Abstract
Background
Prostate cancer (PCa) is the second leading cause of cancer death in men. Several articles have reported that microRNA-21 (miR-21) and microRNA-30c (miR-30c) have diagnostic values for PCa, but the results are inconclusive. In order to precisely assess the diagnostic values of miR-21 and miR-30c for PCa, this meta-analysis is performed.
Methods
Articles were searched in the databases of PubMed, Embase, and Web of Knowledge (search date: September 6, 2018). Studies were included if they were designed to evaluate the diagnostic performance of miR-21 or miR-30c for PCa. Using Stata 12.0 and Meta-Disc 1.4, the pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under curve (AUC) of the summary receiver-operating characteristic (SROC) curve with the corresponding 95% CI were calculated.
Results
Overall, ten studies (six studies for miR-21 and four for miR-30c) involving1,371 participants were included in this meta-analysis. For miR-21, the pooled SEN and SPE were, respectively, 0.91 (95% CI: 0.87–0.94) and 0.88 (95% CI: 0.82–0.93), the pooled PLR and NLR were, respectively, 7.74 (95% CI: 4.81–12.47), 0.1 (95% CI: 0.06–0.15), the DOR was 77.64 (95% CI: 34.64–174.02), AUC of SROC was 0.95 (95% CI: 0.93–0.97). For miR-30c, the pooled SEN and SPE were, respectively, 0.74 (95% CI: 0.65–0.81) and 0.78 (95% CI: 0.72–0.83), the pooled PLR and NLR were, respectively, 3.39 (95% CI: 2.69–4.26), and 0.34 (95% CI: 0.26–0.44), the DOR was 10.06 (95% CI: 6.96–14.55), and AUC of SROC was 0.83 (95% CI: 0.79–0.86).
Conclusion
For PCa, miR-21 is a good diagnostic biomarker and miR-30c is a moderate diagnostic biomarker.
Introduction
As a complex disorder resulting from the combined effects of multiple environmental and genetic factors, prostate cancer (PCa) is the second leading cause of cancer death in men.Citation1 In Europe, it accounts for >92,300 deaths annually.Citation2 As a routine assay test in clinic, prostate-specific antigen (PSA) in serum is not specific for PCa, some evaluated PSA levels may induce false-positive due to infection or hyperplasia. Therefore, it is necessary to find novel effective biomarkers to diagnose PCa.
MicroRNAs, a family of small noncoding RNAs (19±22 nucleotides), are stable and easy to accurately measure. MicroRNAs are differentially expressed in normal tissues and cancers, contributing to cancer development and progression.Citation3 MicroRNA-21(miR-21), an anti-apoptotic agent functioning via p53 network, can target and inhibit tumor suppressor gene PTEN expression to promote PCa cell proliferation and invasion.Citation4,Citation5 MicroRNA-30c (miR-30c) has been acknowledged as a tumor suppressor in various human cancers, such as ovarian cancer, gastric cancer, and PCa.Citation6
With respect to diagnostic abilities of miR-21 and miR-30c for PCa, several studies have been conducted, but their results are inconclusive.Citation7–Citation14 For example, Porzycki et al found specificity (SPE) of miR-21 to diagnose PCa was 0.75, while Yang et al found it could reach 0.93.Citation8,Citation9 What’s more, Huang found sensitivity (SEN) of miR-30c was 0.82, while Chen et al found it was only 0.6.Citation11,Citation14 In order to precisely assess the diagnostic values of miR-21 and miR-30c for PCa, this meta-analysis is performed.
Methods
This meta-analysis was conducted according to the guidelines of the PRISMA statement ().Citation15
Eligibility criteria
This meta-analysis selected the patients diagnosed as PCa and the control consisted of healthy people or patients with benign prostate disease. The index tests, which included miR-21 and miR-30c from serum, plasma, mononuclear cell, and tissue, were assessed by fluorescence quantitative PCR. Reference standard was histopathological test, PCa was diagnosed based on histopathological confirmation.
Studies were included if they met following inclusion criteria: 1) studies were designed to evaluate the diagnostic performance of miR-21 or miR-30c for PCa; 2) PCa was diagnosed based on histopathological confirmation and the control group consisted of healthy people or patients with benign prostate disease; 3) 2×2 contingency tables could be directly extracted or calculated; and 4) articles were written in English.
Search strategy
Articles published prior to September 6, 2018, were systematically searched in the databases of PubMed, Embase, and Web of Knowledge. Search terms were as follows: (“microRNA” or “miRNA” or “miR”) and (“prostate cancer” or “prostate carcinoma”) and (“diagnosis” or “sensitivity” or “specificity”). Search strategy is shown in . In addition, cited references from relevant articles were examined to select other relevant articles. Two reviewers independently searched the articles. Any disagreements between the two reviewers were resolved by discussion.
Study selection
The titles and abstracts of the identified articles were examined by two reviewers. Those articles falling under the exclusion criteria (reviews, case reports, letters, comments, conference abstracts, or meta-analyses; animal studies; duplicate studies) were not considered. The full texts were then rescreened and evaluated more thoroughly for eligibility using the same exclusion criteria. Disagreement between the two reviewers was discussed until a consensus was reached.
Data extraction
The following data were extracted or calculated from retrieved articles by the two reviewers: first author, year of publication, country of participants, specimen type, source of control group, sample size, microRNAs as control for normalization, microRNAs as biomarker, relative expressions in case group compared with control group, diagnostic SEN and SPE values, values of true positive (TP), false positive (FP), false negative (FN) and true negative (TN).
Methodological quality assessment
Two reviewers assessed the methodological quality of included studies according to the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool.Citation16 Patient selection, index test, reference standard, flow, and timing were used to evaluate the risk of bias and the first three domains were applied to assess applicability concerns.
Statistical analysis
Statistical analyses were undertaken utilizing Stata 12.0 (StataCorp, College Station, TX, USA) and Meta-Disc 1.4 (Cochrane Colloquium, Barcelona, Spain). Diagnostic threshold effects were inspected with the Spearman rank correlation analysis. Heterogeneity was measured using Cochrane-Q test and I2 index. P<0.05 for Q test or I2 >50% designated heterogeneity. The pooled SEN and SPE, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic score (DS), diagnostic odds ratio (DOR), and area under curve (AUC) of the summary receiver operating characteristic (SROC) curve with the corresponding 95% CI were calculated using a bivariate binomial mixed model. Deeks’ funnel plot asymmetry analysis was performed to identify publication bias. Sensitivity analysis was conducted to assess the stability.
Results
Literature search and study characteristics
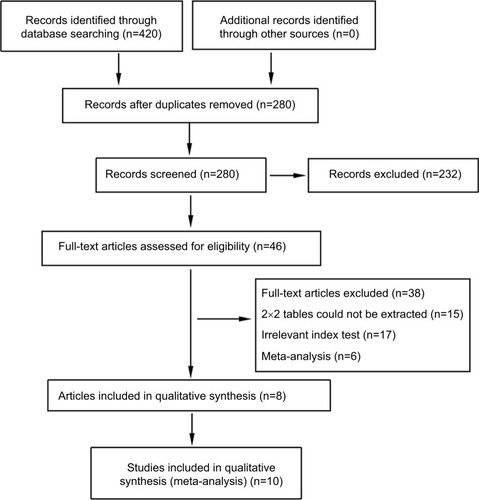
A total of 420 records were identified through database searching. After removing duplicates, the titles and abstracts for 280 records were screened for eligibility. Of these, 46 records were identified and full-text articles were retrieved. Thirty-eight manuscripts were excluded through assessment of the full-text articles and eight remaining articles encompassing ten studies were included in the meta-analysis ().
The ten included studies owned 1,371 participants (737 PCa patients and 634 controls). Their characteristics and the numbers of TP, FP. FN, and TN with the corresponding SEN, SPE are listed in . The number of included studies for miR-21 and miR-30c were, respectively, six and four. Countries of participants for miR-21 involved Poland, China, and Egypt, while miR-30c only involved China. Relative expressions of miR-21 in case group were higher than that in control group. On the contrary, relative expressions of miR-30c were lower in case group. Two studies assessed miR-30c from PCa cancer tissue and PCa adjacent normal tissue,Citation7, Citation11 and other two studies assessed miR-30c from plasma of PCa patients and control group.Citation14 Studies assessed miR-21 from serum, plasma, mononuclear cell of PCa patients, and control group.
Table 1 Characteristics of included studies
Methodological quality assessment
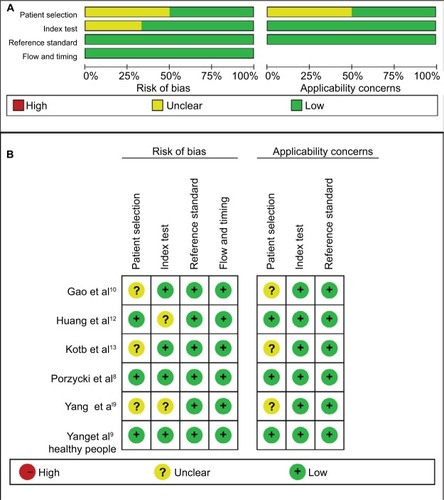
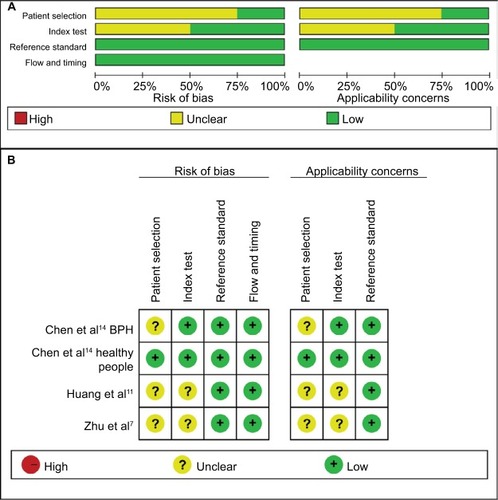
Quality assessment of included studies was conducted using the QUADAS-2 tool ( and ). The majority of included studies satisfied most domains of QUADAS-2. The unclear situation existed in the domains of patient selection and index test because there were variations in control group, specimen, and microRNAs as control for normalization.
Diagnostic values
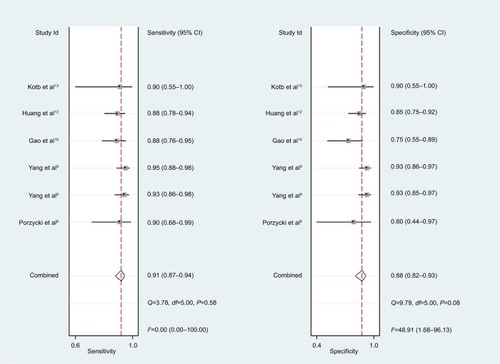
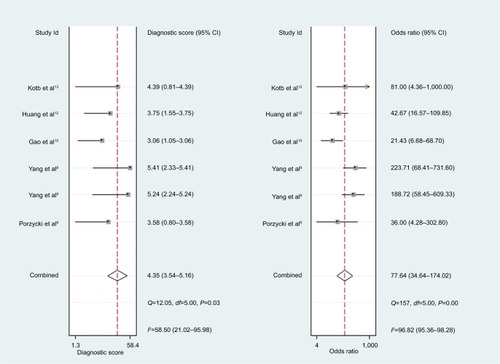
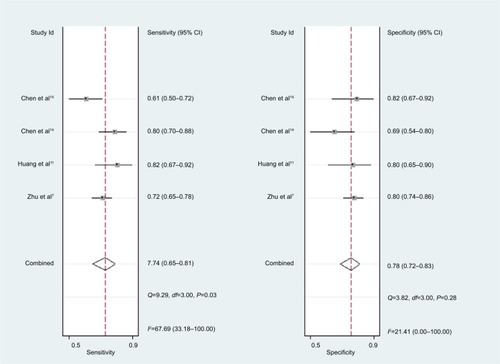
For miR-21, the Spearman rank correlation coefficient was –0.841 (P=0.036), suggesting that there were no diagnostic threshold effects. The pooled SEN and SPE were, respectively, 0.91 (95% CI: 0.87–0.94) and 0.88 (95% CI: 0.82–0.93) (). I2 for SEN and SPE were, respectively, 0% (P=0.58) and 48.9% (P=0.08), showing no significant heterogeneity among studies. The pooled PLR and NLR were, respectively, 7.74 (95% CI: 4.81–12.47) and 0.1 (95% CI: 0.06–0.15) (). The pooled DS and DOR were, respectively, 4.35 (95% CI: 3.54–5.16) and 77.64 (95% CI: 34.64–174.02) (). AUC of SROC curve was 0.95 (95% CI: 0.93–0.97) ().
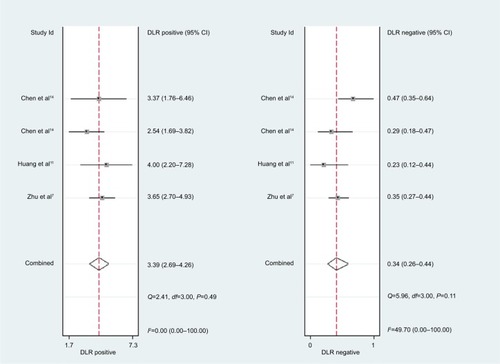
Figure 5 Forest plots of positive likelihood ratio and negative likelihood ratio for miR-21.
Abbreviation: DLR, diagnostic likelihood ratio.
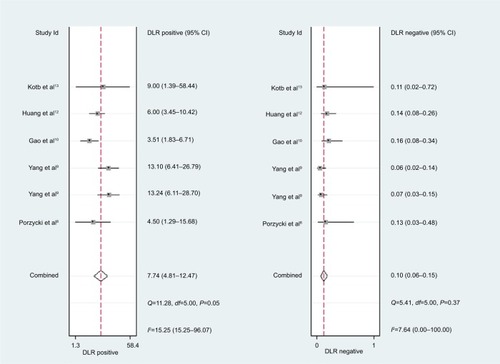
Figure 7 Summary receiver operating characteristic curves.
Note: (A) miR-21, (B) miR-30c.
Abbreviations: AUC, area under curve; SROC, summary receiver- operating characteristic; SENS, sensitivity; SPEC, specificity.
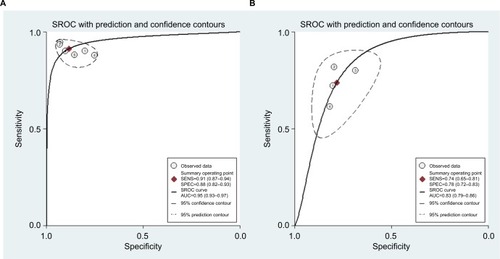
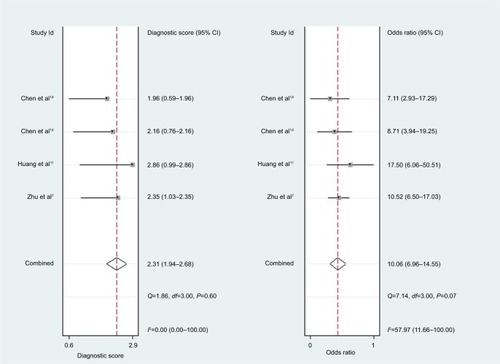
For miR-30c, the Spearman rank correlation coefficient was 0.80 (P=0.2), suggesting no existence of diagnostic threshold effects. The pooled SEN and SPE were, respectively, 0.74 (95% CI: 0.65–0.81) and 0.78 (95% CI: 0.72–0.83) (). I2 for SEN and SPE were, respectively, 67.69% (P=0.03) and 21.41% (P=0.28), revealing that significant heterogeneities existed among studies. The pooled PLR and NLR were, respectively, 3.39 (95% CI: 2.69–4.26) and 0.34 (95% CI: 0.26–0.44) (). The pooled DS and DOR were, respectively, 2.31 (95% CI: 1.94–2.68) and 10.06 (95% CI: 6.96–14.55) (). AUC of SROC curve was 0.83 (95% CI: 0.79–0.86) ().
Publication bias
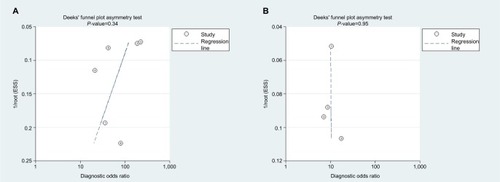
As shown in Deeks’ funnel plots (), publication biases were likely absent with symmetrical funnel shapes. The P-values for the funnel plot asymmetry tests (P=0.43 for miR-21 and P=0.95 for miR-30c) confirmed lack of publication biases.
Sensitivity analysis
Sensitivity analysis was performed by eliminating studies one by one. As shown in , the pooled SEN and corresponding heterogeneities of miR-21 and miR-30c were not dominantly influenced by removing any study.
Discussion
The pooled SEN and SPE of miR-21 were, respectively, 0.91 and 0.88, and the AUC of SROC was 0.95, presenting a good diagnostic ability of miR-21. The pooled PLR of 7.74 indicated that PCa probability increased by 7.74-fold with positive miR-21 result. Conversely, the pooled NLR of 0.1 showed the probability could only be 10% when miR-21 result was negative. As a combination of PLR and NLR, DOR is a meaningful diagnostic index. With a DOR value of 77.64, it was confirmed that miR-21 could be a good diagnostic biomarker for PCa. Sensitivity analysis showed that the conclusion of this meta-analysis was robust. There is a research which suggests that miR-21 and androgen receptor axis can exert oncogenic effects in prostate tumors by downregulating transforming growth factor beta receptor II.Citation17
For miR-30c, this meta-analysis showed that pooled SEN and SPE were, respectively, 0.74 and 0.78; furthermore AUC of SROC was 0.83, presenting a moderate diagnostic ability of miR-30c. Significant heterogeneities were found among included studies. Although sensitivity analysis was carried out, it was still hard to identify which study was the source of heterogeneities.
Three previous meta-analyses (by Yin et al, Ouyang et al, and Yang et al) also reported the diagnostic value of microRNAs for PCa,Citation18–Citation20 we read them carefully with great interest. The number for miR-21 of enrolled studies by a previous meta-analysis (by Yin)Citation18 was less than our meta-analysis. Other two meta-analyses (by Ouyang et al and Yang et al)Citation19,Citation20 only focused on overall microRNAs but not each single microRNA, inevitably brought about significant heterogeneities, lower quality assessments, and meaninglessness in application. Another previous meta-analysis by Song et al provided mean ± SD or fold change of microRNAs expression levels in PCa patients vs controls,Citation21 but did not provide diagnostic information, such as pooled SEN and SPE.
Limitations
There were several limitations in our meta-analysis. First, the situation of unclear risk in the domains of patient selection and index could lower methodological qualities. Second, significant heterogeneities existed among included studies of miR-30c. Despite sensitive analysis being performed, potential sources of heterogeneities were still hard to been found. Third, comparison with blood-based specimen, tissue-based specimen is difficult to obtain in real life. Fourth, the number of included studies were few. It is necessary to adopt standardization of control group, specimen, and index test and to conducted more related studies.
Conclusion
Our meta-analysis showed that, for PCa, miR-21 is a good diagnostic biomarker and miR-30c is a moderate diagnostic biomarker.
Supplementary material
Table S1 PRISMA Checklist
Table S2 Search strategy
Table S3 Sensitivity analysis for pooled sensitivities and corresponding heterogeneities
Disclosure
The authors report no conflicts of interest in this work.
References
- PorzyckiPCiszkowiczESemikMTyrkaMCombination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognitionInt Urol Nephrol20185091619162630014459
- YangBLiuZNingHMicroRNA-21 in peripheral blood mono-nuclear cells as a novel biomarker in the diagnosis and prognosis of prostate cancerCancer Biomark201617222323027434290
- GaoYGuoYWangZAnalysis of circulating miRNAs 21 and 375 as potential biomarkers for early diagnosis of prostate cancerNeoplasma201663462362827268927
- HuangWKangXLCenSWangYChenXHigh-level expression of microRNA-21 in peripheral blood mononuclear cells is a diagnostic and prognostic marker in prostate cancerGenet Test Mol Biomarkers201519946947526247873
- KotbSMosharafaAEssawiMHassanHMeshrefAMorsyACirculating miRNAs 21 and 221 as biomarkers for early diagnosis of prostate cancerTumour Biol20143512126131261725190021
- ZhuCHouXZhuJJiangCWeiWExpression of miR-30c and miR-29b in prostate cancer and its diagnostic significanceOncol Lett20181633140314430127906
- HuangZZhangLYiXYuXDiagnostic and prognostic values of tissue hsa-miR-30c and hsa-miR-203 in prostate carcinomaTumour Biol20163744359436526499781
- ChenZHZhangGLLiHRA panel of five circulating microRNAs as potential biomarkers for prostate cancerProstate201272131443145222298030
References
- CollakFKDemirUOzkanliSKurumEZerkPEIncreased expression of YAP1 in prostate cancer correlates with extraprostatic extensionCancer Biol Med201714440541329372107
- WilsonHCShahSIAbelPDContemporary hormone therapy with LHRH agonists for prostate cancer: avoiding osteoporosis and fractureCent European J Urol2015682165168
- WangWZhangELinCMicroRNAs in tumor angiogenesisLife Sci2015136283526144623
- PapagiannakopoulosTShapiroAKosikKSMicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cellsCancer Res200868198164817218829576
- YangYGuoJXShaoZQmiR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: an experimental studyAsian Pac J Trop Med2017101879128107872
- LiuSLiXZhuangSMiR-30C impedes glioblastoma cell proliferation and migration by targeting SOX9Oncol Res201927216517129495977
- ZhuCHouXZhuJJiangCWeiWExpression of miR-30C and miR-29b in prostate cancer and its diagnostic significanceOncol Lett20181633140314430127906
- PorzyckiPCiszkowiczESemikMTyrkaMCombination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognitionInt Urol Nephrol20185091619162630014459
- YangBLiuZNingHMicroRNA-21 in peripheral blood mono-nuclear cells as a novel biomarker in the diagnosis and prognosis of prostate cancerCBM2016172223230
- GaoYGuoYWangZAnalysis of circulating miRNAs 21 and 375 as potential biomarkers for early diagnosis of prostate cancerNeoplasma201663462362827268927
- HuangZZhangLYiXYuXDiagnostic and prognostic values of tissue hsa-miR-30c and hsa-miR-203 in prostate carcinomaTumor Biol201637443594365
- HuangWKangXLCenSWangYChenXHigh-level expression of microRNA-21 in peripheral blood mononuclear cells is a diagnostic and prognostic marker in prostate cancerGenet Test Mol Biomarkers201519946947526247873
- KotbSMosharafaAEssawiMHassanHMeshrefAMorsyACirculating miRNAs 21 and 221 as biomarkers for early diagnosis of prostate cancerTumor Biol201435121261312617
- ChenZHZhangGLLiHRA panel of five circulating microRNAs as potential biomarkers for prostate cancerProstate201272131443145222298030
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- WhitingPFRutjesAWWestwoodMEQUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studiesAnn Intern Med2011155852953622007046
- MishraSDengJJGowdaPSAndrogen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (Tgfbr2) expression in prostate cancerOncogene201433314097410624037531
- YinCFangCWengHYuanCWangFCirculating microRNAs as novel biomarkers in the diagnosis of prostate cancer: a systematic review and meta-analysisInt Urol Nephrol20164871087109527068818
- OuyangHZhouYZhangLShenGDiagnostic value of microRNAs for urologic cancers: a systematic review and meta-analysisMedicine20159437e127226376375
- YangQZhengYZhuDDiagnostic performance of microRNAs expression in prostate cancerTumor Biol201435101052910538
- SongCJChenHChenLZRuGMGuoJJDingQNThe potential of microRNAs as human prostate cancer biomarkers: a meta-analysis of related studiesJ Cell Biochem201811932763278629095529