids block inflammation by targeting cytokines and proteins, such as reducing the production of IL-1, IL-2, IFN-γ, TNF-a, and granulocyte-macrophage colony-stimulating factor.Citation47
Another substance possibly targeted by topical corticosteroids in RISRs is histamine. Moriyasu et al demonstrated that histamine was involved in the development of radiation-induced erythema and edema. They observed that the irradiation of skin of mast cell-deficient mice never led to erythema and edema and that blockage of H1 receptors with antihistamine inhibited erythema and edema induced by gamma irradiation.Citation52 The long-term application of topical steroids (>6 weeks) has been shown to decrease histamine and deplete mast cells on skin.Citation53
Mometasone furoate (MMF) is a highly potent corticosteroid. Irradiated skin samples treated with topical mometasone showed a significant decrease in the pro-inflammatory mediators.Citation28 A randomized trial assessed the effect of MMF on acute skin-related toxicity in patients accepting breast or chest wall RT. The results suggested that the patients receiving daily MMF treatment during RT might experience reduced acute dermal toxicity compared to the patients receiving placebo.Citation54 Another randomized trial demonstrated that the preventive and sustained use of 0.1% methylprednisolone was better for relieving skin damage caused by radiation therapy than the topical dexpanthenol emollients.Citation55 Another study showed that the preventive and sustained use of 0.1% betamethasone during breast cancer chest wall RT delayed the onset of acute radiation dermatitis.Citation56 A randomized study with betamethasone and two moisturizing creams showed that the control of acute radiation dermatitis with betamethasone cream for breast cancer adjuvant RT was more effective than the moisturizers.Citation57
In the future, topical corticosteroids may be the first-line treatment for the patients undergoing RT to prevent moist desquamation.
Creams and ointments
Trolamine-containing topical emulsions have a history of use for radiation dermatitis, with information on their mechanistic role already reviewed.Citation4 They can remove necrotic tissue, accelerate fibroblast proliferation, reduce vascular changes in vitro, restore CD34 expression, accelerate epithelial cell proliferation, and reduce IL-1 secretion.Citation4 Triethanolamine has been used in clinical practice for >30 years to manage a variety of diseases that affect skin integrity, such as radiation dermatitis and skin wounds.Citation58 No major adverse reactions have been reported with topical emulsions containing triethanolamine. Local tolerability is good, and the potential risk of skin irritation is low.Citation59
Biafine is an oil-in-water topical emulsion containing tri-ethanolamine, approved by the Food and Drug Administration for use in a variety of wound healing environments, including minor abrasions to full-thickness wounds, superficial partial-thickness empyrosis, and radiation dermatitis.Citation60 Application of biafine in a human epidermal wound model showed that wound healing is promoted by enhancing the macrophage recruitment and increasing the ratio of IL-1 to IL-6, measured from the wound exudate collected 24 hours after wounding. IL-1 stimulates fibroblast growth and affects extracellular matrix composition through collagen generation and collagenase activation, while IL-6 promotes epidermal growth and inhibits fibroblast proliferation. Biafine is believed to have a positive effect on the formation of granulation tissue in fibroblasts by inducing the release of IL-1 and reducing the secretion of IL-6.Citation61
Application of biafine on full-thickness resection and burn wound models enhanced re-epithelization and accelerated closure. Histologically, biafine-treated wounds showed greater epidermal maturation, granulation tissue formation, and collagen quantity and quality. Treatment with biafine was associated with larger macrophage infiltration and lower neutrophil presence in the early stages of healing, which is crucial for acute and chronic skin wound healing.Citation62 A study evaluating the effectiveness of biafine as a preventive agent for acute RISRs in women receiving RT of chest wall showed that biafine significantly reduced skin toxicity, although most of the patients developed grade 2 radiation dermatitis; and no delay or interruption in treatment was observed due to dermal toxicity.Citation63
Collectively, these studies and the clinical observations have supported the therapeutic benefit of trolamine-containing topical emulsions for cutaneous ulcers and radiation dermatitis.
Hydrogel and hydrocolloid dressings
Hydrogel or hydrocolloid dressings are generally recommended for the treatment of mild acute skin wounds, from the surface to partial-thickness burns or chronic wounds, such as diabetic foot lesions or pressure sores. They increase the healing rate and reduce infection and pain.Citation64 Hydrogel dressings do not adhere to wounds, and they are easy to clean and reuse. Hydrocolloid dressings are moisture retentive and self-adhesive, and they can be placed for a few days to simplify wound care.Citation65 When the hydrocolloid is placed on the wound, the area in direct contact absorbs the secretion and gradually forms a soft gel to keep the wound surface moist. This moisture promotes cell migration and assists in wound debridement through autolysis. The dressings can also prevent moisture from evaporating, allowing rehydration of the escharotic, which gradually liquefies and separates. Many studies have shown the benefits of the moist environment.Citation66 Moreover, hydrocolloids are impermeable to oxygen and thus create an area of low oxygen tension on the wound surface, which can stimulate angiogenesis, thereby accelerating the growth of granulation tissue.
A few case reports have documented the use of hydro-colloid dressings on refractory wounds, including perineal burns after RT. They reported that hydrocolloid dressings have beneficial effects on healing, pain relief, comfort, and ease of use.Citation67 Margolin et al conducted a study on 20 patients with hydrocolloidal dressing. The aim of the study was to determine whether moist occlusion healing improved the healing time, safety, and comfort for radiation-induced wounds. There was no obvious infection of the wound, and the average healing time is 12 days. It indicated that hydrocolloid dressing is effective in the treatment of radiation-induced skin wetting.Citation65
Moist desquamation is the main cause of patient suffering, causing pain or discomfort and increasing the risk of infection. Gentian violet (GV) has been effective in the treatment of moist desquamation caused by RT.Citation68 A trial compared GV and hydrogel dressings in treating moist desquamation caused by RT. The result showed that hydrogel dressings cured the moist desquamation induced by RT and are more tolerant than GV.Citation69 In another trial, 60 patients receiving RT for head and neck cancer were assigned to hydrogel dressings or routine clinical practice for skin reactions. The result revealed that hydrogel dressings have a higher healing rate and less frequent occurrence of severe skin reactions than routine clinical practice.Citation70
Therefore, hydrogel dressings appear to be promising in the treatment of acute radiation-induced skin wounds.
Mesenchymal stem cells (MSCs)
MSCs are important for regenerative medicine because of their strong ability of cytokine secretion, immunoregulation, and multipotential differentiation. The multipotential lineage of MSCs is characterized by the capacity for extracorporeal expansion and the ability to differentiate into bone, cartilage, and adipose tissues.Citation71 MSCs from bone marrow have the highest proliferative capacity and maintain their pluripotency even after 50 passages.Citation72 MSCs have demonstrated accelerated healing of excisional wounds and thermal burns.Citation73–Citation76 Studies have shown that MSCs have protective effects on radiation-induced lung injury and vascular injury.Citation72,Citation77 More importantly, a lot of clinical trials have shown that intravenous injection of allogeneic human bone marrow MSCs (BMMSCs) is safe for patients.Citation78
The role of MSCs in repairing different forms of RISRs has been extensively studied in animal models. Several groups have reported that locally injected MSCs are effective in healing ulcerative radiation wounds.Citation79–Citation81 Donor MSCs implanted in radiation lesions of irradiated mice differentiated into skin cells, leading to a partial understanding of the basic mechanisms of radiation-damaged skin regeneration observed in regenerating skin and hair follicle tissue. MSCs have been proposed to stimulate vascularization of these skin lesions, thereby increasing oxygen supply and helping immune cell recruitment.Citation82 Shen et al revealed that the protective effect of MSCs are mainly through the inhibition of radiation-induced OS and inflammation, including the downregulation of TNF-a, ICAM-1, TGF-β, and CTGF and the upregulation of antioxidant enzymes hemeoxygenase-1 and catalase in the radiation-induced damage model.Citation72
Studies have also shown that the immunoregulatory properties of BMMSCs contribute to skin healing.Citation83 An experiment demonstrated that the systemic infusions of BMMSCs could durably alter the progression of radiation-induced fibrosis by altering the macrophage phenotype and suppressing local inflammation. Immunohistochemical analysis showed that BMMSCs reduced the pro-inflammatory IL-1β levels, activated CD80+ macrophages, and increased the amount of anti-inflammatory IL-10 when irradiated mice were treated with systemic BMMSCs infusions.Citation84 Currently, clinical data on the effects of MSCs on RISRs in humans are lacking. One Chinese case study reported about the treatment of a radiation with a combination of MSCs and hematopoietic stem cells, leading to the regeneration of radiation-induced skin ulcerations after 36 days.Citation85
MSCs may be a promising therapeutic approach to treat RISRs because of their powerful therapeutic function. But in recent years some people have suggested that cells called MSC may not really be stem cells.Citation86 Whether MSCs can become the standard treatment for RISRs requires further study and experimental confirmation.
Hyperbaric oxygen therapy (HBOT)
Hyperbaric oxygen is defined as 100% oxygen at two to three times the atmospheric pressure at sea level, which can result in arterial oxygen tension in excess of 2,000 mmHg and oxygen tension in tissue of almost 400 mmHg. Such doses of oxygen have many beneficial biochemical, cellular, and physiologic effects.Citation87 HBOT is a relatively unknown treatment for delaying RISRs except for several small series and case reports that have been published.Citation88,Citation89 The role of hyperbaric oxygen in acute and subacute radiation injuries has not been well-studied or established, although researchers have shown interest in pursuing this application.Citation90
Hyperbaric oxygen is thought to have complex effects on immunity, oxygen transportation, and hemodynamics, resulting in favorable therapeutic effects by reducing hypoxia and edema as well as enabling normal host responses to infection and ischemia.Citation91 Since vascular obliteration and stromal fibrosis are the consistent cause and manifestation of radiation injury, the impact of hyperbaric oxygen in stimulating angiogenesis is an important mechanism against radiation injury. HBOT can induce neovascularization in hypoxic tissues.Citation92 MarxCitation93 have demonstrated enhanced vascularity and cellularity in heavily irradiated tissues after HBOT. They also demonstrated serial improvement in transcutaneous oxygen measurements of patients receiving hyperbaric oxygen as an indirect measure of increased vascular density.
According to the Undersea and Hyperbaric Medical Society, indications for HBOT include arterial insufficiency, refractory osteomyelitis, delayed radiation-induced soft tissue injury, and bone necrosis. HBOT has been recommended as a treatment for delayed radiation injury.Citation94 In a randomized trial, a positive correlation was reported between tissue oxygenation and various markers of wound healing.Citation95
In one case study, necrotic lesions induced by accidental radiation exposure improved after 140 sessions of HBOT without any adverse effects.Citation96 Another study reported about the experience of delayed radiation injury in a single HBOT-treated gynecologic cancer patients. These patients with necrotic ulceration derive the most benefit from HBOT, and two of the four patients had completely healed ulcers. There was an improvement of >50% in the other two patients.Citation97 In another case, a female patient received treatment with HBOT a total of 101 times over the course of 1 year for a refractory skin ulcer after radical mastectomy and RT, and she completely recovered from the radiation-induced skin ulcer.Citation98
HBOT has been applied as a therapy for delayed radiation injury for >30 years and considered as a good alternative for conservative treatment.
Superoxide dismutase (SOD)
OS is one of the main causes of RISRs. Antioxidant systems include enzymes, such as SOD, and glutathione peroxidase as well as peptides, such as glutathione. SOD is the first line of defense against OS pathological conditions because SOD can scavenge the ROS.Citation33,Citation99 Liposomal SOD is thought to down-regulate TGF-β expression in myofibroblasts and functions as an anti-inflammatory agent and antioxidant.Citation24
Synthetic SOD with low molecular weight, low toxicity, low cost, and biological stability have opened new opportunities for therapeutic interventions against RISRs.Citation100 A novel cyclized analog called EUK-207 is a synthetic SOD, which has a useful role in many OS models. A remarkable mitigation effect of EUK-207 on radiation-induced injury was noticed, including improvement in wound healing. By using 30 Gy of radiation dose without drug treatment, severe skin lesions could be induced that do not heal. At 48 hours after IR, EUK-207 was administered by hypodermic injection pump for up to 90 days. The rats treated with EUK-207 had reduced wet desquamation, lowered tissue inflammation, and enhanced wound contraction within 1 month.Citation101
Clinical regression of fibrosis was seen at 2-month follow-up in a clinical trial of 34 patients treated with six intramuscular injections of SOD over a 3-week period.Citation102 Reversal of radiation-induced skin fibrosis by liposomal Cu/Zn-SOD and Mn-SOD was observed after 6-month IR in an experimental swine model. It allowed correct tissue regeneration in established post-irradiation fibrotic regions.Citation103
Therefore, SOD can be used as a new therapeutic method for the treatment of RISRs by generating an anti-OS environment.
Low-intensity laser
Laser therapy has been used as an assistant in the tissue repair process. Laser therapy photo activates cellular mechanisms, leading to the normalization of the affected region by promoting a reduction of edema, analgesia induction, and an acceleration of tissue repair.Citation104,Citation105 Therapeutic lasers provide low-energy density, which is sufficient for the target cells to stimulate the membrane or organelles. Laser radiation is absorbed by cytochromes in the mitochondria and converted into energy (ATP) by the cell, which plays a role in the acceleration or stimulation of protein synthesis and cell proliferation. Thus, the cell enters a state of photoperiod activation, in which it seeks to establish a normal state in the affected area, such as in the repair phase after tissue injury.Citation106
Rezvani et alCitation107 reported that low-intensity light exposure could reduce the necrosis of the dermis after X-ray exposure in animals. In a previous case report, three patients had undergone mastectomy for breast cancer and had refractory radiation ulcers on the skin. The radiation ulcers were treated with 30 mW helium-neon laser three times weekly and healed completely within 7.5–8 weeks.Citation108
For some intractable chronic RISRs, low-density laser therapy may become an advanced method in the future.
Prevention of RISRs
Prevention of RISRs is an important consideration in the pre- and post-RT period. First, proper skin hygiene is essential. Skin should be washed with mild soaps and lukewarm water to help maintain skin barrier, and it will decrease the risk for acute RISRs.Citation109 Wear loose fitting clothing, avoid sun exposure, avoid metallic topical products, and use water-based lipid-free moisturizers may help prevent post-RT complication.Citation110 In addition to having therapeutic effects, topical corticosteroids have long been used to prevent RISRs. Corticosteroid use recommends application of low to medium potency steroids to the treatment field one to two times a day after each RT session to reduce the severity of acute RISRs and decrease the severity of symptoms.Citation4 Oral Wobe-Mugus (a proteolytic enzyme made from a mixture of 100 mg papain, 40 mg trypsin, and 40 mg chymotrypsin) has been shown to reduce the odds for developing RT-induced skin toxicity by as much as 87% in two non-blind RCTs vs no medication.Citation111,Citation112
Conclusion
Acute and chronic RISRs are the common side effects of RT. Inflammatory response and OS are the two main mechanisms of RISRs. The treatment of acute RISRs needs the formulation of relevant treatment strategies based on the degree of reactions. Traditional treatments, including topical steroids, creams, ointments, and hydrogel dressings, have been widely used in clinics. They have proved to have considerable therapeutic effects. Innovative treatment of acute RISRs still needs further study and confirmation. The treatment of chronic RISRs is more difficult. Many advanced treatments such as MSC, HBOT, SOD, and laser therapy are promising. However, a large number of clinical trials and studies are still required to validate their efficacy.
Disclosure
This report was supported in part by grants from the Norman Bethne Program of Jilin University (2015225 to Ying Xin and 2015203 to Xin Jiang), the Education Department of Jilin Province Foundations (2016-448 to Xin Jiang), the Jilin Provincial Science and Technology Foundations (20180414039 GH to Ying Xin and 220160414055 GH to Xin Jiang), and the Health and Family Planning Commission of Jilin Province Foundations (2016Q034 to Ying Xin and 2015Q010 to Xin Jiang). The authors report no other conflicts of interest in this work.
References
- VazAFPinto-NetoAMCondeDMCosta-PaivaLMoraisSSEstevesSBQuality of life of women with gynecologic cancer: associated factorsArch Gynecol Obstet2007276658358917564721
- ChapelAFrancoisSDouayLBenderitterMVoswinkelJFifteen years of preclinical and clinical experiences about biotherapy treatment of lesions induced by accidental irradiation and radiotherapyWorld J Stem Cells201353687223951388
- MendelsohnFADivinoCMReisEDKersteinMDWound care after radiation therapyAdv Skin Wound Care200215521622412368711
- HymesSRStromEAFifeCRadiation dermatitis: clinical presentation, pathophysiology, and treatment 2006J Am Acad Dermatol2006541284616384753
- MartinMTVulinAHendryJHHuman epidermal stem cells: role in adverse skin reactions and carcinogenesis from radiationMutat Res2016770Pt B34936827919341
- ArchambeauJOPeznerRWassermanTPathophysiology of irradiated skin and breastInt J Radiat Oncol Biol Phys1995315117111857713781
- PorockDFactors influencing the severity of radiation skin and oral mucosal reactions: development of a conceptual frameworkEur J Cancer Care (Engl)2002111334311966833
- HuHNahasSGattiRAAssaying radiosensitivity of ataxia-telangiectasiaMethods Mol Biol2017159911128477107
- BernierJPoortmansPClinical relevance of normal and tumour cell radiosensitivity in BRCA1/BRCA2 mutation carriers: a reviewBreast201524210010625557581
- ShanleySMcReynoldsKArdern-JonesAAcute chemotherapy-related toxicity is not increased in BRCA1 and BRCA2 mutation carriers treated for breast cancer in the United KingdomClin Cancer Res200612237033703817145825
- ParkHChoiDHNohJMAcute skin toxicity in Korean breast cancer patients carrying BRCA mutationsInt J Radiat Biol2014901909423957571
- NajafiMMotevaseliEShiraziAMechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implicationsInt J Radiat Biol201894433535629504497
- ZhaoWRobbinsMEInflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implicationsCurr Med Chem200916213014319149566
- StraubJMNewJHamiltonCDLominskaCShnayderYThomasSMRadiation-induced fibrosis: mechanisms and implications for therapyJ Cancer Res Clin Oncol2015141111985199425910988
- BrayFNSimmonsBJWolfsonAHNouriKAcute and chronic cutaneous reactions to ionizing radiation therapyDermatol Ther (Heidelb)20166218520627250839
- PeterRUDiagnosis and treatment of cutaneous radiation injuriesPanizzonandRGSeegenschmiedtMHRadiation Treatment and Radiation Reactions in DermatologyBerlin, HeidelbergSpringer Berlin Heidelberg2015185188
- JankoMOntiverosFFitzgeraldTJDengADeCiccoMRockKLIL-1 generated subsequent to radiation-induced tissue injury contributes to the pathogenesis of radiodermatitisRadiat Res2012178316617222856653
- HanRWuDDengSLiuTZhangTXuYNLRP3 inflammasome induces pyroptosis in lung tissues of radiation-induced lung injury in miceXi Bao Yu Fen Zi Mian Yi Xue Za Zhi20173391206121129089078
- ShinDLeeGSohnSHRegulatory T cells contribute to the inhibition of radiation-induced acute lung inflammation via bee venom phospholipase A2 in miceToxins (Basel)201685131
- AhmadIMuneerKMChangMEUltraviolet radiation-induced downregulation of SERCA2 mediates activation of NLRP3 inflammasome in basal cell carcinomaPhotochem Photobiol20179341025103328120514
- LamkanfiMEmerging inflammasome effector mechanismsNat Rev Immunol201111321322021350580
- AllamRLawlorKEYuECMitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome primingEMBO Rep201415998299024990442
- BentzenSMPreventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathologyNat Rev Cancer20066970271316929324
- MartinMLefaixJ-LDelanianSTGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target?Int J Radiat Oncol Biol Phys200047227729010802350
- TibbsMKWound healing following radiation therapy: a reviewRadiother Oncol1997422991069106919
- PohlersDBrenmoehlJLöfflerITGF-beta and fibrosis in different organs – molecular pathway imprintsBiochim Biophys Acta20091792874675619539753
- FlandersKCMajorCDArabshahiAInterference with transforming growth factor-beta/Smad3 signaling results in accelerated healing of wounds in previously irradiated skinAm J Pathol200316362247225714633599
- ChenMFChenWCLaiCHHungCHLiuKCChengYHPredictive factors of radiation-induced skin toxicity in breast cancer patientsBMC Cancer201010150820860847
- MeeranSMAkhtarSKatiyarSKInhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammationJ Invest Dermatol200912951258127019020550
- ChekiMYahyapourRFarhoodBCOX-2 in radiotherapy: a potential target for radioprotection and radiosensitizationCurr Mol Pharmacol201811317318329468988
- YahyapourRMotevaseliERezaeyanAReduction-oxidation (redox) system in radiation-induced normal tissue injury. Molecular Mechanisms and Implications in Radiation TherapeuticsClin Transl Oncol201820897598829318449
- NajafiMShiraziAMotevaseliEGerailyGNorouziFHeidariMRezapoorSThe melatonin immunomodulatory actions in radiotherapyBiophys Rev20179213914828510090
- VorotnikovaERosenthalRATriesMDoctrowSRBraunhutSJNovel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiationRadiat Res2010173674875920518654
- BaeYSOhHRheeSGYooYDRegulation of reactive oxygen species generation in cell signalingMol Cells201132649150922207195
- DentPYacoubAFisherPBHaganMPGrantSMAPK pathways in radiation responsesOncogene200322375885589612947395
- DrummondGRSelemidisSGriendlingKKSobeyCGCombating oxidative stress in vascular disease: NADPH oxidases as therapeutic targetsNat Rev Drug Discov201110645347121629295
- PandayASahooMKOsorioDBatraSNADPH oxidases: an overview from structure to innate immunity-associated pathologiesCell Mol Immunol201512152325263488
- AktanFiNOS-mediated nitric oxide production and its regulationLife Sci200475663965315172174
- OhtaSMatsudaSGunjiMKamogawaAThe role of nitric oxide in radiation damageBiol Pharm Bull20073061102110717541161
- PuginJHow tissue injury alarms the immune system and causes a systemic inflammatory response syndromeAnn Intensive Care2012212722788849
- FreyBRückertMDelochLRühlePFDererAFietkauRGaiplUSImmunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseasesImmunol Rev2017280123124829027224
- YahyapourRAminiPRezapoorSTargeting of inflammation for radiation protection and mitigationCurr Mol Pharmacol201811320321029119941
- LópezEGuerreroRNúñezMIEarly and late skin reactions to radiotherapy for breast cancer and their correlation with radiation-induced DNA damage in lymphocytesBreast Cancer Res200575R69069816168114
- LomaxMEFolkesLKO’NeillPBiological consequences of radiation-induced DNA damage: relevance to radiotherapyClin Oncol (R Coll Radiol)2013251057858523849504
- DedonPCTannenbaumSRReactive nitrogen species in the chemical biology of inflammationArch Biochem Biophys20044231122214989259
- JaiswalMLarussoNFBurgartLJGoresGJInflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanismCancer Res200060118419010646872
- MeghrajaniCFCoHCAng-TiuCMRoaFCTopical corticosteroid therapy for the prevention of acute radiation dermatitis: a systematic review of randomized controlled trialsExpert Rev Clin Pharmacol20136664164924164612
- BoströmALindmanHSwartlingCBerneBBerghJPotent corticosteroid cream (mometasone furoate) significantly reduces acute radiation dermatitis: results from a double-blind, randomized studyRadiother Oncol200159325726511369066
- KupperTSThe activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responsesJ Invest Dermatol1990946 Suppl146S150S2141048
- BrachMAGrussHJKaishoTAsanoYHiranoTHerrmannFIonizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa BJ Biol Chem199326812846684728473290
- BeetzAMesserGOppelTvan BeuningenDPeterRUKindPInduction of interleukin 6 by ionizing radiation in a human epithelial cell line: control by corticosteroidsInt J Radiat Biol199772133439246192
- MoriyasuSYamamotoKKureyamaNOkamuraKIkedaTYamatodaniAInvolvement of histamine released from mast cells in acute radiation dermatitis in miceJ Pharmacol Sci2007104218719017558180
- BartonJLavkerRMSchechterNMLazarusGSTreatment of urticaria pigmentosa with corticosteroidsArch Dermatol198512112151615234062333
- MillerRCSchwartzDJSloanJAMometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4Int J Radiat Oncol Biol Phys20117951460146620800381
- SchmuthMWimmerMAHoferSTopical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind studyBr J Dermatol2002146698399112072066
- OmidvariSSabooriHMohammadianpanahMTopical beta-methasone for prevention of radiation dermatitisIndian J Dermatol Venereol Leprol2007733209
- UlffEMarotiMSerupJFalkmerUA potent steroid cream is superior to emollients in reducing acute radiation dermatitis in breast cancer patients treated with adjuvant radiotherapy. A randomised study of betamethasone versus two moisturizing creamsRadiother Oncol2013108228729223827771
- Del RossoJQBikowskiJTrolamine-containing topical emulsion: clinical applications in dermatologyCutis200881320921418441842
- HarperJLFranklinLEJenretteJMAgueroEGSkin toxicity during breast irradiation: pathophysiology and managementSouth Med J2004971098999315558927
- GlesingerRCohenADBogdanov-BerezovskyAKriegerYRosen-bergLA randomized controlled trial of silver sulfadiazine, biafine, and saline-soaked gauze in the treatment of superficial partial-thickness burn wounds in pigsAcad Emerg Med200411433934215064205
- CoulombBFriteauLDubertretLBiafine applied on human epidermal wounds is chemotactic for macrophages and increases the IL1/IL6 ratioSkin Pharmacol Physiol1997105–6281287
- KrauszAEAdlerBLLandriscinaARosenJMMusaevTNosanchukJDFriedmanAJBiafine topical emulsion accelerates excisional and burn wound healing in miceArch Dermatol Res2015307758359425794496
- SzumacherEWightonAFranssenEPhase II study assessing the effectiveness of Biafine cream as a prophylactic agent for radiation-induced acute skin toxicity to the breast in women undergoing radiotherapy with concomitant CMF chemotherapyInt J Radiat Oncol Biol Phys2001511818611516855
- WasiakJClelandHCampbellFSpinksADressings for superficial and partial thickness burnsCochrane Database Syst Rev20133CD002106
- MargolinSGBrenemanJCDenmanDLLachapellePWeckbachLAronBSErratum: management of radiation-induced moist skin desquamation using hydrocolloid dressingCancer Nurs1990134267280
- FisherJScottCStevensRRandomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13Int J Radiat Oncol Biol Phys20004851307131011121627
- PanasitiVDevirgiliisVBorroniRGManagement of skin ulcers in a patient with mycosis fungoidesDermatol Online J200612216
- NaylorWMallettJManagement of acute radiotherapy induced skin reactions: a literature reviewEur J Oncol Nurs20015422123312849619
- GollinsSGaffneyCSladeSSwindellRRCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamationJ Wound Care200817626827518666721
- CensabellaSClaesSOrlandiniMBraekersRThijsHBulensPRetrospective study of radiotherapy-induced skin reactions in breast cancer patients: reduced incidence of moist desquamation with a hydroactive colloid gel versus dexpanthenolEur J Oncol Nurs201418549950424877859
- DominiciMLe BlancKMuellerIMinimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statementCytotherapy20068431531716923606
- ShenYJiangXMengLXiaCZhangLXinYTransplantation of bone marrow mesenchymal stem cells prevents radiation-induced artery injury by suppressing oxidative stress and inflammationOxid Med Cell Longev201820183113
- LandryYLeOMaceKARestivoTEBeauséjourCMSecretion of SDF-1alpha by bone marrow-derived stromal cells enhances skin wound healing of C57BL/6 mice exposed to ionizing radiationJ Cell Mol Med2010146B1594160419725920
- JacksonWMNestiLJTuanRSMesenchymal stem cell therapy for attenuation of scar formation during wound healingStem Cell Res Ther2012332022668751
- HeoSCJeonESLeeIHKimHSKimMBKimJHTumor necrosis factor-a-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanismsJ Invest Dermatol201113171559156721451545
- DingJHoriKZhangRMarcouxYHonardoustDShankowskyHATredgetEEStromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS)Wound Repair Regen201119556857822092795
- KleinDSteensJWiesemannAMesenchymal stem cell therapy protects lungs from radiation-induced endothelial cell loss by restoring superoxide dismutase 1 expressionAntioxid Redox Signal2017261156358227572073
- XinYJiangXWangYInsulin-producing cells differentiated from human bone marrow mesenchymal stem cells in vitro ameliorate streptozotocin-induced diabetic hyperglycemiaPLoS One2016111e014583826756576
- AgayDScherthanHForcheronFGrenierNHérodinFMeinekeVDrouetMMultipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig modelExp Hematol2010381094595620600578
- AkitaSAkinoKHiranoAOhtsuruAYamashitaSMesenchymal stem cell therapy for cutaneous radiation syndromeHealth Phys201098685886220445394
- LatailladeJJDoucetCBeyENew approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapyRegen Med20072578579417907931
- NicolayNHLopez PerezRDebusJHuberPEMesenchymal stem cells – a new hope for radiotherapy-induced tissue damage?Cancer Lett2015366213314026166559
- de MayoTCongetPBecerra-BayonaSSossaCLGalvisVArango-RodríguezMLThe role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic micePLoS One2017126e017753328594903
- HortonJAHudakKEChungEJWhiteAOScrogginsBTBurkeenJFCitrinDEMesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammationStem Cells201331102231224123897677
- GuoMDongZQiaoJSevere acute radiation syndrome: treatment of a lethally 60Co-source irradiated accident victim in China with HLA-mismatched peripheral blood stem cell transplantation and mesenchymal stem cellsJ Radiat Res201455220520923979075
- CaplanAIMesenchymal stem cells: time to change the nameStem Cells Transl Med201761445145128452204
- TibblesPMEdelsbergJSHyperbaric-oxygen therapyN Engl J Med1996334164216488628361
- BaertJCarpentierPGarciaRBHyperbaric oxygen treatment for radiation ulcer of the bladderBr J Urol1998819299309666791
- WarrenDCFeehanPSladeJBCianciPEChronic radiation proctitis treated with hyperbaric oxygenUndersea Hyperb Med19972431811849308141
- CarlUMHartmannKAHyperbaric oxygen treatment for symptomatic breast edema after radiation therapyUndersea Hyperb Med19982542332349883492
- GrimPSHyperbaric oxygen therapyJAMA199026316221622202181162
- FeldmeierJJHyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 updateUndersea Hyperb Med20123961121113923342770
- MarxREOsteoradionecrosis: a new concept of its pathophysiologyJ Oral Maxillofac Surg19834152832886572704
- FeldmeierJJHeimbachRDDavoltDACourtWSStegmannBJSheffieldPJHyperbaric oxygen as an adjunctive treatment for delayed radiation injury of the chest wall: a retrospective review of twenty-three casesUndersea Hyperb Med19952243833938574126
- KaurSPawarMBanerjeeNGargREvaluation of the efficacy of hyperbaric oxygen therapy in the management of chronic nonhealing ulcer and role of periwound transcutaneous oximetry as a predictor of wound healing response: a randomized prospective controlled trialJ Anaesthesiol Clin Pharmacol2012281707522345950
- YildizSCimsitMIlgezdiSUzunGGumusTQyrdediTDalciDHyperbaric oxygen therapy used to treat radiation injury: two case reportsOstomy Wound Manage20065251416
- FinkDChettyNLehmJPMarsdenDEHackerNFHyperbaric oxygen therapy for delayed radiation injuries in gynecological cancersInt J Gynecol Cancer200616263864216681739
- EnomotoMYagishitaKOkumaKHyperbaric oxygen therapy for a refractory skin ulcer after radical mastectomy and radiation therapy: a case reportJ Med Case Rep2017111528049509
- Batinić-HaberleIRebouçasJSSpasojevićISuperoxide dismutase mimics: chemistry, pharmacology, and therapeutic potentialAntioxid Redox Signal201013687791820095865
- RebouçasJSSpasojevic´IBatinic´-HaberleIPure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biologyJ Biol Inorg Chem200813228930218046586
- RosenthalRAFishBHillRPSalen Mn complexes mitigate radiation injury in normal tissuesAnticancer Agents Med Chem201111435937221453241
- DelanianSBailletFHuartJLefaixJLMaulardCHoussetMSuccessful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trialRadiother Oncol199432112207938674
- LefaixJLDelanianSLeplatJJSuccessful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental studyInt J Radiat Oncol Biol Phys19963523053128635938
- TumiltySMunnJAbbottJHMcDonoughSHurleyDABaxterGDLaser therapy in the treatment of achilles tendinopathy: a pilot studyPhotomed Laser Surg2008261253018248158
- YasukawaAHruiHKoyamaYNagaiMTakakudaKThe effect of low reactive-level laser therapy (LLLT) with helium-neon laser on operative wound healing in a rat modelJ Vet Med Sci200769879980617827885
- da SilvaJPda SilvaMAAlmeidaAPLombardi JuniorIMatosAPLaser therapy in the tissue repair process: a literature reviewPhotomed Laser Surg2010281172119764898
- RezvaniMRobbinsMEHopewellJWWhitehouseEMModification of late dermal necrosis in the pig by treatment with multi-wavelength lightBr J Radiol1993667821451498457828
- SchindlASchindlMPernerstorfer-SchönHMossbacherUSchindlLLow intensity laser irradiation in the treatment of recalcitrant radiation ulcers in patients with breast cancer–long-term results of 3 casesPhotodermatol Photoimmunol Photomed2000161343710721863
- RoyIFortinALarochelleMThe impact of skin washing with water and soap during breast irradiation: a randomized studyRadiother Oncol200158333333911230896
- BernierJBonnerJVermorkenJBConsensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neckAnn Oncol200819114214917785763
- DalePSTamhankarCPGeorgeDDaftaryGVCo-medication with hydrolytic enzymes in radiation therapy of uterine cervix: evidence of the reduction of acute side effectsCancer Chemother Pharmacol200147SupplS29S3411561869
- GujralMSPatnaikPMKaulRParikhHKConradtCTamhankarCPDaftaryGVEfficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancersCancer Chemother Pharmacol200147SupplS23S2811561868
