Abstract
Background
Despite the continuing increase in the breast cancer incidence rate among Saudi Arabian women, no breast cancer risk-prediction model is available in this population. The aim of this research was to develop a risk-assessment tool to distinguish between high risk and low risk of breast cancer in a sample of Saudi women who were screened for breast cancer.
Methods
A retrospective chart review was conducted on symptomatic women who underwent breast mass biopsies between September 8, 2015 and November 8, 2017 at King Abdulaziz Medical City, Riyadh, Saudi Arabia.
Results
A total of 404 (63.8%) malignant breast biopsies and 229 (36.2%) benign breast biopsies were analyzed. Women ≥40 years old (aOR: 6.202, CI 3.497–11.001, P=0.001), hormone-replacement therapy (aOR 24.365, 95% CI 8.606–68.987, P=0.001), postmenopausal (aOR 3.058, 95% CI 1.861–5.024, P=0.001), and with a family history of breast cancer (aOR 2.307, 95% CI 1.142–4.658, P=0.020) were independently associated with an increased risk of breast cancer. This model showed an acceptable fit and had area under the receiver-operating characteristic curve of 0.877 (95% CI 0.851–0.903), with optimism-corrected area under the curve of 0.865.
Conclusion
The prediction model developed in this study has a high ability in predicting increased breast cancer risk in our facility. Combining information on age, use of hormone therapy, postmenopausal status, and family history of breast cancer improved the degree of discriminatory accuracy of breast cancer prediction. Our risk model may assist in initiating population-screening programs and prompt clinical decision making to manage cases and prevent unfavorable outcomes.
Introduction
The incidence of breast cancer is increasing in Saudi Arabia and is currently the most common cancer among women,Citation1 accounting for 28.7% of all cancers reported among females in the country.Citation2 According to the Saudi Cancer Registry, the age-standardized incidence in 2001 was 11.8 per 100,000 females,Citation3 which increased to 22.7 in 2014.Citation2 A Saudi Arabian study in 2008 reported that the incidence of breast cancer was expected to increase by 350% in 2025.Citation4 The apparent increase in breast cancer incidence is possibly attributable to genetic, environmental, and dietary factors.Citation1
Breast cancer remains the major cause of death among women in Saudi Arabia.Citation1 This poor prognosis could be due to diagnosis at an advanced stage.Citation5 Early detection of breast cancer is known to improve patients’ survival and clinical outcomes.Citation6 Also, early detection can be aided by applying risk-assessment tools to stratify patients with greater risk of breast cancer.Citation7 This may aid the screening process to improve individual clinical decision making. Numerous breast cancer risk-prediction models have been developed and validated in various female populations to aid diagnosis decision making and improve accuracy.Citation8–Citation10
Gene expressions for breast cancer in Saudi Arabia are different from those in the North American population.Citation11 As such, the generalizability of the current existing models may be suitable for specific populations where they have been validated, but may not be suitable to project the risk of breast cancer in women in Saudi Arabia. To our knowledge, there is a great need to develop and validate a risk-prediction model in Saudi Arabia to stratify patients at high risk effectively and achieve better understanding of existing risk-prediction models.
The aim of this study was to develop a risk-assessment tool to distinguish between high risk and low risk of breast cancer in a sample of women screened for breast cancer at King Abdulaziz Medical City, Riyadh (KAMC-RD), Ministry of National Guard – Health Affairs (MNG-HA), Saudi Arabia. The model assessed the contribution of demographic, clinical, and reproductive factors and breast-imaging findings in predicting breast cancer. We hypothesized that a number of factors would be associated with increased risk of breast cancer in women who had undergone a biopsy of breast mass at KAMC-RD, MNG-HA.
Methods
A single center retrospective chart review was conducted on 637 symptomatic women who underwent a biopsy of breast mass between September 8, 2015, and November 8, 2017, at KAMC-RD, MNG-HA. The study received ethical approval from the institutional review board at the MNG-HA (SP17/027/R). Due to the study design, patient consent to review their medical records was not required as per the institutional review board.
Data were retrieved on women’s age at biopsy of breast mass, body-mass index (kg/m2), diabetes, hypertension, asthma, lung disease, hyperlipidemia, use of oral contraceptives, use of hormone-replacement therapy (HRT) to treat signs and symptoms, change in size or skin of breast, family history of breast cancer, and history of genital disease. These data were recorded as yes/no. Breast cancer in Saudi Arabia predominantly impacts women who are over 40 years of age.Citation12 Women’s age at biopsy was classified into two groups: <40 years and 40 years or more. This cutoff value was defined by the Youden index, as it produced sensitivity of 93% and specificity of 57.2%. In accordance with the World Health Organization, a body-mass index ≥30 kg/m2 was used to define obesity.Citation13
Data were extracted on postmenopausal status (yes/no) from medical charts and age ≥50 years was used as a cutoff to define postmenopausal status in cases of unavailable data on postmenopausal status. This cutoff was used because two previous reports estimated mean age at menopause to be around 50 years among women in Saudi Arabia.Citation14,Citation15 Data were retrieved on imaging tests used to screen for breast cancer, including breast ultrasound, mammography, and magnetic resonance imaging (MRI). A positive screening for breast cancer on mammography, ultrasound, or MRI was classified as abnormal diagnostic imaging. A total of 637 symptomatic women underwent breast biopsy during the study period. Data were excluded for four women, because they had no available breast-biopsy findings on their records.
Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Due to the study design, patient consent to review their medical records was not required as per the institutional review board at the Ministry of National Guard – Health Affairs.
Statistical analysis
Data were analyzed using STATA version 12 (StataCorp, College Station, TX, USA). An overall summary of sample characteristics and across-group analyses is presented in .
Table 1 Women’s characteristics
P-values of χ2/independent-sample t-tests and unadjusted OR were reported to test whether specific sample characteristics were associated with breast cancer. Accuracy was evaluated for each predictor alone in identifying breast cancer by area under the receiver-operating characteristic (ROC) curve (AUC) analysis and 95% CI ().
Table 2 Bivariate analysis: factors associated with increased breast cancer risk
Development of a risk-prediction model to classify breast cancer risk was undertaken using the stepwise logistic regression model (). The model evaluated 14 potential factors that could be associated with increased risk of breast cancer, and were evaluated at α≤0.05. The Hosmer–Lemeshow test was used to assess the goodness of fit of the final model, with P>0.05, suggesting that the final model fit the data well.
Table 3 ROC analysis: accuracy of individual factors
The overall performance of the final model was evaluated by AUC and compared with the individual predictors in the final model (). The AUC of the final model was internally corrected using a bootstrap resampling approach by generating 100 random samples drawn with replacements from the original sample (n=633). This was performed to validate the AUC result on 100 replicates. Average optimism and SD were calculated. A risk-probability calculator was developed to predict the risk of developing breast cancer, which is a function of the important selected factors.
Results
A total of 633 women who underwent breast biopsy were analyzed, which included 404 (63.8%) who had had a malignant breast biopsy and 229 (36.2%) who had had a benign breast biopsy. The mean age was 49.3±15.6 years, ranging 16–97 years. The majority (94.5%) of our sample had at least one abnormal imaging examination. presents the sample characteristics.
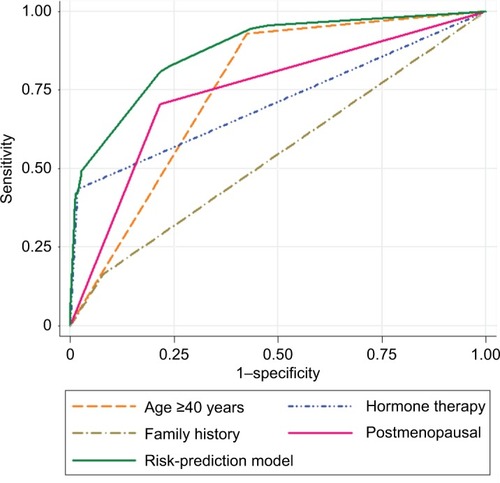
The unadjusted risk () of being diagnosed with breast cancer tended to increase with age ≥40 years, obesity, diabetes, hypertension, asthma, lung disease, hyperlipidemia, postmenopause, use of HRT, changes in size or skin of breast, family history of breast cancer, and history of genital disease (P≤0.05). ROC-curve analyses were used to quantify discriminatory accuracy of individual predictors for classifying the risk of breast cancer (). AUC ranged between 0.509 (abnormal diagnostic imaging) to 0.751 (age ≥40 years). Age ≥40 years, postmenopause, and use of menopausal hormone therapy showed higher discriminatory accuracy (AUC >0.7) compared with other individual predictors (). A prediction model that combines various patients’ information to increase the discriminatory accuracy for classifying the risk of breast cancer was used.
From a total of 14 predictive factors, four significant independent risk factors () were identified to predict high risk of breast cancer. These were age ≥40 years, HRT, postmenopause, and family history of breast cancer. We noted that women >40 years had higher odds of developing breast cancer (aOR 6.202, CI 3.497–11.001; P=0.001). Women who used menopausal hormone therapy had 24.365 times the odds of breast cancer than women who did not use menopausal hormone therapy (aOR 24.365, 95% CI 8.606–68.987; P=0.001). Women who had a family history of breast cancer were twice as likely to develop breast cancer as those who had no had family history of breast cancer (aOR 2.307, 95% CI 1.142–4.658, P=0.020). Postmenopausal women were associated with higher odds of developing breast cancer (aOR 3.058, 95% CI 1.861–5.024, P=0.001). According to the goodness-of-fit test, the model showed an acceptable fit (Hosmer–Lemeshow, P=0.053). The model showed substantial discriminatory accuracy for classifying the risk of breast cancer (AUC 0.877, 95% CI 0.851–0.903). In 100 bootstrap resamples, the overall accuracy of these samples was also high, with an AUC of 0.870 with mean optimism of 0.002 and SD 0.012. The corrected estimate of discriminatory accuracy was 0.877–0.012=0.865. From these results, a breast cancer risk calculator was developed (Supplementary material).
Table 4 Multivariate analysis: factors associated with increased breast cancer risk
A probability of 0.72 was defined as the optimal operating point to discriminate between high risk and low risk of breast cancer, with sensitivity, specificity, and Youden index of 80.7%, 78.5%, and 0.592, respectively ( and ).
Discussion
This study proposes a breast cancer prediction model based on a retrospective cohort of 633 symptomatic women who underwent breast biopsy at KAMC-RD, MNG-HA, Saudi Arabia. The predictive accuracy of breast cancer risk classification into high risk and low risk has not been previously reported in the Saudi population. The developed model integrates four risk factors: age ≥40 years, postmenopausal status, HRT, and family history of breast cancer. The discriminatory accuracy of each factor alone was: 0.751, 0.744, 0.707, and 0.541 (AUC), respectively. However, combining information on age, HRT, postmenopausal status, and family history of breast cancer increased the degree of discriminatory accuracy of breast cancer prediction (0.877) with optimism-corrected AUC of 0.865.
Age ≥40 years was associated with increased risk of breast cancer. This is comparable to several previous existing risk-prediction models, where older women are much more likely than younger women to be diagnosed with breast cancer.Citation7,Citation16–Citation20 Our findings are also in agreement with annual data reported by the Saudi Health Council, where the incidence of breast cancer was higher in women aged >40 years.Citation2,Citation3 Targeting and monitoring women aged >40 years may improve early detection,Citation7,Citation21 and improve the survival rate from breast cancer.Citation22
HRT use was the strongest predictor and associated with the highest risk of breast cancer. This association was in agreement with a number of earlier studies.Citation23–Citation26 Barlow et al reported that the model could be improved by adding the use of HRT.Citation23 The use and duration of hormone therapy must be justified and monitored, as it can be used as part of preventive health strategies or as a modifiable factor to reduce the risk of developing breast cancer.
In agreement with several earlier studies,Citation25–Citation29 postmenopausal women have a higher risk of breast cancer. The results also show agreement with previous models,Citation23,Citation27,Citation29 wherein family history of breast cancer was significantly associated with breast cancer. Breast cancer-screening strategies should target postmenopausal women and women with a family history of breast cancer to reduce risk. These study findings might become a prerequisite for a large prospective study, and then for the development of preventive health programs to reduce the high risk of breast cancer in these groups.
Strength and limitations
We identified several limitations in the present study. Association does not necessarily indicate causation. Due to the nature of the study design, some potential risk factors for breast cancer were not available to be included in the model (eg, breast density, age at menarche, age at first pregnancy, number of children). Although it was a large retrospective cohort, validation was performed internally, rather than in a separate prospective cohort. The results of this prediction model may be suitable only for symptomatic women who were treated at KAMC-RD, MNG-HA and underwent breast-mass biopsy.
Despite these limitations, it appears promising that combining information on demographic, clinical, and reproductive factors, ie, age, hormone use, postmenopausal status, and family history of breast cancer, produced substantial improvement in the discriminative ability of the model com pared to individual factors. The model confirmed findings in other published risk-prediction models and may contribute to clinical decision making on the diagnostic pathway and prompt a screening program.
Conclusion
The prediction model developed in this study had a high ability to predict increased breast cancer risk in our facility. Risk assessment was improved by combining information on age, menopausal hormone therapy, being postmenopausal, and family history of breast cancer. This model can be used in risk assessment for identifying high-risk groups and improving the early-diagnosis process. Our risk model may assist in initiating population screening programs and prompt clinical decision-making to manage cases and prevent unfavorable outcomes. External validation is required to assess the effectiveness of the model in a prospective cohort and then develop primary preventive strategies for breast cancer in Saudi Arabia.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Abbreviations
| MNG-HA | = | Ministry of National Guard-Health Affairs |
| KAMC-RD | = | King Abdulaziz Medical City, Riyadh |
| CI | = | confidence interval |
| ROC | = | receiver-operating characteristic |
| AUC | = | area under the ROC curve |
| MRI | = | magnetic resonance imaging |
| BMI | = | body-mass index |
Acknowledgments
The authors would like to thank King Abdullah International Medical Research Center, Riyadh, Saudi Arabia, for approving this study.
Supplementary material
Table S1 Breast cancer risk calculator
Table S2 Optimal operating point to discriminate between high risk and low risk of breast cancer
Disclosure
The authors report no conflicts of interest in this work.
References
- Al-RikabiAHusainSIncreasing prevalence of breast cancer among Saudi patients attending a tertiary referral hospital: a retrospective epidemiologic studyCroat Med J201253323924322661137
- Cancer Incidence Report Saudi Arabia2014Saudi Cancer Registry Available from: https://nhic.gov.sa/eServices/Documents/2014.pdfAccessed 18 February, 2018
- National Cancer Registry (Saudi Arabia)Saudi Arabia Cancer Incidence Report 2001Riyadh, Saudi ArabiaNational Cancer Registry (Saudi Arabia)2005 Available from: http://ghdx.healthdata.org/record/saudi-arabia-cancer-incidence-report-2001Accessed 18 February, 2018
- IbrahimEMZeeneldinAASadiqBBEzzatAAThe present and the future of breast cancer burden in the Kingdom of Saudi ArabiaMed Oncol200825438739318317955
- Al TamimiDMShawarbyMAAhmedAHassanAKAlodainiAAProtein expression profile and prevalence pattern of the molecular classes of breast cancer - a Saudi population based studyBMC Cancer201010122320492711
- BalabramDTurraCMGobbiHSurvival of patients with operable breast cancer (Stages I-III) at a Brazilian public hospital - a closer look into cause-specific mortalityBMC Cancer2013131123282137
- GailMHBrintonLAByarDPProjecting individualized probabilities of developing breast cancer for WHITE females who are being examined annuallyJ Natl Cancer Inst19898124187918862593165
- LeeSMParkJHParkHJImplications of systematic review for breast cancer predictionCancer Nurs2008315E40E46
- AnothaisintaweeTTeerawattananonYWiratkapunCKasamesupVThakkinstianARisk prediction models of breast cancer: a systematic review of model performancesBreast Cancer Res Treat2012133111022076477
- MeadsCAhmedIRileyRDA systematic review of breast cancer incidence risk prediction models with meta-analysis of their performanceBreast Cancer Res Treat2012132236537722037780
- AmemiyaYBacopulosSAl-ShawarbyMA comparative analysis of breast and ovarian cancer-related gene mutations in Canadian and Saudi Arabian patients with breast cancerAnticancer Res20153552601261025964535
- EzzatAAIbrahimEMRajaMAAl-SobhiSRostomAStuartRKLocally advanced breast cancer in Saudi Arabia: high frequency of stage III in a young populationMed Oncol19991629510310456657
- World Health OrganizationObesity: preventing and managing a global epidemicWHO Technical Report Series No. 894GenevaWorld Health Organization2000
- GreerWSandridgeALChehabeddineRSThe frequency distribution of age at natural menopause among Saudi Arabian womenMaturitas200346426327214625123
- AldughaitherAAlmutairyHAlateeqMMenopausal symptoms and quality of life among Saudi women visiting primary care clinics in Riyadh, Saudi ArabiaInt J Womens Health2015764526170720
- CostantinoJPGailMHPeeDValidation studies for models projecting the risk of invasive and total breast cancer incidenceJ Natl Cancer Inst199991181541154810491430
- AndersonSJAhnnSDuffKNSABP Breast Cancer Prevention Trial risk assessment program, version 2NSABP Biostatistical Center Technical Report814199212
- DiteGSMahmoodiMBickerstaffeAUsing SNP genotypes to improve the discrimination of a simple breast cancer risk prediction modelBreast Cancer Res Treat2013139388789623774992
- ChayWYOngWSTanPHValidation of the Gail model for predicting individual breast cancer risk in a prospective nationwide study of 28,104 Singapore womenBreast Cancer Res2012141R1922289271
- BreyerJZWendlandEMKopsNLCaleffiMHammesLSAssessment of potential risk factors for breast cancer in a population in southern BrazilBreast Cancer Res Treat2018169112513129344741
- PitmanJAMcgintyGBSomanRRDrotmanMBReichmanMBArleoEKScreening mammography for women in their 40S: the potential impact of the American cancer Society and U.S. Preventive Services Task Force breast cancer screening recommendationsAJR Am J Roentgenol2017209369770228504572
- FonteinDBde GlasNADuijmMAge and the effect of physical activity on breast cancer survival: a systematic reviewCancer Treat Rev201339895896523608116
- BarlowWEWhiteEBallard-BarbashRProspective breast cancer risk prediction model for women undergoing screening mammographyJ Natl Cancer Inst200698171204121416954473
- GreenLEDinhTASmithRAAn estrogen model: the relationship between body mass index, menopausal status, estrogen replacement therapy, and breast cancer riskComput Math Methods Med2012201218
- WangFDaiJLiMRisk assessment model for invasive breast cancer in Hong Kong womenMedicine20169532e451527512870
- Leon GuerreroRTNovotnyRWilkensLRRisk factors for breast cancer in the breast cancer risk model study of Guam and SaipanCancer Epidemiol201750Pt B22123329120829
- ErikssonMCzeneKPawitanYLeiflandKDarabiHHallPA clinical model for identifying the short-term risk of breast cancerBreast Cancer Res20171912928288659
- CrookePSJustenhovenCBrauchHEstrogen metabolism and exposure in a genotypic-phenotypic model for breast cancer risk predictionCancer Epidemiol Biomarkers Prev20112071502151521610218
- ParkBMaSHShinAKorean risk assessment model for breast cancer risk predictionPLoS One2013810e7673624204664