Abstract
Background
While emerging evidence indicates that circHIPK3 is critically involved in tumorigenesis and the development of several cancers, its role in prostate cancer (PCa) is not clearly understood.
Materials and methods
Human PCa samples and their matched normal adjacent tissues were obtained from 26 patients to assess the expression of circHIPK3 and its relationship with PCa prognosis. A series of in vitro and in vivo functional experiments were carried out to elucidate the role of circHIPK3 in PCa progression and its underlying molecular mechanisms.
Results
In this study, we found that circHIPK3 was overexpressed in PCa tissues and that higher circHIPK3 expression was associated with tumor stage. Moreover, circHIPK3 knockdown markedly inhibited the proliferation, migration, and invasion of PCa cells in vitro and impaired tumor growth in vivo. Bioinformatics analysis and luciferase reporter assays demonstrated that circHIPK3 could promote MCL1 expression by interacting with miR-193a-3p in PCa. Finally, rescue assays illustrated that circHIPK3 knockdown could partially reverse the effects of MCL1 overexpression.
Conclusion
In summary, our study illustrated, for the first time, that circHIPK3-mediated miR-193a-3p-MCL1 signaling promotes PCa development and progression, providing a novel therapeutic target for PCa.
Introduction
Approximately almost 1.3 million new cases of prostate cancer (PCa) were diagnosed in 2018 and PCa was responsible for an estimated 359,000 associated deaths worldwide in 2018, making it the second most frequently diagnosed cancer and the fifth leading cause of cancer death in men.Citation1 Clinically, at the time of diagnosis, ~70% of patients present with organ-confined low- or intermediate-risk PCa. However, the 5-year relative survival rate decreases to 30% in the event of distant metastasis.Citation2 Therefore, it is of great value to gain a better understanding of the molecular mechanism underlying the development and progression of PCa and identify novel therapeutic targets.
Circular RNA (circRNA) is a conserved and stable type of endogenous noncoding RNA that is formed by back-splicing events of precursor mRNA.Citation3 Growing evidences show that circRNAs are implicated in a wide range of physiological or pathological processes such as tumorigenesis, by regulating cell survival, proliferation, and metastasis.Citation4 CircHIPK3 (circRNA ID: hsa_circ_0000284) is a product of HIPK3 gene’s exon2 splicing and consists of 1,099 nucleotides in length.Citation5 It has been reported that circHIPK3 may function through sponging some miRNAs and regulate the progression of many cancers, including gastric cancer,Citation6 gallbladder cancer,Citation7 ovarian cancer,Citation8 colorectal cancer,Citation9 and liver cancer.Citation10 However, whether circHIPK3 harbors miRNAs with regulatory roles in PCa is still unknown.
In our study, we identified that circHIPK3 was upregulated in PCa and increased circHIPK3 levels predict poor prognosis in PCa patients. Moreover, we showed that circH-IPK3 knockdown suppresses PCa cell proliferation, migration, and invasion. In mechanism, we found that circHIPK3 could function as a ceRNA through harboring miR-193a-3p to abolish the suppressive effect on target oncogene MCL1, which promoted PCa growth and metastasis. Therefore, our study for the first time demonstrated the novel role of circHIPK3 in PCa, and the circHIPK3/miR-193a-3p/MCL1 signaling pathway might be a promising therapeutic target for PCa treatment.
Materials and methods
Patient samples
Human PCa samples and their matched normal adjacent tissues were obtained from 26 patients at Beijing Chaoyang Hospital of Capital Medical University. None of the patients had received preoperative radiotherapy, chemotherapy or any other medical intervention before surgery. All tissues were placed immediately in liquid nitrogen after removal from the PCa patients and stored at −80°C until use. All tumors and paired nontumor tissues were confirmed by two experienced pathologists. This study was approved by the Human Research Ethics Committee of Beijing Chaoyang Hospital, and written informed consent was obtained from all participants prior to sample collection. All procedures performed involving human participants in this study were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Cell culture and transfection
The human prostate epithelial cell line RWPE-1 and PCa cell lines (LNCaP, PC3, DU145, 22Rv1) were purchased from Shanghai Institutes for Biological Sciences, Shanghai, China. Cells were cultured in RPMI-1640 medium (10-040-CVR; Corning Incorporated, Corning, NY, USA) supplemented with 10% FBS (35-015-CV; Corning), penicillin/streptomycin (1:100; Sigma-Aldrich Co., St Louis, MO, USA), and 4 mM l-glutamine (Sigma-Aldrich Co.) and placed in an incubator containing 95% air and 5% CO2 at 37°C. Small interfering RNAs (si-circHIPK3), miR-193a-3p mimic, miR-193a-3p inhibitor, and their negative controls were synthesized by GenePharma (Shanghai, China) and transfected into cultured PC3 and DU145 cells using RNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA). The sequences of si-circHIPK3 were as follows: sense GGUACUACAGGUAUGGCCUTT; antisense AGGCCAUACCUGUAGUACCGA.
Real-time PCR (qRT-PCR) assay
All patient samples or PCa cell total RNAs were isolated using Trizol reagent (Thermo Fisher Scientific). Quantification of extracted RNA was performed using NanoDrop. cDNA synthesis was performed using PrimeScriptRT reagent kit (Takara Bio Inc., Kusatsu, Japan) using 1 µg of total RNA. Real-time PCR was conducted using a Bio-Rad CFX96 system with SYBR green to determine the mRNA expression level of the gene of interest. Expression levels were quantified using the 2−ΔΔCt method, and U6 or GAPDH was used as the internal control. The primer sequences used in this study were as follows: circHIPK3, forward 5′-TTCAACATATCTACAATCTC-GGT-3′ and reverse 5′-ACCATTCACATAGGTCCGT-3′; HIPK3, forward 5′-CTTATGCCCGTCAAGGTCAAA-3′ and reverse 5′-ACAAGTATGGTTACGGTGCTG-3′; MCL1, forward 5′-TGCTTCGGAAACTGGACATCA′ and reverse 5′-TAGCCACAAAGGCACCAAAG-3′; GAPDH, forward 5′-GCACCGTCAAGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′; U6, forward 5′-AGCCCGCACTCAGAACATC-3′ and reverse 5′-GCCACCAAGACAATCATCC-3′.
Cell proliferation assay
Cell proliferation assays were performed using the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). The transfected cells were seeded in 24-well plates at a density of 2×103 cells per well and cultured for 1, 2, 4, or 6 days. A volume of 10 µL of CCK-8 solution was added to each well, and the plates were incubated at 37°C for 1 hour. The OD450 was measured at a wavelength of 450 nm using a microplate reader instrument (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Wound-healing assay
Twenty-four hours after transfection, the plates were scratched using a sterile pipette tip to generate a wound through the confluent monolayer. The wound-healing speed was determined after 24 hours and normalized to the wound size length at 0 hour.
Cell invasion assays
Cell invasion was evaluated by using Transwell cell culture chambers with an 8 µm pore size according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). About 1×105 cells were suspended in serum-free RPMI-1640 medium with Matrigel in the upper chamber, whereas the complete RPMI-1640 medium with 10% FBS was placed into the lower chamber. After 24 hours of incubation, cells in the bottom chamber were fixed with methanol for 30 minutes and stained with 0.5% crystal violet for 30 minutes. The number of invading cells was calculated under the microscope in five random fields and are shown as the average per field.
Colony formation assay
Cells were seeded at a density of 1×103 cells in 60 mm dishes and incubated at 37°C for 1 week. Cell colonies were fixed with methanol for 30 minutes and stained with 0.5% crystal violet for 30 minutes. Colonies were observed under a microscope.
Western blotting assay
Cells were washed twice with cold PBS and lysed in RIPA buffer and equal amounts of protein lysates (30 µg per treatment in each lane) were loaded onto the 10% SDS-PAGE gels, then transferred onto polyvinylidene difluoride membranes (PVDF; EMD Millipore, Billerica, MA, USA). After PVDF membranes were blocked with 5% skim milk for 1 hour, they were sequentially incubated with primary antibodies, HRP-conjugated secondary antibodies, and visualized using an ECL system (Thermo Fisher Scientific). The primary antibodies used in the study were listed below: MCL1 (#ab32087; Abcam, Cambridge, UK) and GAPDH (#sc-166574; Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Dual luciferase reporter assay
PCa cells were cotransfected with wild-type or mutated circHIPK3 or MCL1 3′UTR reporter plasmids, and with miR-193a-3p mimics or negative controls. After transfection cultured for 48 hours, firefly and renilla luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer’s instructions.
RNA immunoprecipitation assay
PCa cells were lysed in complete RNA immunoprecipitation (RIP) lysis buffer supplemented with RNase inhibitor, and the cell extract was incubated with magnetic beads conjugated with anti-Argonaute 2 (AGO2, #ab32381; Abcam) or a control anti-IgG antibody (EMD Millipore) overnight at 4°C. The RNA/antibody complex was washed three times with RIP buffer supplemented with RNase inhibitor and Proteinase K. The RNA was extracted using Trizol (Thermo Fisher Scientific) according to the manufacturer’s protocol and was subjected to qRT-PCR analysis.
Biotin-coupled miRNA capture assay
The biotin-labeled circHIPK3 and control probes were synthesized by GenePharma (Shanghai, China). In brief, biotin-labeled circHIPK3 and control probes were transfected into PC3 cells at a final concentration of 20 nmol/L overnight. The biotin-coupled RNA complex was then pulled down by incubating the cell lysate with streptavidin-coated magnetic beads (Thermo Fisher Scientific). Subsequently, RNA was extracted using Trizol Reagent (Thermo Fisher Scientific) and evaluated by qRT-PCR analysis.
Animal experiments
All animal experiments were approved by the Capital Medical University Experimental Animal Care Committee. Male BALB/c nude mice (4 weeks old) were maintained under specific pathogen-free conditions and manipulated according to protocols approved by the Capital Medical University Experimental Animal Care Commission. Subsequently, 5×106 si-circHIPK3 or control PC3 cells were subcutaneously injected into the flanks of each mouse. Tumor size was measured every 1 week by caliper to determine tumor volume. Four weeks later, the mice were euthanized, and tumors were removed and weighed.
Statistical analyses
Statistical analyses were performed with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA), and the results are presented as the mean ± SD. Student’s t-test was used to analyze the differences between two groups and one-way ANOVA was used for multiple comparisons. P<0.05 was considered statistically significant.
Results
CircHIPK3 silencing inhibits PCa cell proliferation and invasion
To identify whether circHIPK3 plays a role in PCa, we first explored the expression level of circHIPK3 in 26 pair-matched PCa tissues and adjacent normal tissues by qRT-PCR. PCR results showed that circHIPK3 levels were markedly upregulated in PCa tissues, compared to normal tissues (). In addition, we found that circHIPK3 expression was significantly associated with pathological T stage (). According to these clinical data, we proposed that circHIPK3 may play an oncogenic role in the proliferation and progression of PCa. To explore the biological function of circHIPK3 in vitro, we analyzed its expression in four PCa cell lines. Consistently, circHIPK3 expression was significantly upregulated in PCa cells (LNCaP, PC3, DU145, 22Rv1) compared to the human prostate epithelial cell line RWPE-1 ().
Figure 1 CircHIPK3 silencing inhibits PCa cell proliferation and invasion.
Notes: (A) Relative expression of circHIPK3 in PCa tissue samples and their paired noncancerous tissue samples measured by qRT-PCR (Student’s t-test). (B) Relative expression of circHIPK3 in TI–TII PCa samples and TIII–TIV PCa samples measured by qRT-PCR (Student’s t-test). (C) Relative expression of circHIPK3 in PCa cell lines was measured by qRT-PCR (Student’s t-test). (D) Relative circHIPK3 and HIPK3 mRNA expression was detected after transfection in PC3 and DU145 cells by qRT-PCR (Student’s t-test). (E) Wound-healing assays were used to detect cell migration capacities of PC3 and DU145 cells after transfection (Student’s t-test). (F) Transwell assays were used to detect cell invasion capacities of PC3 and DU145 cells after transfection (Student’s t-test). (G) CCK-8 assays were used to detect cell viability of PC3 and DU145 cells after transfection (Student’s t-test). (H) Colony formation assays were used to detect cell viability of PC3 and DU145 cells after transfection (Student’s t-test). Quantified values were mean ± SD of at least three independent experiments. *P<0.05, **P<0.01.
Abbreviations: CCK-8, Cell-Counting Kit-8; NC, negative control; PCa, prostate cancer; qRT-PCR, quantitative real-time reverse transcription PCR.
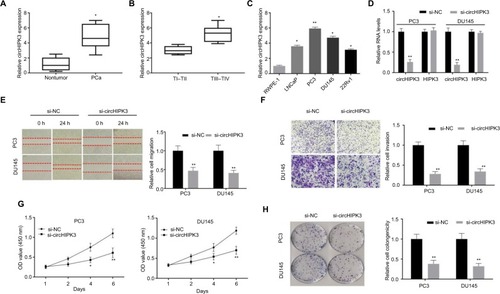
To explore the function of circHIPK3 in PCa, we first silenced circHIPK3 in PC3 and DU145 cells by transfection with siRNA targeting circHIPK3. The PCR results indicated that circHIPK3 expression was significantly decreased in PC3 and DU145 cells after transfection and that the expression of its host gene, HIPK3, was not decreased (). We then used wound-healing assays and Transwell invasion assays to analyze whether circHIPK3 knockdown affects PCa cell migration and invasion. As shown in and , these assays indicated that circHIPK3 silencing markedly repressed PC3 and DU145 cell migration and invasion. Furthermore, to explore whether circHIPK3 was involved in cellular proliferation, we performed CCK-8 and colony formation assays. The CCK-8 assay results showed that PCa cell viability was markedly suppressed after knocking down circHIPK3 (). Consistently, colony formation assay results demonstrated that circHIPK3 silencing decreased the colony formation abilities of PC3 and DU145 cells (). Taken together, these findings revealed that circHIPK3 silencing inhibits the proliferation, migration, and invasion of PCa cells.
MiR-193a-3p is targeted by circHIPK3 in PCa cells
We then sought to explore the possible molecular mechanism of circHIPK3 in PCa. It has been reported that the functions of circRNAs are mainly related to their intracellular localization and that circHIPK3 preferentially localizes in the cytoplasm of many cancer cells.Citation5,Citation10 To determine the subcellular localization of circHIPK3 in PCa, we performed qRT-PCR assays. The results demonstrated that the circular form of circHIPK3 was mainly located in the cytoplasm of both PC3 and DU145 cells (). Then, we conducted RIP with an antibody against AGO2 in PC3 and DU145 cells. The results demonstrated that circHIPK3 was significantly enriched by the AGO2 antibody (), which suggested that circHIPK3 may act as a binding platform for AGO2 and miRNAs. According to the above results, circHIPK3 might serve as competing endogenous RNAs (ceRNAs) to bind to and inhibit the function of miRNAs function.
Figure 2 miR-193a-3p is targeted by circHIPK3 in PCa cells.
Notes: (A) Cytoplasmic and nuclear RNA fractions were isolated from PC3 and DU145 cells. Relative circHIPK3 expression levels in the cell cytoplasm or nucleus were examined by qRT-PCR. GAPDH was used as the cytoplasmic control, and U6 was used as the nuclear control. (B) AGO2 RIP assays were used to detect circHIPK3 binding with AGO2 complex in PC3 and DU145 cells. (C) Biotin-coupled miRNA capture assays were used to detect which miRNA binds with circHIPK3 in PC3 cells. (D) Diagrammatic sketch of the binding sites for miR-193a-3p in circHIPK3. Luciferase reporter assay showed that ectopic expression of miR-193a-3p suppressed the activity of circHIPK3 in PC3 and DU145 cells (Student’s t-test) (E) Relative circHIPK3 expression was detected after transfection in PC3 and DU145 cells by qRT-PCR (Student’s t-test). (F) Relative miR-193a-3p expression was detected after transfection in PC3 and DU145 cells by qRT-PCR (Student’s t-test). Quantified values were mean ± SD of at least three independent experiments. **P<0.01.
Abbreviations: AGO2, Argonaute 2; Mut, mutant; NS, no significance; qRT-PCR, quantitative real-time reverse transcription PCR; RIP, RNA immunoprecipitation; WT, wild-type.
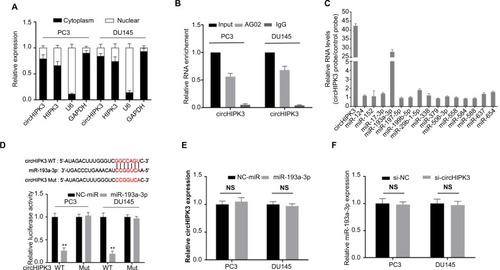
To screen for potential miRNAs that could bind to circH-IPK3 in PCa, we analyzed two public databases (miRanda and RegRNA2.0) and found 15 potential miRNAs that had a potential binding site for circHIPK3 in both databases. To verify which miRNAs could actually bind to circHIPK3 in PCa, we performed a circHIPK3 pull-down assay using biotin-coupled probes specifically against circHIPK3 and then analyzed the 15 candidate miRNA levels in the complex. Interestingly, we found a specific enrichment of miR-193a-3p compared with the controls, whereas the other miRNAs demonstrated no enrichment () in the complex, indicating that miR-193a-3p is a circHIPK3-associated miRNA in PC3 cells. To further confirm this result, we performed a dual luciferase assay using miR-193a-3p mimics. The results showed that luciferase activity was reduced in PC3 and DU145 cells cotransfected with wild-type circH-IPK3 and miR-193a-3p mimics compared with that of the control (). Meanwhile, we mutated the target site of miR-193a-3p in circHIPK3 from the luciferase reporter, and the luciferase activity did not significantly change in cells cotransfected with mutant circHIPK3 and miR-193a-3p mimics (). Furthermore, silencing circHIPK3 in PCa cells did not affect the expression of miR-193a-3p (), and transfection of miR-193a-3p mimics also did not affect the expression of circHIPK3 (), which indicated that circHIPK3 and miR-193a-3p may not be digested by each other. All of these experimental results suggest that circHIPK3 may function as a sponge for miR-193a-3p without affecting its expression in PCa.
MiR-193a-3p exerts its role by regulating MCL1 expression in PCa
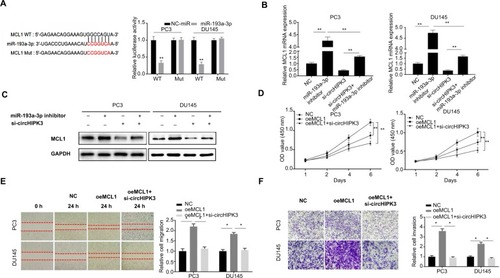
MiRNAs have been widely shown to regulate gene expression by targeting the 3′UTR region of mRNAs. Thus, we searched for the potential target genes of miR-193a-3p by bioinformatics analysis using the TargetScan database and miRanda database. Based on the predictions of those databases, we identified MCL1, an oncogene that could promote the growth and metastasis of many cancers, as a potential target gene of miR-193a-3p. To confirm this hypothesis in PCa, we then performed a luciferase reporter assay to validate the interaction between miR-193a-3p and the 3′UTR of MCL1 mRNA. The results showed that transfection of miR-193a-3p mimics could reduce the activity of a luciferase reporter carrying the MCL1-3′UTR of WT compared to NC-miR (). Furthermore, we mutated the target sites for miR-193a-3p from the 3′UTR of MCL1 mRNA and the luciferase activity did not significantly change in cells cotransfected with mutant MCL1-3′UTR and miR-193a-3p mimics (). As expected, inhibiting miR-193a-3p led to elevated MCL1 mRNA and protein expression while circHIPK3 silencing suppressed MCL1 mRNA and protein expression in PC3 and DU145 cells ( and ). Moreover, inhibition of circHIPK3 could partly counteract the effect of the miR-193a-3p inhibitor on MCL1 expression ( and ). Thus, we speculated that circHIPK3 might regulate MCL1 expression by sponging miR-193a-3p in PCa cells. To investigate whether circHIPK3-mediated upregulation of MCL1 contributes to PCa progression, we then performed CCK-8, wound healing, and Transwell assays. According to the CCK-8 assays, we found that MCL1 overexpression significantly increased the proliferation of PC3 and DU145 cells and that circHIPK3 silencing markedly reverse the increased cell proliferation induced by MCL1 overexpression (). Similarly, based on the migration and Transwell assays results, we also demonstrated that MCL1 overexpression significantly increased the migration and invasion of PCa cells and that circHIPK3 knockdown significantly offset the increased cell migration and invasion ( and ). Taken together, our data demonstrated that MCL1 targeted by miR-193a-3p is essential for circHIPK3-mediated PCa progression.
Figure 3 MiR-193a-3p exerts its role by the regulation of MCL1 expression in PCa.
Notes: (A) Putative binding site for miR-193a-3p in MCL1 3′UTR. Luciferase reporter assay showed miR-193a-3p mimics suppressed the activity of WT MCL1 3′UTR. However, there was no statistical effect on luciferase activity when MUT MCL1 3′UTR and miR-193a-3p mimics were cotransfected (Student’s t-test). (B) Relative MCL1 mRNA expression was detected after transfection in PC3 and DU145 cells by qRT-PCR (one-way ANOVA). (C) Relative MCL1 protein expression was detected after transfection in PC3 and DU145 cells by WB. (D) CCK-8 assays were used to detect cell viability of PC3 and DU145 cells after transfection (one-way ANOVA). (E) Wound-healing assays were used to detect cell migration capacities of PC3 and DU145 cells after transfection (one-way ANOVA). (F) Transwell assays were used to detect cell invasion capacities of PC3 and DU145 cells after transfection (one-way ANOVA). Quantified values were mean ± SD of at least three independent experiments. Magnification ×100. *P<0.05, **P<0.01.
Abbreviations: CCK-8, Cell-Counting Kit-8; Mut, mutant; NC, negative control; qRT-PCR, quantitative real-time reverse transcription PCR; WB, Western blot; WT, wild-type.
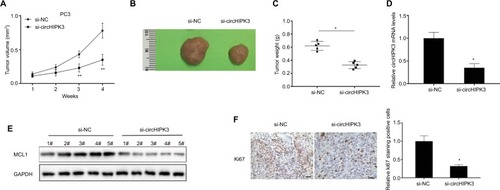
CircHIPK3 silencing suppressed PCa growth in vivo
In vitro experiments have demonstrated the function of circH-IPK3 in PCa. We further utilized an in vivo nude mouse model to investigate the effects of circHIPK3 on PCa tumor growth. PC3 cells transfected with si-circHIPK3 or a control siRNA were subcutaneously injected into nude mice. The results showed that circHIPK3 knockdown significantly suppressed PC3 tumor volumes ( and ) and tumor weights (), compared to those of the control group. Moreover, the expression levels of circHIPK3 and MCL1 were significantly decreased in tumor tissues of the si-circHIPK3 group at the end of this experiment ( and ). Finally, immunohistochemistry results revealed that tumor tissues collected from the si-circHIPK3 group had fewer proliferation marker Ki-67-positive cells when compared with those of the control group (). Therefore, these data suggested that circHIPK3 knockdown could suppress PCa growth in vivo.
Figure 4 CircHIPK3 silencing suppressed PCa growth in vivo.
Notes: (A) Tumor volumes were monitored every 1 week (Student’s t-test). (B) Representative tumors from two groups of nude mice after injection of si-circHIPK3 PC3 cells or controls cells. (C) Tumor weights were measured (Student’s t-test). (D) Relative circHIPK3 expression was detected in two groups of tumors by qRT-PCR (Student’s t-test). (E) Relative MCL1 protein expression was detected in two cell groups by WB. (F) Ki67 expression and relative positive cell numbers were determined by immunohistochemistry in each group (Student’s t-test). Scale bars: 20 µm. Quantified values were mean ± SD of at least three independent experiments. *P<0.05, **P<0.01.
Abbreviations: NC, negative control; WB, Western blot; qRT-PCR, quantitative real-time reverse transcription PCR.
Discussion
PCa is the second most commonly diagnosed malignancy in men and account for 13.5% of newly diagnosed cancers worldwide in 2018.Citation1 Although at the time of diagnosis most men present with localized PCa, current clinical diagnostic biomarkers such as prostate-specific antigen are not precise enough to distinguish between PCa and other benign prostatic diseases such as benign prostatic hyperplasia and prostatitis.Citation11 Moreover, these biomarkers do not optimally triage individual patients into risk groupings that can be used to determine how aggressively the cancer should be treated.Citation12
Accumulating evidence suggests that circRNAs may be used as tumor biomarkers and regulators because of their crucial involvement in various biological processes.Citation4 However, to date, only two circRNAs have been explored in PCa.Citation13,Citation14 Kong et al identified that circSMARCA5 was upregulated in four PCa cell lines compared to a normal prostate cell line and in 21 PCa tissue samples compared with corresponding normal prostate tissues. Moreover, this study also found that circSMARCA5 expression was significantly induced after DHT treatment and that circSMARCA5 may act as a potential pro-oncogenic circRNA in vitro by promoting the PCa cell cycle and inhibiting PCa cell apoptosis. Dai et al found that circRNA-MYLK is upregulated in PCa cell lines in comparison with normal prostate cell lines and is highly upregulated in 17 PCa tissues in comparison with corresponding normal prostate tissues. In addition, this study found that circRNA-MYLK knockdown significantly inhibited PCa cell proliferation, migration, and invasion. The group further showed that the upregulation of circRNA-MYLK may act as an miRNA sponge binding with miR-29a. These two findings had proved that circRNAs have been discovered to play a functional role in regulating PCa development and progression.
CircHIPK3, which originates from exon 2 of the HIPK3 gene, is a highly conserved and stable circRNA in many cell lines and tissues.Citation5 Previous studies have demonstrated that circHIPK3 plays an important role in tumorigenesis and that circHIPK3 silencing in tumors significantly inhibits the cancer cell proliferation, migration, and invasion.Citation5–Citation10,Citation15,Citation16 For example, Kai et al indicated that circHIPK3 was upregulated in many cancer tissues and that circHIPK3 knockdown inhibited HuH-7, HCT-116, and HeLa cell proliferation through sponging miR-124.Citation5 Jin et al found that circHIPK3 upregulation in glioma correlated with tumor progression and that circHIPK3 could influence the malignant behaviors of glioma cells via the circHIPK3/miR-654/IGF2BP3 regulatory network.Citation15 Ke et al showed that circHIPK3 was highly expressed in nasopharyngeal carcinoma (NPC) tissues and cell lines and that its expression levels could act as a prognostic marker in NPC patients.Citation16 In addition, their findings demonstrated that circHIPK3 facilitated NPC progression by protecting ELF3 from miR-4288-mediated silencing.Citation16 Chen et al demonstrated that circHIPK3 was upregulated in hepatocellular carcinoma tissues and that silencing circHIPK3 inhibited cell proliferation and migration by downregulating the miR-124-AQP3 axis.Citation10 However, the role of circHPIK3 in PCa remains unclear. Thus, our aim was to reveal the function of circHIPK3 in PCa.
In this study, we found that circHIPK3 was upregulated in PCa and predicted poor prognosis among PCa patients. Functionally, we demonstrated that circHIPK3 knockdown markedly inhibited PCa cell proliferation, migration, and invasion in vitro and decreased tumor growth in vivo. Through bioinformatics prediction and biotin-coupled miRNA capture, we discovered that miR-193a-3p binds with circHIPK3 in PCa cells. Most published studies indicate that miR-193a-3p may function as a tumor-suppressing miRNA in many cancers, including PCa.Citation17–Citation19 Functionally, miR-193a-3p can inhibit cell proliferation, migration, and invasion in many cancers by suppressing MCL1.Citation20–Citation23 In addition, MCL1 was expressed at higher levels in PCa tumors and promoted PCa cell growth, migration, and invasion.Citation24,Citation25 In the present study, we identified circHIPK3 as an miR-193a-3p sponge that upregulates the expression of the oncogene MCL1, promoting PCa progression.
However, our study has limitations. First, only 26 pairs of PCa tissues were analyzed in this study because of the limited number of available PCa samples. Second, all the patient samples are from only one hospital. A larger number of PCa samples should be tested at multiple centers to further confirm the conclusions of this study. Third, we look forward to further research to explore why and how circHIPK3 was upregulated in PCa, which will provide a deeper understanding of the molecular mechanism.
Conclusion
In conclusion, our study first revealed the novel function of circHIPK3 in PCa progression. We illustrated that circH-IPK3-mediated miR-193a-3p-MCL1 signaling promote PCa development and progression, providing a novel therapeutic target for PCa therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrayFFerlayJSoerjomataramISiegelRLTorreLAJemalAGlobal cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin201868639442430207593
- SchymuraMJSunLPercy-LaurryAProstate cancer collaborative stage data items–their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010Cancer2014120Suppl 233758377025412388
- ChenLLThe biogenesis and emerging roles of circular RNAsNat Rev Mol Cell Biol201617420521126908011
- KristensenLSHansenTBVenøMTKjemsJCircular RNAs in cancer: opportunities and challenges in the fieldOncogene201837555556528991235
- ZhengQBaoCGuoWCircular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAsNat Commun2016711121527050392
- ChengJZhuoHXuMRegulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancerJ Transl Med201816121630068360
- KaiDYannianLYitianCDinghaoGXinZWuJCircular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124Biochem Biophys Res Commun2018503286386929928876
- LiuNZhangJZhangLYWangLCircHIPK3 is upregulated and predicts a poor prognosis in epithelial ovarian cancerEur Rev Med Pharmacol Sci201822123713371829949144
- ZengKChenXXuMCircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7Cell Death Dis20189441729549306
- ChenGShiYLiuMSunJcircHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinomaCell Death Dis20189217529415990
- CarterHBProstate-specific antigen (PSA) screening for prostate cancer: revisiting the evidenceJAMA2018319181866186829800999
- SartorOde BonoJSMetastatic prostate cancerN Engl J Med2018378764565729412780
- DaiYLiDChenXCircular RNA myosin light chain kinase (MYLK) promotes prostate cancer progression through modulating miR-29a expressionMed Sci Monit2018243462347129798970
- KongZWanXZhangYAndrogen-responsive circular RNA circ-SMARCA5 is up-regulated and promotes cell proliferation in prostate cancerBiochem Biophys Res Commun201749331217122328765045
- JinPHuangYZhuPZouYShaoTWangOCircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signalingBiochem Biophys Res Commun201850331570157430057315
- KeZXieFZhengCChenDCircHIPK3 promotes proliferation and invasion in nasopharyngeal carcinoma by abrogating miR-4288-induced ELF3 inhibitionJ Cell Physiol201923421699170630070690
- GrossiISalviAAbeniEMarchinaEDe PetroGBiological function of MicroRNA193a-3p in health and diseaseInt J Genomics2017201710113
- LingZWangXTaoTInvolvement of aberrantly activated HOTAIR/EZH2/miR-193a feedback loop in progression of prostate cancerJ Exp Clin Cancer Res201736115929141691
- LiuYXuXXuXMicroRNA-193a-3p inhibits cell proliferation in prostate cancer by targeting cyclin D1Oncol Lett20171455121512829142597
- NakanoHYamadaYMiyazawaTYoshidaTGain-of-function microRNA screens identify miR-193a regulating proliferation and apoptosis in epithelial ovarian cancer cellsInt J Oncol20134261875188223588298
- WilliamsMKirschnerMBChengYYmiR-193a-3p is a potential tumor suppressor in malignant pleural mesotheliomaOncotarget2015627234802349526125439
- WuYWangHLncRNA NEAT1 promotes dexamethasone resistance in multiple myeloma by targeting miR-193a/MCL1 pathwayJ Biochem Mol Toxicol2018321e22008
- HuangYLuoHLiFLINC00152 down-regulated miR-193a-3p to enhance MCL1 expression and promote gastric cancer cells proliferationBiosci Rep2018383BSR2017160729339419
- AraiSJonasOWhitmanMACoreyEBalkSPChenSTyrosine kinase inhibitors increase MCL1 degradation and in combination with BCLXL/BCL2 inhibitors drive prostate cancer apoptosisClin Cancer Res201824215458547030021909
- WangBDCeniccolaKYangQIdentification and functional validation of reciprocal microRNA-mRNA pairings in African American prostate cancer disparitiesClin Cancer Res201521214970498426089375
