Abstract
Purpose
This study was designed to evaluate the prognostic value of the combination of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (neutrophil/platelet-to-lymphocyte ratio [NLR–PLR]) in patients with hepatocellular carcinoma (HCC) who receive transarterial chemoembolization (TACE) therapy.
Patients and methods
Data from 216 patients who were diagnosed with HCC after TACE therapy were retrospectively collected. R software was used to analyze the time-dependent receiver operating characteristic (ROC) curves and to compare the area under the ROC curves (AUROCs).
Results
The long-term survival rates were significantly higher for patients with lower values than those with higher values of NLR, PLR, and NLR–PLR. The mean overall survival decreased gradually with increases in the NLR–PLR score (P<0.0001). The AUROC values of the NLR–PLR score were consistently higher than those of NLR and PLR.
Conclusion
This study showed that the NLR–PLR score might be a useful predictor for patients with HCC who receive TACE therapy.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies and is the third most lethal of all human cancers.Citation1 Curative approaches, such as hepatic resection, liver transplantation, and radio-frequency ablation, are the recommended treatment modalities for early HCC.Citation2–Citation4 However, the majority of patients with HCC is diagnosed at an intermediate to advanced stages. Transarterial chemo-embolization (TACE) and sorafenib are recommended for these patients, respectively, according to the Barcelona Clinic Liver Cancer (BCLC) staging classification, adopted by the American Association for the Study of Liver Disease and European Association for the Study of the Liver guidelines.Citation5,Citation6 Moreover, it was shown that the efficacy of sorafenib treatment was limited and costly.Citation7 Therefore, rather than sorafenib, TACE was the most frequent first-line treatment for patients with BCLC stage C disease in real-life clinical practice for the management of HCC.Citation8 In this study, TACE, which delivers chemotherapeutic drugs to the tumor while blocking tumor-feeding arteries, is used for patients with intermediate to advanced stages HCC.Citation9,Citation10
Several reports have highlighted prognostic indicators for patients with HCC, including serum α-fetoprotein (AFP), tumor size, vascular invasion, and extrahepatic spread.Citation11 However, the heterogeneity of the study populations led to variation. What is more, these indicators may not be specific risk factors for HCC patients who receive TACE therapy, which would indicate whether a given patient could benefit from this treatment and stratify patients according to their risk levels. Thus, the search for an effective biomarker that can be used to precisely predict the disease prognosis and to inform an optimal personalized treatment strategy is necessary and urgent to improve clinical outcomes.
In 1863, the close relationship between inflammation and malignancy was first described by Virchow, who observed the presence of leukocytes in tumor tissue;Citation12,Citation13 since this discovery, accumulated evidence has indicated that the presence of a systemic inflammatory response and malnutrition is associated with a poor prognosis for various malignancies.Citation14,Citation15 Moreover, the prognostic value of the systemic inflammatory response in patients with various cancers can be reflected by the combination of hematological components. The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have been widely used as evaluation tools for the inflammatory and immune responses and are reportedly prognostic factors in various malignancies.Citation16–Citation18 Furthermore, higher values of NLR and PLR are associated with unfavorable clinical features in patients with HCC.Citation19–Citation22 However, few reports have addressed the relationship between the combination of NLR and PLR and the prognosis of patients with HCC who received TACE therapy. In this study, we aimed to elucidate the prognostic impact of the combination of NLR and PLR for patients with HCC who received TACE therapy.
Patients and methods
Patients
Consecutive patients who were newly diagnosed with HCC after TACE therapy performed between January 2007 and July 2015 at the Department of Hepatobiliary and Pancreatic Surgery of Sun Yat-sen University Cancer Center were enrolled in this study. All patients with HCC included in this study were categorized as BCLC stage B and C in this study. The criteria for inclusion were as follows: 1) treatment with TACE as the initial therapy; 2) three courses of TACE treatment; and 3) Child-Pugh A or B liver function. Exclusion criteria included the following: 1) treatments such as hepatic resection, liver transplantation, radio-frequency treatment, or other HCC treatments before or after TACE; 2) Child-Pugh C liver function; 3) inadequate renal function (serum creatinine and serum blood urea nitrogen > the upper limit of normal); 4) obstructive jaundice; 5) diagnosis of second tumors; or 6) lost to follow-up. This study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Clinical data collection
All clinical and radiological data were retrieved from medical records. Hematologic examination and imaging examinations, including AFP, liver ultrasonography, contrast-enhanced dynamic computed tomography (CT), magnetic resonance imaging (MRI), and hepatic arterial angiography, were used in the clinical diagnosis. The diagnosis of HCC was established on the typical features of HCC identified by two radiological images or one radiological image combined with the elevated AFP level (≥400 ng/mL) or pathologic evidence.Citation23
Clinical and radiological parameters, including age, gender, white blood cell count, platelet (PLT) count, neutrophil cell count, lymphocyte cell count, PLT count, AFP, alanine transaminase, aspartate aminotransferase (AST), total bilirubin, ALP, gamma-glutamyl transpeptidase (GGT), albumin (ALB), C-reactive protein (CRP), hepatitis B surface antigen (HBsAg), NLR, PLR, hepatitis B virus DNA load, tumor diameter, tumor number, vascular invasion, HBV infection, performance status, BCLC stage, and Cancer of the Liver Italian Program (CLIP) score were measured and analyzed. Clinical and radiological data were retrieved at the time of diagnosis before the initial TACE was initiated. Vascular invasion was defined as the presence of thrombus adjacent to the tumor in the portal system or the hepatic vein system with a vague boundary that was confirmed by at least two imaging modalities in this study.Citation24 Apart from NLR and PLR, several inflammation-based factors have also been explored, including the combination of CRP and white cell count as the prognostic index (PI),Citation25 a combination of serum CRP and ALB in the modified Glasgow Prognostic Score (mGPS),Citation26 and a combination of ALB and lymphocyte count in the prognostic nutritional index (PNI).Citation21
Treatment procedure
Three courses of uniform treatment protocols were performed for each patient. The Seldinger technique was used, as previously reported.Citation27 Three hundred milligrams of carboplatin (Bristol-Myers Squibb, New York, NY, USA), 50 mg of epirubicin (Pharmorubicin, Pfizer, Wuxi, Jiangsu, P. R. China), and 6 mg of mitomycin (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, Zhejiang, P. R. China) were used in conventional chemoembolization. The delivered dose of Lipiodol (Lipiodol Ultra-Fluide; Andre Guerbet Laboratories, France) was determined in accordance with the location, size, and number of tumors, ranging from 5 to 30 mL.
Follow-up
Patients were followed up at least once every 2 months during the first years and once every 3 months thereafter. The AFP test, liver ultrasonography, CT, and MRI were selectively performed as needed. Overall survival (OS) was defined as the duration (in days) from the date of the first TACE until death or last follow-up in this study. The median follow-up period was 431.11 days.
Statistical analysis
Statistical analysis was performed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). Continuous data are expressed as the means and ranges, and categorical data are shown as frequencies and proportions. Student’s t-test was used to compare continuous variables. The chi-squared test and Fisher’s exact test were used to compare the categorical variables.
Univariate and multivariate analyses were performed to assess the significance of the differences in the clinical or radiological data. The associated 95% CI was calculated. The Kaplan–Meier method was used to analyze OS. Significant differences between the groups were identified using the log-rank test. The survival curves were performed using MedCalc software version 11.4.2.0 (http://www.medcalc.be). A two tailed P-value <0.05 was considered statistically significant.
NLR or PLR was estimated by dividing an absolute neutrophil or PLT count by an absolute lymphocyte count which were obtained within 1 week before the initial TACE. Time-dependent receiver operating characteristic (ROC) curves were performed for the assessability of both NLR and PLR to predict long-term survival and to determine the optimal cutoff value for these two variables.Citation28,Citation29 The analyses of ROC curves and comparisons of the areas under the ROC curves (AUROCs) were performed using R software version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org) with the “survival ROC” package and the “survival ROC.C” package.
Results
Optimal cutoff values for the variables
The NLR and PLR were calculated by dividing the neutrophil or PLT counts by the lymphocyte counts. The mean values of the neutrophil, PLT, and lymphocyte counts were 4.26×109/L, 194.37×109/L, and 1.61×109/L, respectively. The analysis of the time-dependent ROC was used to decide the optimal cutoff values for the NLR and PLR, which were 1.77 and 94.62, respectively, along with the strongest Youden index for the OS prediction. The threshold for each clinical and radiological dataset was utilized as the cutoff values for these variables.
Patient characteristics
A total of 216 patients who were newly diagnosed with HCC and had received TACE as the initial therapy were included in this study. There were 200 male (92.6%) and 16 female (7.4%) patients with a median age of 53 years in the whole study cohort. Enlarged tumors and multiple tumors were common in this study. Additionally, 30.3% of the patients had vascular invasion, and 5.5% of the patients were sorted into the metastatic tumor group. Hepatitis B infection was the most common cause of HCC, and HBsAg was positive in ~97.2% of the enrolled patients. Most patients had good reserve liver function with Child-Pugh A and good performance status, with scores of 0–1 (). Elevated scores of neutrophil/platelet-to-lymphocyte ratio (NLR–PLR) were more frequently observed in HCC patients of BCLC stage C or high values of CLIP scores, while no differences in values of NLR–PLR were observed in relation to BCLC stages or CLIP scores (P>0.05).
Table 1 Relationship between clinic and radiological factors and NLR–PLR score
NLR and PLR were divided into two groups: <1.77 and ≥1.77 for NLR and <94.62 and ≥94.62 for PLR. Among the 216 patients, an elevated NLR or PLR was observed in 167 (77.3%) patients or 141 (65.3%) patients, respectively. When NLR was combined with PLR, combined NLR–PLR was generated. According to NLR–PLR, patients were assigned different scores: patients with NLR <1.77 and PLR <94.62 were assigned a score of 1; patients with NLR <1.77 and PLR ≥94.62 or NLR ≥1.77 and PLR <94.62 were assigned a score of 2; and patients with NLR ≥1.77 and PLR ≥94.62 were assigned a score of 3. It was shown that a higher NLR–PLR score was associated with elevated CRP (P<0.001), elevated ALP (P=0.021), and a larger tumor size (P<0.001; ). In addition, the correlations of inflammation parameters were also conducted. As shown in , there were significantly positive correlations between NLR–PLR and NLR, PLR, PI, mGPS, and CRP. In contrast, a significantly negative correlation was also observed between NLR–PLR and PNI (P<0.05, ).
Table 2 Correlations of inflammation parameters for HCC patients after TACE therapy
OS and prognostic factors
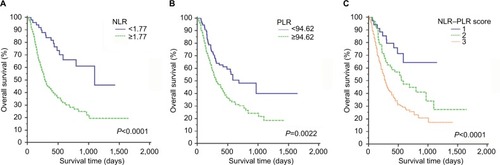
The estimated 1-, 2-, and 3-year OS rates for all patients were 61.3%, 44.2%, and 40.5%, respectively. The median OS was 17 months. The long-term survival rates were significantly higher for patients with lower values of NLR than for patients with higher values of NLR (P<0.0001, ). Patients with PLR <94.62 also had better long-term survival than patients with PLR ≥94.62 (P=0.0022, ). The mean OS decreased gradually along with increasing NLR–PLR. Moreover, the long-term survival rates of patients were significantly stratified by NLR–PLR scores. The 3-year OS rates of patients with NLR–PLR scores of 1, 2, and 3 were 64.3%, 27.3%, and 17.2%, respectively (P<0.0001, ).
Figure 1 Kaplan–Meier curves for OS in patients who were diagnosed with HCC after TACE therapy; patients were stratified according to the inflammation-based prognostic scores.
Note: (A) NLR; (B) PLR; and (C) NLR–PLR.
Abbreviations: HCC, hepatocellular carcinoma; NLR, neutrophil-to-lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; TACE, transarterial chemoembolization.
In the univariate survival analysis, the NLR–PLR score (HR, 1.85; 95% CI, 1.41–2.42; P<0.001) was significantly associated with OS. Other significant prognostic parameters included the CRP, AST, ALB, ALP, GGT, AFP, tumor diameter, and vascular invasion (). After the multivariate Cox proportional hazards analysis, we found that the pretreatment NLR–PLR score (HR, 1.33; 95% CI, 1.00–1.77; P=0.045), the pretreatment AFP levels (HR, 1.68; 95% CI, 1.18–2.40; P=0.004), the tumor diameter (HR, 6.77; 95% CI, 2.43–18.44; P<0.001), and vascular invasion (HR, 2.03; 95% CI, 1.43–2.88; P<0.001) were independent predictors of OS ().
Table 3 Univariate and multivariate analyses of OS in the study cohort
Prognostic value of the NLR–PLR score
Additionally, the prognostic capacities of the NLR, PLR, and NLR–PLR scores were compared by analyzing the AUROC values to show whether the NLR–PLR score was at least equivalent or superior to the NLR or PLR scores. The ROC curves for OS prediction were calculated for the patients at 1, 2, and 3 years of follow-up (). As shown in , the AUROC values of the NLR–PLR score were consistently higher than those of NLR and PLR. The NLR–PLR score showed a better distinguishing power for predicting the prognosis of patients with HCC who were treated with TACE compared with NLR or PLR alone. The NLR–PLR score divided patients into subgroups more precisely.
Figure 2 Comparisons of the AUROC values for OS stratified by the inflammation-based prognostic scores at 1-year (A), 2-year (B), and 3-year (C).
Abbreviations: AUROC, area under the ROC curves; NLR, neutrophil-to-lymphocyte ratio; NLR–PLR, neutrophil/platelet-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival.
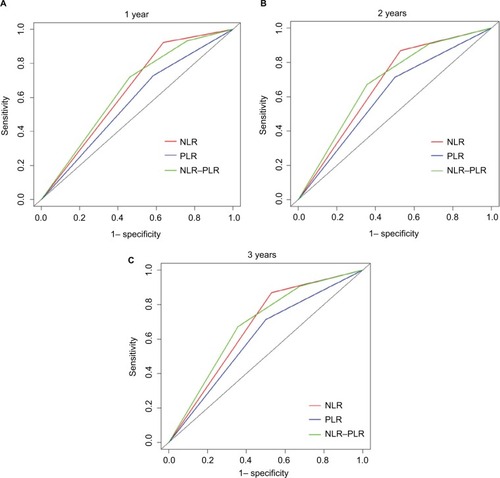
Table 4 Comparison of the AUROC values among the NLR, PLR, and NLR–PLR scores
Discussion
This study demonstrated that increased pretreatment NLR, PLR, and NLR–PLR scores predicted an unfavorable prognosis for OS in patients who were diagnosed with HCC and had received TACE therapy. Additionally, similar to previous studies,Citation21,Citation30,Citation31 the NLR–PLR score had superior discriminative capacity for predicting prognosis compared with NLR or PLR.
Accumulated evidence shows that the systemic inflammatory response is associated with poor prognosis in multiple types of cancers.Citation32–Citation34 Several epidemiological studies have suggested that chronic inflammation may cause various malignancies.Citation33 Moreover, HCC not only develops at sites of inflammation caused by the hepatitis B virus infectionCitation35,Citation36 but also triggers regional inflammatory responses, which release inflammatory cytokines that promote the formation of an inflammatory microenvironment around the tumor.Citation32,Citation37,Citation38 Multiple reportsCitation39–Citation41 have argued that the chemicals and embolization agent in TACE can stimulate lymphocytes to become active. Furthermore, TACE treatment can sensitize p53-negative HCC cells to the cytotoxic effects of cisplatin and can result in increased HCC cell death.Citation42 Synergistic cytotoxic effects in vitro and significant inhibition of tumor growth in vivo were observed in preclinical HCC models when TACE was administered.Citation21,Citation43 Peripheral inflammatory cells (ie, lymphocytes, neutrophils, and PLTs) were associated with the progression and prognosis of various types of cancer. Additionally, the NLR, PLR, and NLR–PLR scores, which represent the systematic inflammatory response, have potential value as prognostic factors for patients with HCC who received TACE therapy.
Inflammatory processes in the tumor microenvironment play important roles in tumor cell proliferation and cancer prognosis. The growth and survival factors that are released from inflammatory cells can stimulate tumor formation, progression, angiogenesis, invasion, and metastasis.Citation44,Citation45 The paradoxical roles of lymphocyte immune cells and circulating neutrophils in the inflammatory processes act as crucial opposing regulators in cancer occurrence.Citation46 Neutrophils, which are a major component of the leukocyte population, produce pro-angiogenic factors, including vascular endothelial growth factors (VEGFs), to stimulate tumor development and progression.Citation32 This phenomenon is partly explained by the upregulation of cyclooxygenase-2 or the suppression of an antitumor adaptive immune response.Citation47–Citation49 Similarly, an elevated PLT level can increase the production of VEGF, increasing angiogenesis and promoting tumor growth.Citation50 However, lymphocytes are important components in cancer immune surveillance and can suppress tumor maturation.Citation51 A decreased lymphocyte count is associated with an insufficient immunologic reaction to the tumor, which consequently enables tumor progression and metastasis.Citation52 Additionally, several studies have suggested that adaptive immune cells, such as B-lymphocytes, CD8+cytotoxic T-lymphocytes, and CD4+ helper T-lymphocytes, play extremely important roles in the modulation of cancer development through tumor cell lysis.Citation46,Citation53
The NLR and PLR values are determined from the neutrophil and PLT counts, respectively, relative to the lymphocyte count, and these measurements may reflect inflammation and the immune response during tumor formation. An elevated NLR or PLR may reflect the presence of a chronic systemic inflammatory response. Patients with high values of NLR or PLR are relatively lymphocytopenic resulting in a poorer lymphocyte-mediated immune response to malignancy and therefore an increased potential for tumor recurrence and reduced survival. Numerous reports have demonstrated that NLR and PLR are useful for predicting the prognosis of various types of tumor.Citation30 Moreover, cross-sectional studies have revealed that an elevated NLR or PLR is closely associated with poor survival,Citation14,Citation20 which is consistent with the findings of our study.
In addition, we found that an elevated NLR–PLR score was associated with increased tumor size, which showed that the inflammation-based prognostic score paralleled tumor progression. Additionally, an elevated AFP level, an enlarged tumor, and vascular invasion were also associated with poor OS by multivariate analysis in our study, which is consistent with previous studies.Citation54 Apart from AFP, tumor size, and vascular invasion, the NLR–PLR score was identified as an independent indicator for OS. Moreover, significantly associations were shown between NLR–PLR and other inflammation-based factors, including PI, mGPS and PNI,Citation21,Citation25,Citation26 which were associated with indexes, such as ALB, CRP, and ALP.Citation55–Citation57 It was also indicated that an elevated NLR–PLR score was also associated with elevated CRP and elevated ALP in this study. However, CRP and ALP were both excluded after the multivariate analysis, indicating that the NLR–PLR score was a more powerful predictor than CRP and ALP and was representative as an inflammation-based prognostic factor in patients with HCC, compared with other inflammation-based factors. There were some previous studies which focused on inflammation-based factors,Citation58,Citation59 such as NLR and PLR in HCC patients after TACE therapy, while they only discussed the prognostic effect of a single factor. Similarly, in our study, the Kaplan–Meier analysis showed that elevated NLR or PLR was associated with poor OS, these parameters were suboptimal for predicting OS. The NLR–PLR score divided the patients into subgroups more accurately in Kaplan–Meier survival analysis. Compared with values of AUROC in similar studies, a higher value for NLR–PLR scores indicated that the combined effect was greater than the individual effect of either variable alone. Another strength of our study was that it comprised a patients group whose management and follow-up were relatively homogenous. Excluding patients who received other treatments was important in removing potential confounders. Although a body of evidence existed suggesting that a high NLR or PLR was associated with adverse prognoses of patients with HCC, to date no reports focused on the combination of NLR and PLR targeting specific therapies in patients with HCC. The combined results of the AUROC and Kaplan–Meier survival curves strongly support the prognostic value of the NLR–PLR score in patients with HCC after TACE therapy for the first time. Further research is needed to evaluate the value of NLR–PLR in other patients groups with HCC and attempt independent validation of NLR–PLR for patient risk stratification.
Study limitations
This study has several limitations. First, this retrospective analysis relied on a single institutional dataset of HCC patients who were treated with TACE and there was no external validation for our study. The geographic and institutional heterogeneity that existed among these patients might affect the results of the study. Second, there might be other reasonable cutoff values for variables from other studies. Therefore, a large-scale prospective validation study is needed to confirm these results.
Conclusion
The predictive powers of NLR, PLR, and NLR–PLR were compared in patients with HCC after TACE therapy in this study. To the best of our knowledge, this study is the first to reveal that the NLR–PLR score can predict the prognosis of HCC for patients who receive TACE therapy. The NLR–PLR score is a routine parameter that is easily available and well standardized, and an elevated NLR–PLR score might correlate with a more aggressive disease phenotype. Thus, the NLR–PLR score might provide evidence for individualized treatment.
Acknowledgments
This work was supported by grants from School of Sociology and Anthropology-Sun Yat-sen University Cancer Center Joint Foundation on Medical Humanities (No 201804). We acknowledge the Medical Records Department for collecting the survival data of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- FornerALlovetJMBruixJHepatocellular carcinomaLancet201237998221245125522353262
- RazaASoodGKHepatocellular carcinoma review: current treatment, and evidence-based medicineWorld J Gastroenterol201420154115412724764650
- ZhangJZhouZGHuangZXProspective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinomaChin J Cancer20163512526956022
- BruixJShermanMPractice Guidelines Committee, American Association for the Study of Liver DiseasesManagement of hepatocellular carcinomaHepatology20054251208123616250051
- European Association for the Study of the Liver, European Organisation for Research and Treatment of CancerEASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaJ Hepatol201256490894322424438
- PalmerDHSorafenib in advanced hepatocellular carcinomaN Engl J Med20083592324982499
- ParkJWChenMColomboMGlobal patterns of hepatocellular carcinoma management from diagnosis to death: the bridge studyLiver Int20153592155216625752327
- LlovetJMBruixJSystematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survivalHepatology200337242944212540794
- BruixJShermanMAmerican Association for the Study of Liver DiseasesManagement of hepatocellular carcinoma: an updateHepatology20115331020102221374666
- LlovetJMBurroughsABruixJHepatocellular carcinomaLancet200336293991907191714667750
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- FerreriAJMIllerhausGZuccaECavalliFInternational Extranodal Lymphoma Study GroupFlows and flaws in primary central nervous system lymphomaNat Rev Clin Oncol20107812
- TempletonAJKnoxJJLinXChange in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacyEur Urol201670235836426924770
- TempletonAJMcNamaraMGŠerugaBPrognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysisJ Natl Cancer Inst20141066dju12424875653
- DengMMaXLiangXZhuCWangMAre pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer?Oncotarget2017823372003720728380461
- RossiSBassoMStrippoliAAre markers of systemic inflammation good prognostic indicators in colorectal cancer?Clin Colorectal Cancer201716426427428412137
- SongWTianCWangKZhangRJZouSBPreoperative platelet lymphocyte ratio as independent predictors of prognosis in pancreatic cancer: a systematic review and meta-analysisPLoS One2017126e017876228575033
- ZhaoYSiGZhuFPrognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysisOncotarget2017814228542286228206965
- DongLBaiKCaoYHuangQLvLJiangYPrognostic value of pre-operative platelet to lymphocyte ratio in patients with resected primary hepatocellular carcinomaClin Lab201662112191219628164677
- HeCBLinXJInflammation scores predict the survival of patients with hepatocellular carcinoma who were treated with transarterial chemoembolization and recombinant human type-5 adenovirus H101PLoS One2017123e017476928355305
- YangTZhuJZhaoLLymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resectionJ Surg Oncol2017115671872828127774
- BruixJShermanMLlovetJMClinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the LiverJ Hepatol200135342143011592607
- LeeYHHsuCYHuangYHVascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impactJ Clin Gastroenterol201448873474124100755
- WangDSLuoHYQiuMZComparison of the prognostic values of various inflammation based factors in patients with pancreatic cancerMed Oncol20122953092310022476808
- ProctorMJMorrisonDSTalwarDAn inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome studyBr J Cancer2011104472673421266974
- LuoJGuoRPLaiECTransarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative studyAnn Surg Oncol201118241342020839057
- GalunDBogdanovicADjokic KovacJBulajicPLoncarZZuvelaMPreoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative-intent surgery for hepatocellular carcinoma: experience from a developing countryCancer Manag Res20181097798829765248
- FlussRFaraggiDReiserBEstimation of the Youden index and its associated cutoff pointBiom J200547445847216161804
- PedrazzaniCMantovaniGFernandesEAssessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancerSci Rep201771149428473700
- KitanoYYamashitaYIYamamuraKEffects of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios on survival in patients with extrahepatic cholangiocarcinomaAnticancer Res20173763229323728551669
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- Bard-ChapeauEANguyenATRustAGTransposon mutagenesis identifies genes driving hepatocellular carcinoma in a chronic hepatitis B mouse modelNat Genet2014461243224316982
- JiangDKSunJCaoGGenetic variants in Stat4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinomaNat Genet2013451727523242368
- EndigJBuitrago-MolinaLEMarhenkeSDual role of the adaptive immune system in liver injury and hepatocellular carcinoma developmentCancer Cell201630230832327478039
- MohsAKuttkatNReißingJFunctional role of CCL5/RAN-TES for HCC progression during chronic liver diseaseJ Hepatol201766474375328011329
- WangYZhengCLiangBHepatocellular necrosis, apoptosis, and proliferation after transcatheter arterial embolization or chemo-embolization in a standardized rabbit modelJ Vasc Interv Radiol201122111606161221959058
- ChaoYWuCYKuoCYCytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolizationHepatol Int20137388389226201926
- McNallyMEMartinezAKhabiriHInflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolizationAnn Surg Oncol201320392392822965570
- SiebenMHerzerKZeidlerMKilling of p53-deficient hepatoma cells by parvovirus H-1 and chemotherapeutics requires promyelocytic leukemia proteinWorld J Gastroenterol200814243819382818609705
- LiYYuDCChenYA hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicinCancer Res200161176428643611522637
- de VisserKEEichtenACoussensLMParadoxical roles of the immune system during cancer developmentNat Rev Cancer200661243716397525
- JaiswalMLarussoNFBurgartLJGoresGJInflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanismCancer Res200060118419010646872
- IshigamiSNatsugoeSTokudaKPrognostic value of intratumoral natural killer cells in gastric carcinomaCancer200088357758310649250
- DannenbergAJSubbaramaiahKTargeting cyclooxygenase-2 in human neoplasia: rationale and promiseCancer Cell20034643143614706335
- SchoppmannSFBirnerPStöcklJTumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesisAm J Pathol2002161394795612213723
- LiuCHChangSHNarkoKOverexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic miceJ Biol Chem200127621185631856911278747
- WiesnerTBuglSMayerFHartmannJTKoppHGDifferential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancerClin Exp Metastasis201027314114920182908
- DunnGPOldLJSchreiberRDThe immunobiology of cancer immunosurveillance and immunoeditingImmunity200421213714815308095
- StotzMPichlerMAbsengerGThe preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancerBr J Cancer2014110243544024357796
- ZouWImmunosuppressive networks in the tumour environment and their therapeutic relevanceNat Rev Cancer20055426327415776005
- ZhangNGuJYinLIncorporation of alpha-fetoprotein (AFP) into subclassification of BCLC C stage hepatocellular carcinoma according to a 5-year survival analysis based on the SEER databaseOncotarget2016749813898140127835609
- LiZXueTQChenXYPredictive values of serum VEGF and CRP levels combined with contrast enhanced MRI in hepatocellular carcinoma patients after TACEAm J Cancer Res20166102375238527822426
- WuSJLinYXYeHXiongXZLiFYChengNSPrognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resectionInt J Surg201636Pt A14315127793641
- RekikSGuyotEBhaisMThe CRP level and state score predict survival in cirrhotic patients with hepatocellular carcinoma treated by transarterial embolizationDig Liver Dis20164891088109227375209
- LiuCJiaBSZouBWNeutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolizationMedicine20179645e851229137051
- TianXCLiuXLZengFRChenZWuDHPlatelet-to-lymphocyte ratio acts as an independent risk factor for patients with hepatitis B virus-related hepatocellular carcinoma who received transarterial chemoembolizationEur Rev Med Pharmacol Sci201620112302230927338055
