Abstract
Purpose
O-linked N-acetylglucosamine (O-GlcNAc or O-GlcNAcylation) is a post-translational modification, which plays a vital role in the progression of various cancers. The purpose of the present study was to assess O-GlcNAcylation in human renal cell carcinoma (RCC).
Methods
O-GlcNAcylation levels and O-GlcNAc-transferase (OGT) expression in human RCC cell lines and 10 paired clinical tissues were detected by Western blot and Immunohistochemis-try. Then, the effects of O-GlcNAcylation on RCC cell proliferation in vitro were investigated by Cell Counting Kit-8 assay. A xenograft assay was performed to assess the in vivo effects of OGT knockdown in RCC cells. Cell apoptosis and cell cycle assays were performed by flow cytometry. Co-immunoprecipitation assays were used to assess epidermal growth factor receptor (EGFR) O-GlcNAcylation and the interaction between OGT and EGFR.
Results
O-GlcNAcylation levels and OGT expression were increased in RCC, and the high amounts correlated with poor patient prognosis. OGT knockdown significantly suppressed RCC cell proliferation in vitro and in vivo. Notably, EGFR was modulated by O-GlcNAcylation and directly interacted with OGT.
Conclusion
These findings provide novel insights into the oncogenic roles of O-GlcNAcylation and OGT in the development of RCC, indicating that OGT might be used as a target for RCC therapy in the future.
Introduction
In 2018, it was expected that about 63,340 patients would be newly diagnosed with renal cell carcinoma (RCC) in the US, which is among the most lethal human genitourinary cancers.Citation1,Citation2 However, about 30% of RCC cases have metastases at initial diagnosis.Citation3 Due to its resistance to chemotherapy and radiotherapy, targeted therapy remains the first-line treatment option for these advanced RCC patients.Citation4 Most RCC cases show a notable clinical response; however, the treatment effects of targeted agents are limited due to the development of drug-resistant phenotypes.Citation5 Hence, it is necessary to further assess the mechanisms involved in RCC and improve diagnosis and therapy strategies for this malignancy.
O-linked N-acetylglucosamine (O-GlcNAc or O-GlcNAcylation) is a post-translational modification, which is considered a new cancer hallmark based on multiple studies in the past decade.Citation6 O-GlcNAc synthesis is catalyzed by O-GlcNAc-transferase (OGT) while the group is removed by O-GlcNAcase (OGA).Citation7 As the substrate of O-GlcNAc, UDP-GlcNAc is synthesized from glycolytic metabolites through the hexosamine biosynthetic pathway (HBP) and add GlcNAc to serine or threonine residues of target proteins.Citation8–Citation10 Numerous biological processes are influenced by O-GlcNAcylation, such as cell cycle progression, signal transduction, and metabolism.Citation11 Being dynamic and reversible, aberrant O-GlcNAc modulation is involved in the formation and progression of many diseases, especially carcinogenesis.Citation8 The biological effects of O-GlcNAc in cancer development are mostly via O-GlcNAcylation of proteins such as p53 and β-catenin.Citation12,Citation13 For example, the oncogene c-Myc is O-GlcNAcylated, which could inhibit its phosphorylation and consequently suppress proteasome mediated degradation.Citation14 Increased levels of O-GlcNAcylation have been reported in various cancers, including prostate cancer,Citation15 colon cancer,Citation16 liver carcinoma,Citation17 breast cancer,Citation14 and leukemia.Citation18,Citation19 However, the potential roles of O-GlcNAc in renal cancer remain undefined.
The number of proteins containing the O-GlcNAc modification has steadily increased over the past years. As a receptor tyrosine kinase (RTK), the epidermal growth factor receptor (EGFR) is always overexpressed in various cancers thus contributing to carcinogenesis by improving the invasive potential and metastatic behavior.Citation20 EGFR is indeed overexpressed in RCC and is associated with cell cycle and tumorigenesis.Citation21–Citation24 Overexpression of EGFR in RCC could also lead to the upregulation of VEGF, which is involved in the angiogenic phenotype.Citation25 Therefore, upregulated EGFR is considered an available molecular target for RCC therapy.Citation26–Citation28
The present study aimed to assess the expression and function of OGT as well as O-GlcNAcylation levels in RCC. Moreover, the potential mechanisms were explored.
Materials and methods
Antibodies and reagents
O-GlcNAc specific antibodies (RL2) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against OGT and other primary antibodies were purchased from Abcam (Cambridge, MA, USA).
Cell culture and lentiviral shRNA infection
The human normal renal tubular epithelial HK-2 and RCC 786-O, A498, ACHN, and CAKI-2 cell lines were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CCCAS, Shanghai, China). 786-O cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA). A498, ACHN, and CAKI-2 cells were cultured in RMPI 1640 (Thermo Fisher Scientific), while HK-2 cells were cultured in Keratinocyte Medium (KM, ScienCell, Carlsbad, CA, USA). All cell culture media were supplemented with 10% Fetal Bovine Serum (FBS, Hyclone, Logan, UT, USA), and cells were cultured at 37°C with 5% CO2.
The lentivirus expressing shRNA against OGT was produced by Jiman Co. (Shanghai, China). 786-O and A498 cells were infected with LV-sh-OGT or LV-sh-NC and selected by puromycin (Sigma–Aldrich, St. Louis, MO, USA). The expression levels of OGT and O-GlcNAc were examined at the RNA and protein levels.
Immunohistochemical staining
All tissues were formalin-fixed and embedded in paraffin blocks. Then, 4 µm sections were dewaxed in xylene and rehydrated in graded ethanol. Antigen retrieval was performed in pre-heated Tris-EDTA buffer for 20 minutes. Methanol containing 0.3% H2O2 was used to block endogenous peroxidase activity for 8 minutes. Next, bovine serum albumin (BSA) was used to block the sections for 30 minutes before incubation with the primary antibody overnight at 4°C. The sections were then incubated with horseradish peroxidase (HRP)-conjugated rabbit-anti-mouse IgG for 30 minutes. This was followed by treatment with the DAB+ EnVision System (Thermo Fisher Scientific) and counterstaining with Mayer/hematoxylin.
Cell proliferation in vitro
The shRNA sequence of OGT is shown in . The proliferation ability of renal cancer cells was evaluated by Cell Counting Kit-8 (CCK8) assay (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, 786-O and A498 cells were transfected with sh-NC or sh-OGT for 48 hours and seeded into 96-well plates at a density of 5×103 per well. After incubation for different times (0 hour, 24 hours, 48 hours, 72 hours, and 96 hours), 10 µL CCK-8 reagent was added to each well and further incubated for 4 hours. Finally, cell proliferation was determined by measuring absorbance at 450 nm on a plate reader.
Table 1 The shRNA sequence of OGT
Xenograft assays in nude mice
Stably transfected 786-O (LV-sh-OGT and LV-sh-NC) cells (3×106 in 0.2 mL PBS) were implanted subcutaneously into the dorsal flank of male BALB/c nude mice (6 weeks old). The tumorigenic potential of cells was evaluated 3 weeks after inoculation. The mice were sacrificed by pentobarbital overdose (1%), and tumors were excised and weighed. Animal care and experiments were carried out under the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. All animal studies were approved by the Ethics Committees of Shanghai Tenth People’s Hospital.
Protein extraction and western blot
Total protein was extracted with RIPA lysis buffer containing protease and phosphatase inhibitors. The Bio-Rad protein assay system was used to measure total protein levels according to the manufacturer’s instructions. Equal amounts of protein were separated by SDS-PAGE and transferred onto nitrocellulose (NC) membranes (Bio-Rad). The blots were probed with primary antibodies overnight and incubated with secondary antibodies for 1 hour at room temperature, before signal visualization.
Co-immunoprecipitation
About 2 mg of cell lysate in 1 mL RIPA buffer was incubated with 2 µg of anti-EGFR antibody with shaking at 4°C overnight. Samples were then mixed with 30 µL of protein A/G-agarose (Santa Cruz) and incubated with shaking at 4°C for 2 hours. Agarose beads were washed five times in RIPA buffer. Immunoprecipitates were then eluted in 2 X SDS sample loading buffer and separated by SDS-PAGE.
Cell cycle distribution analysis
Cells were harvested and fixed with 70% ice-cold ethanol overnight. Then, cells were centrifuged and resuspended in PBS containing propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA), RNase (100 µg/mL) and Triton X-100 (0.2%) for 30 minutes. Finally, cell cycle distribution was analyzed by flow cytometry.
Statistical analysis
SPSS version 23.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Data are mean ± SD from three independent experiments. The Wilcoxon signed-rank test, Student’s t-test, or chi-squared test was used to assess group differences. P<0.05 was considered statistically significant.
Ethics statement
The study was approved by the Ethics Committees of Shanghai Tenth People’s Hospital and written informed consent was obtained from all participating individuals in the study.
Results
Hyper-O-GlcNAcylation and OGT overexpression occur in renal cell carcinoma
First, we examined O-GlcNAc modification levels and OGT expression in human RCC (786-O, ACHN, CAKI-1, and A498) cell lines compared with the human normal renal tubular epithelial HK-2 cell line. Total O-GlcNAcylation and the expression of OGT was increased in RCC cells (). In addition, OGT expression was elevated in RCC tissues (). We also assessed the protein levels of OGT in RCC and adjacent normal tissues by immunohistochemistry (IHC). OGT signals were more intense in tumor tissues compared with adjacent normal samples (). The Oncomine database (https://www.oncomine.org/) was selected to analyze the levels of OGT in RCC and normal renal tissues. The results indicated that OGT mRNA expression levels were obviously elevated in RCC tissues compared with normal ones ().
Figure 1 Hyper-O-GlcNAcylation and OGT Overexpression Occur in renal cell carcinoma
Notes: (A) Expression levels of O-GlcNAcylation and OGT in RCC (786-O, ACHN, CAKI-1, and A498) cell lines compared with the human normal renal tubular epithelial HK-2 cell line. (B) Expression levels of OGT in RCC and adjacent normal tissues were detected by Western blot. (C) Representative images of immunohistochemical staining for OGT expression in RCC and adjacent normal tissues. (D) Oncomine expression analysis of OGT in human RCC and normal renal tissues. (E) Comparison of OGT expression in Fuhrman grades I–IV of RCC tissue samples by IHC staining (left panel, magnification ×100; right panel, magnification ×200). (F) Kaplan–Meier analysis of the correlation between OGT expression and overall survival in RCC patients. (G) Overall survival in RCC patients with high or low expression of OGT from TCGA datasets.
Abbreviations: ccRCC, clear cell RCC; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-GlcNAc-transferase; RCC, renal cell carcinoma; TCGA, The Cancer Genome Atlas.
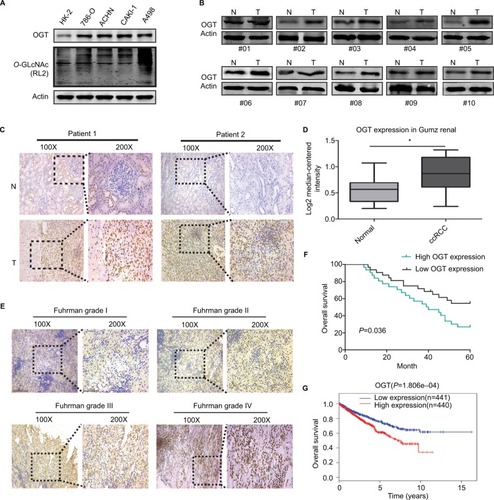
In addition, IHC data showed that OGT staining in RCC was positively correlated with the Fuhrman grade (). Next, we explored whether OGT expression was correlated with patient prognosis in RCC. Kaplan–Meier analysis showed that RCC patients with higher OGT expression levels had relatively poorer prognosis compared with those showing lower OGT expression (). Similarly, the TCGA datasets also supported the notion that high OGT levels predicted shorter survival time in RCC ().
Taken together, these findings suggested that hyper-O-GlcNAcylation and OGT overexpression occurred in renal cell carcinoma; moreover, OGT expression could serve as a prognostic marker of RCC progression.
Reduction of OGT level inhibits the growth of RCC in vitro and in vivo
O-GLcNAcylation plays an important role in the proliferation of various cancers. Meanwhile, suppressing OGT expression inhibits tumor formation and growth.Citation6,Citation29,Citation30 Therefore, we assessed whether hyper-O-GlcNAcylation and OGT overexpression are associated with RCC cell proliferation. Stable OGT knockdown in 786-O and A498 cells was successfully performed (). First, we demonstrated that OGT knockdown effectively attenuated the proliferation of RCC cells as determined by CCK8 assay ().
Figure 2 OGT suppression inhibits RCC growth in vitro and in vivo.
Notes: (A) Expression levels of OGT and O-GlcNAcylation after OGT knockdown in 786-O and A498 cells. (B, C) CCK8 assay was used to assess the proliferation of 786-O and A498 cells after OGT knockdown. (D) Representative images and animal weights. (E) Weights of the excised tumors derived from nude mice are shown. (F) Representative images of H&E staining and Ki-67 immunohistochemical detection in the excised tumors derived from nude mice. Data are mean ± SD from three independent experiments. *P<0.05. Magnification ×200.
Abbreviations: CCK8, Cell Counting Kit-8; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-GlcNAc-transferase; NC, negative control; RCC, renal cell carcinoma.
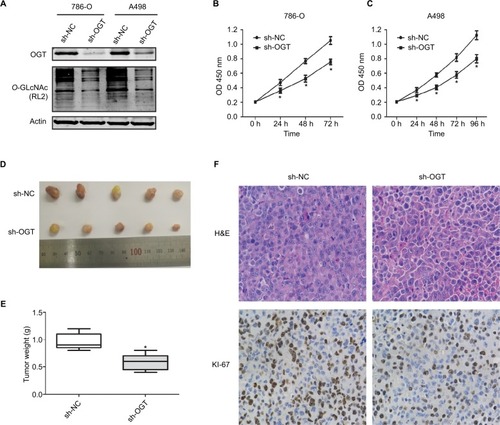
We next examined whether OGT suppression in RCC cells inhibits the tumorigenic phenotype in vivo. Stably transfected 786-O (LV-sh-OGT and LV-sh-NC) cells were injected into male BALB/c nude mice. A total of 3 weeks after implantation, the tumor-bearing mice were sacrificed, and tumors were harvested and weighted. Then, immunohistological examination of Ki-67 expression was performed. Significantly reduced tumor size and weight were observed in OGT knockdown 786-O cells (). As shown in , less Ki-67-positive cells were found in tumors from the OGT knockdown group compared with control samples, indicating a tumor-suppressing potential.
OGT suppression promotes RCC cell apoptosis and inhibits cell cycle progression
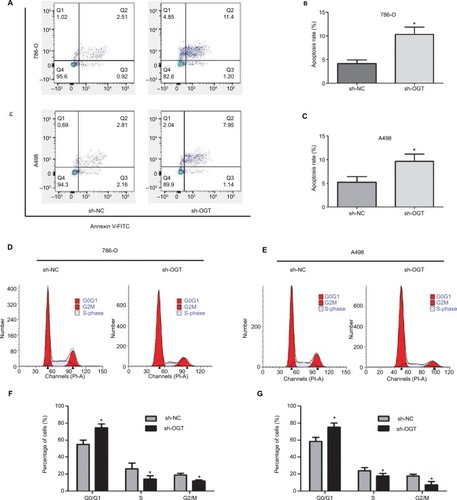
The effects of O-GLcNACylation on RCC cell apoptosis and cell cycle distribution were examined by flow cytometry. The results showed increased apoptosis of 786-O and A498 cells after OGT downregulation (). Moreover, cell cycle analysis indicated that the G0/G1 cell population was increased while the S and G2/M phases were decreased following OGT knockdown (). These data indicated that OGT downregulation inhibited RCC cell proliferation probably via apoptosis promotion and cell cycle inhibition.
Figure 3 OGT suppression promotes RCC cell apoptosis and inhibits the cell cycle.
Notes: (A–C) Apoptotic rates in 786-O and A498 cells as measured by flow cytometry following OGT knockdown. (D–G) Effects of OGT knockdown on cell cycle distribution in T24 and UMUC-3 cells, as determined by flow cytometry. Data are mean ± SD from three independent experiments. *P<0.05.
Abbreviations: FITC, Fluorescein isothiocyanate; OGT, O-GlcNAc-transferase;NC, negative control; PI, propidium iodide; RCC, renal cell carcinoma.
OGT suppression reduces EGFR expression
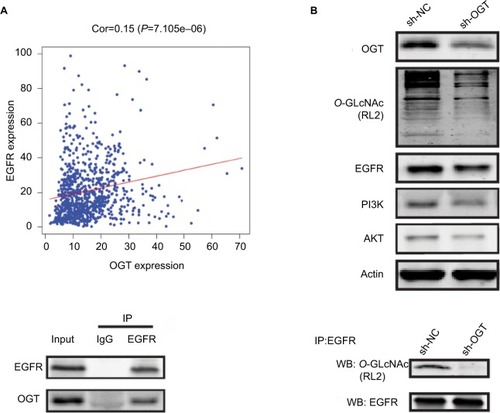
It has been reported that OGT interacts with EGFR, which can be O-GlcNAcylated.Citation31 Meanwhile, EGFR is overexpressed in RCC and shows correlation with poor prognosis in clear cell RCC.Citation32,Citation33 Thus, we first assessed whether EGFR is O-GlcNAcylated in RCC. reveals the correlation between OGT expression and EGFR in TCGA datasets. In this study, EGFR and its downstream PI3K/AKT pathway was downregulated in OGT knockdown 786-O cells (). We next found that EGFR and OGT could co-immunoprecipitate in 786-O cells (). Moreover, EGFR was O-GlcNAcylated in 786-O cells (). These results suggested EGFR O-GlcNAcylation in RCC.
Figure 4 OGT suppression downregulates EGFR.
Notes: (A) Correlation between OGT expression and EGFR in TCGA datasets. (B) Effect of OGT knockdown on EGFR and PI3K/AKT expression in RCC cells. (C) Interaction between OGT and EGFR in 786-O cells, as determined by co-immunoprecipitation and immunoblot. (D) 786-O cells were treated with sh-NC or sh-OGT followed by EGFR immunoprecipitation with anti-EGFR antibodies; the resulting EGFR immunoprecipitates were assessed by WB for EGFR and O-GlcNAc (RL2) detection.
Abbreviations: Cor, correlation; EGFR, epidermal growth factor receptor; IP, immunoprecipitation; OGT, O-GlcNAc-transferase; O-GlcNAc, O-linked N-acetylglucosamine; NC, negative control; RCC, renal cell carcinoma TCGA, The Cancer Genome Atlas; WB, Western blot.
Discussion
In recent years, the role of O-GlcNAcylation in tumorigenesis has attracted increasing attention, with multiple related publications in various human malignancies such as prostate cancer,Citation15 colon cancer,Citation16 liver carcinoma,Citation17 breast cancer,Citation14 and leukemia.Citation18,Citation19 This study for the first time demonstrated that O-GlcNAcylation levels and OGT expression were markedly higher in RCC cell lines and tissue samples compared with respective normal controls. Meanwhile, high OGT expression was associated with the Fuhrman grade and poor patient prognosis in RCC. Functional assays revealed that silencing of OGT effectively inhibited RCC cell proliferation and cell cycle transition accompanied by increased apoptosis. These results suggest that hyper-O-GlcNAcylation and OGT upregulation function as an oncogenic factor in the development of RCC.
Excess food intake is considered an important cancer risk factor, and obesity was reported to be a risk factor for many cancer types, including breast, colon, liver, and endometrial cancers.Citation34,Citation35 A fraction (2%–3%) of glucose entering the cell is applied to the hexosamine biosynthetic pathway (HBP), which controls O-GlcNAcylation of cellular proteins.Citation6 Similar to phosphorylation, O-GlcNAcylation is a reversible post-translational modification that influences almost all cellular processes.Citation8,Citation36 O-GlcNAcylation is mainly upregulated in cancer, and such upregulation is positively correlated with tumor grade or metastasis. However, discrepancies concerning the levels of O-GlcNAc and its cycling enzymes have emerged.Citation14,Citation16,Citation17,Citation37–Citation39
RCC is considered one of the most vascularized human cancers;Citation40 therefore, antiangiogenic therapies are well characterized in RCC.Citation41,Citation42 Upregulated EGFR has been reported to modulate cell functions in RCC, including cell cycle, cell migration, cell invasion and vascularization.Citation21–Citation25,Citation43 In addition, previous study has demonstrated that EGFR is O-GLcNAcylated and directly interacts with OGT in A431 cells.Citation31 The PI3K/AKT pathway was reported to be associated with RCC progression and was a promising drug target.Citation44,Citation45 In the present study, we for the first time reported that EGFR undergoes such post-translational modification and can be co-immunoprecipitated with OGT in human RCC 786-O cells. We also demonstrated that EGFR and its downstream PI3K/AKT pathway was downregulated in OGT knockdown 786-O cells.
Limitation
The present study did not assess whether EGFR is directly involved in OGT-associated effects on RCC cell functions, which deserves further investigation.
Conclusion
In summary, we for the first time reported that O-GlcNAcylation and OGT expression are upregulated and correlated with poor patient prognosis in RCC. In addition, OGT knockdown attenuates RCC cell proliferation in vitro and in vivo. We further found that EGFR is O-GlcNAcylated and interacts with OGT. Taken together, these findings provide novel insights into the oncogenic roles of O-GlcNAcylation and OGT in the development of RCC, and therefore OGT might be used as a target for RCC therapy in the future.
Acknowledgments
This study was supported by National Science Foundation of China (No. 81472389).
Disclosure
The authors report no conflicts of interest in this work.
References
- PruthiDKOomahSLuVQuality and quantity in kidney cancer surgery: the role of nonneoplastic kidney and kidney volumetrics in predicting postoperative renal functionAm J Clin Pathol2019151110811530212840
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- CindoloLPatardJ-JChiodiniPComparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European studyCancer200510471362137116116599
- CalvoESchmidingerMHengDYCGrünwaldVEscudierBImprovement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapyCancer Treat Rev20165010911727664394
- HengDYMackenzieMJVaishampayanUNPrimary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapyAnn Oncol20122361549155522056973
- FardiniYDehennautVLefebvreTIssadTO-GlcNAcylation: a new cancer hallmark?Front Endocrinol2013499
- TrincaGMGoodmanMLPapachristouEKO-GlcNAc-Dependent regulation of progesterone receptor function in breast cancerHorm Canc2018911221
- OzcanSAndraliSSCantrellJELModulation of transcription factor function by O-GlcNAc modificationBiochim Biophys Acta201017995–635336420202486
- MaZVossellerKCancer metabolism and elevated O-GlcNAc in oncogenic signalingJ Biol Chem201428950344573446525336642
- MaZVocadloDJVossellerKHyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cellsJ Biol Chem201328821151211513023592772
- HartGWHousleyMPSlawsonCCycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteinsNature200744671391017102217460662
- YangWHKimJENamHWModification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stabilityNat Cell Biol20068101074108316964247
- YangWHParkSYNamHWNFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditionsProc Natl Acad Sci U S A200810545173451735018988733
- GuYMiWGeYGlcNAcylation plays an essential role in breast cancer metastasisCancer Res201070156344635120610629
- LynchTPFerrerCMJacksonSRShahriariKSVossellerKReginatoMJCritical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasisJ Biol Chem201228714110701108122275356
- MiWGuYHanCO-GlcNAcylation is a novel regulator of lung and colon cancer malignancyBiochim Biophys Acta20111812451451921255644
- ZhuQZhouLYangZO-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantationMed Oncol201229298599321461968
- DingXJiangWZhouPMixed lineage leukemia 5 (MLL5) protein stability is cooperatively regulated by O-GlcNAc transferase (OGT) and ubiquitin specific protease 7 (USP7)PLoS One20151012e014502326678539
- ShiYTomicJWenFAberrant O-GlcNAcylation characterizes chronic lymphocytic leukemiaLeukemia20102491588159820668475
- MendelsohnJAntibody-mediated EGF receptor blockade as an anticancer therapy: from the laboratory to the clinicCancer Immunol Immunother200352534234612700950
- UhlmanDLNguyenPManivelJCEpidermal growth factor receptor and transforming growth factor alpha expression in papillary and nonpapillary renal cell carcinoma: correlation with metastatic behavior and prognosisClin Cancer Res1995189139209816062
- HofmockelGRiessSBassukasIDDämmrichJEpidermal growth factor family and renal cell carcinoma: expression and prognostic impactEur Urol19973144784849187911
- MochHSauterGBuchholzNEpidermal growth factor receptor expression is associated with rapid tumor cell proliferation in renal cell carcinomaHum Pathol19972811125512599385930
- KallioJPHirvikoskiPHelinHMembranous location of EGFR immunostaining is associated with good prognosis in renal cell carcinomaBr J Cancer20038971266126914520458
- LeQ-TDenkoNCGiacciaAJHypoxic gene expression and metastasisCancer Metastasis Rev2004233–429331015197330
- YangJCBevacizumab for patients with metastatic renal cancer: an updateClin Cancer Res20041018 Pt 26367S6370S15448032
- MotzerRJMichaelsonMDRedmanBGActivity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinomaJ Clin Oncol2006241162416330672
- RatainMJEisenTStadlerWMPhase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinomaJ Clin Oncol200624162505251216636341
- ItkonenHMMinnerSGuldvikIJO-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-myc in human prostate cancer cellsCancer Res201373165277528723720054
- ItkonenHMGoradSSDuveauDYInhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolismOncotarget2016711124641247626824323
- StatevaSRVillaloboAO-GlcNAcylation of the human epidermal growth factor receptorOrg Biomol Chem201513308196820426108188
- DorđevićGMatušan IlijašKHadžisejdićIMaričićAGrahovacBJonjićNEGFR protein overexpression correlates with chromosome 7 polysomy and poor prognostic parameters in clear cell renal cell carcinomaJ Biomed Sci20121914022475688
- MerseburgerASHennenlotterJSimonPMembranous expression and prognostic implications of epidermal growth factor receptor protein in human renal cell cancerAnticancer Res2005253B1901190716158924
- DossusLKaaksRNutrition, metabolic factors and cancer riskBest Pract Res Clin Endocrinol Metab200822455157118971118
- RobertsDLDiveCRenehanAGBiological mechanisms linking obesity and cancer risk: new perspectivesAnnu Rev Med201061130131619824817
- HartGWCopelandRJGlycomics hits the big timeCell2010143567267621111227
- SlawsonCPidalaJPotterRIncreased N-acetyl-beta-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetyl-glucosamine containing proteinsBiochim Biophys Acta20011537214715711566258
- KrześlakAFormaEBernaciakMRomanowiczHBryśMGene expression of O-GlcNAc cycling enzymes in human breast cancersClin Exp Med2012121616521567137
- KrześlakAWójcik-KrowirandaKFormaEBieńkiewiczABryśMExpression of genes encoding for enzymes associated with O-GlcNAcylation in endometrial carcinomas: clinicopathologic correlationsGinekol Pol2012831222622384635
- LôrinczTTímárJSzendrôiMAlterations of microvascular density in bone metastases of adenocarcinomasPathol Oncol Res200410314915315448750
- PantuckAJZengGBelldegrunASFiglinRAPathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathwayClin Cancer Res20039134641465214581333
- RiniBISmallEJBiology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinomaJ Clin Oncol20052351028104315534359
- LuZJiangGBlume-JensenPHunterTEpidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinaseMol Cell Biol200121124016403111359909
- DiJHGaoKYQuDBYangJZhengJNRap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in human renal cell carcinomaTumor Biol20173971010428317701653
- LiuFShangliZHuZCAV2 promotes the growth of renal cell carcinoma through the EGFR/PI3K/Akt pathwayOncotargets Ther20181162096216
