Abstract
Background: Glioma patients with mutant isocitrate dehydrogenase have improved survival; this could be in part due to the suppressive effect of mutant IDH on the level of chronic inflammation. This study aimed to prospectively analyze the association of IDH1 mutation status with preoperative levels of blood inflammatory markers: neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), C-reactive protein (CRP), and red cell distribution width (RDW) in gliomas.
Patients and methods: Receiver operating characteristic curves for cutoff value determination, various bivariate tests, and survival analyses (Kaplan–Meier curves and Cox regression) were performed.
Results: Patients with mutant IDH1 had reduced levels of NLR (P<0.032) and CRP (P<0.008). Moreover, these patients showed better median overall survival compared to those without IDH1 mutation (P<0.000). In univariate analysis, IDH1 mutation status (P<0.000), NLR (P<0.000), PLR (P<0.008), and CRP (P<0.001) were among the factors associated with survival. By multivariate analysis, IDH1 mutation (P<0.044) and NLR<2.65 (P<0.022) remained independent factors associated with better survival; other independent variables were tumor grade (P<0.000) and location in noneloquent area (P<0.015).
Conclusion: The obtained results show that IDH1 mutation is associated with lower levels of chronic inflammation that could account for an improved prognosis in this group of patients.
Introduction
Glioma patients with mutations in isocitrate dehydrogenase (IDH-mt) have a higher overall survival (OS) rate compared to those with IDH-wildtype (IDH-wt) tumors; this was confirmed in a recent meta-analysis of 55 observational studies. Citation1 Therefore, understanding the processes triggered by IDH mutations will help identify factors that provide a better prognosis in glioma patients.
Wildtype IDH1 and 2 enzymes catalyze the conversion of isocitrate to α-ketoglutarate (α-KG) in the cytoplasm and mitochondria, respectively. The most common IDH mutation in gliomas is IDH1R132H. Citation2,Citation3 The mutated enzyme loses its ability to produce α-KG; moreover, it acquires a neomorphic activity to catalyze the reduction of α-KG to D-stereoisomer of 2-hydroxyglutarate (D-2-HG). Citation4 The current understanding of the downstream-regulated processes is presented in recent reviews. Citation5,Citation6
The role of α-KG as a co-substrate for α-KG-dependent prolyl-4-hydroxylases (PHDs) is particularly interesting in the scope of our study. These enzymes act as negative regulators of the stability of hypoxia-inducible factor (HIF), a key transcriptional factor that mediates the cellular response to hypoxia. Citation7 Activation of HIF in hypoxic tumor microenvironment is a driving force of tumorigenesis. Citation8 IDH1 mutation appears to influence the HIF stability in two opposite ways. Citation5 A reduction in α-KG levels in IDH1-mt cells may lead to inhibition of PHD activity and thereby to HIF stabilization. Citation9,Citation10 On the other hand, when D-HG accumulates as a result of the IDH1-mt enzyme’s activity, it may function as an agonist of α-KG for PHDs, promoting HIF degradation and thus slowing the tumor growth. Citation11
HIF-1α is involved in the activation of tumor-associated inflammatory signaling. Citation12 Hypoxic and necrotic areas of a growing tumor produce more and more proinflammatory mediators, thereby recruiting more immune cells. These tumor-infiltrating immune cells acquire increasingly tumor-promoting properties, resulting in tumor progression, invasion, and angiogenesis. Citation13 Thus, it was shown that the extent of neutrophil infiltration was correlated with tumor grade in glioma samples. Moreover, the circulating neutrophil count was elevated above the normal range in glioma patients. Citation14,Citation15 A recent review concluded that elevated tumor-infiltrating neutrophils, elevated peripheral blood neutrophils, and elevated neutrophil–lymphocyte ratio (NLR) have been associated with poor survival in the main human cancers. Citation16 In addition, there is increasing evidence that cancer-related chronic inflammatory conditions trigger uncontrolled platelet activation which consequently contributes to tumor growth, angiogenesis, metastasis, and cancer-associated thrombosis. Citation17
The IDH1 mutation-dependent mechanism of downregulation of HIF expressionCitation5 suggests that the mutant tumors have lower inflammation levels in comparison with the IDH1-wt gliomas. Indeed, Amankulor et alCitation18 showed that IDH1-mt tumor-bearing mice have a ~100-fold higher concentration of 2-HG, lower neutrophil chemotaxis, and longer survival time compared to IDH1-wt animals. Moreover, systemic neutrophil depletion caused a significant increase in survival of IDH1-wt mice without any significant effect on IDH1-mt tumors which already attracted a lower number of neutrophils at basal levels. This proposed mechanism is also in agreement with the study of Unruh et alCitation19 which showed a potent antithrombotic effect of IDH1 mutation both in glioma tissue and in the peripheral circulation.
In this study, we aimed to investigate whether IDH1 mutation could be associated with reduced chronic inflammation in glioma patients. For that purpose, the levels of common inflammatory markers NLR, platelet–lymphocyte ratio (PLR),Citation20 C-reactive protein (CRP),Citation21 and red cell distribution width (RDW)Citation22,Citation23 were analyzed in both patient groups, with and without IDH1 mutation. Furthermore, the prognostic role of these markers and that of IDH1-mt was evaluated in the same patients by using Kaplan–Meier and Cox regression analyses. In addition, the impact of other potential clinicopathological factors on survival was assessed.
Patients and methods
Patients
A total of 159 patients with newly diagnosed gliomas were enrolled between February 2015 and December 2016 at National Center for Neurosurgery (Astana, Kazakhstan). The glial tumors were diagnosed according to the 2016 World Health Organization Classification of Tumors of the Central Nervous System. Histological assessment of tissue samples was independently examined by two experienced neuropathologists. The following inclusion criteria were applied: age ≥18 years, normal hemoglobin level (120–180 g/L), absence of active infection, hematological, and autoimmune disorders, and absence of recent steroid treatment.
The clinicopathological characteristics analyzed were age, gender, IDH1R132H status, tumor grade, tumor side (left vs right vs middle vs other), tumor main functional involvement (eloquent vs near-eloquent vs noneloquent), Karnofsky performance score (KPS, <70 vs ≥70), and preoperative full blood count (FBC). The extent of resection was not included in the analysis because most tumors were high-grade gliomas located in eloquent area; therefore, the decision-making was preferably based on the preservation of cognitive functions and quality of life rather than the improvement of survival. OS was defined as the time interval from histologic diagnosis to death or last follow-up (October 2017) for surviving patients.
Patients with low-grade gliomas were generally treated only surgically, and some patients had indications for radiotherapy. In patients with high-grade gliomas, surgery was often followed by radiotherapy and/or chemotherapy; some high-grade gliomas patients were treated by surgery alone, because of the severity of their condition.
All patients gave written informed consent to the study protocol, which was approved by the Ethics Commission of National Centre for Neurosurgery (IORG0008395). This study was conducted in accordance with the Declaration of Helsinki.
IDH1 immunohistochemistry
Immunohistochemical staining with the anti-IDH1R132H mouse monoclonal antibody H09 (Dianova, Hamburg, Germany) was performed on formalin-fixed, paraffin-embedded tissue sections following the standard protocol recommended by the manufacturer.
Blood samples analysis
Preoperative blood samples were routinely collected for FBC within 1–2 days before surgery. FBC including neutrophil, lymphocyte, platelet counts, and RDW was measured using an automated hematology analyzer (Abbott CD-1800; Abbott Laboratories, Abbott Park, IL, USA). Serum CRP concentrations were determined on a Cobas Integra 600 analyzer (Roche, Tokyo, Japan). NLR and PLR were calculated, respectively, as neutrophil and platelet counts divided by lymphocyte count using standard units.
Statistical analysis
Continuous variables are presented as mean±standard error, and categorical variables are presented as frequencies and percentages. Relationships among variables were analyzed using bivariate tests including Pearson’s Chi-square, Spearman’s rho, and independent-samples Mann–Whitney U test. Receiver operating characteristic (ROC) curves were plotted for NLR, PLR, RDW, CRP, and age. The area under curve (AUC) was used as an estimation of diagnostic accuracy and Youden index was calculated to determine the optimal cutoff points. Kaplan–Meier OS curves stratified by the cutoff values were plotted, and survival differences between the groups were analyzed using the log-rank test. Univariate and multivariate Cox regression analyses were performed to determine prognostic variables. Statistical analyses were carried out using the SPSS statistical software package, version 20.0 (IBM Corporation, Armonk, NY, USA). The significance level was set at P<0.05.
Results
Patient characteristics
Seventy-two (45%) women and 87 men were included in the study. The mean age at diagnosis was 44.94±0.93 years, with a median of 46 years (range, 22‒67). Grade II gliomas included 20 cases (15%). Their distribution was as follows: diffuse astrocytoma, IDH-mt (4), diffuse astrocytoma, IDH-wt (2), oligodendroglioma, not otherwise specified (NOS) (8), and oligoastrocytoma, NOS (6). Grade III gliomas were presented by anaplastic oligodendroglioma, NOS (38), anaplastic oligoastrocytoma, NOS (7), anaplastic astrocytoma, IDH-mt (5), and anaplastic astrocytoma, IDH-wt (3). Finally, the grade IV group included glioblastoma, IDH-mt (10) and glioblastoma, IDH-wt (76). The majority of tumors were right/left-sided and located in eloquent or near-eloquent areas. Median tumor volume was 62 cm3 (mean 75.13±4.68 cm3, range 3.4–294 cm3). IDH1R132H was detected in a total of 79 (50%) samples. Most patients (59%) had KPS >70.
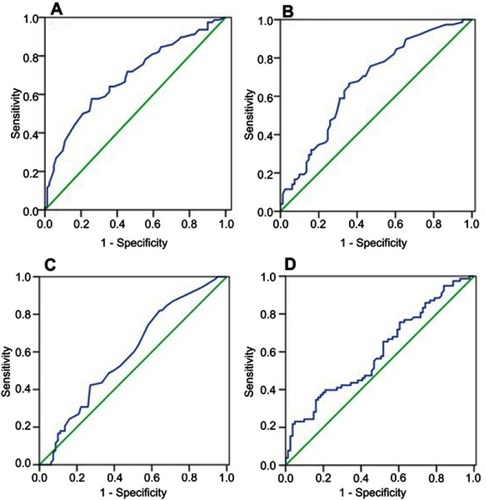
The mean preoperative NLR was 3.64±0.22 (median, 2.7; 0.60‒14.70), the mean PLR was 147.18±5.85 (median, 131.9; 47‒71.9),the mean RDW was 13.21±0.10 (median, 12.90; 11.10‒18.70), and the mean CRP was 3.59±0.43 mg/L (median, 1.41; 0.02–42.71). The optimal cutoff points determined from ROC curves () were: 2.65 for NLR, 166.85 for PLR, 12.75 for RDW, and 50.5 for age. Though the AUC values were low (<0.7), the tests were statistically significant (). In addition, our findings are in line with the literature. There is no consensus on the cutoffs of NLR, PLR, and RDW; various cutoff values have been used in previous studies. For example, in a meta-analysis on head and neck cancer,Citation24 the overall median NLR cutoff for OS was 2.895, ie very close to the 2.65 value determined in our study. Considering the prognostic role of PLR, 8 studies on solid tumorsCitation25 have used the cutoffs between 150 and 200 very close to the 166.85 value found in our study. As for RDW in cancer, the studies are rare and cutoff values are also heterogeneous. Two studies can be citedCitation26,Citation27 as examples where cutoff points determined by ROC analysis were close of 12.75 found in the present work (12.2 and 12.9, respectively). This study failed to obtain the optimal cutoff value for CRP (AUC 0.569, 95% CI 0.479–0.659, P<0.135). Therefore, the CRP cutoff value of 5 mg/L was chosen based on literature data. Citation28
Figure 1 ROC curves for: (A) age (AUC 0.686, 95% CI 0.604–0.769, P<0.000), (B) NLR (AUC 0.673, 95% CI 0.590–0.757, P<0.000), (C) PLR (AUC 0.599, 95% CI 0.511–0.687, P<0.032), and (D) RDW (AUC 0.591, 95% CI 0.503–0.680, P<0.047).
Abbreviations: ROC, receiver operating characteristic; AUC, area under curve; NLR, neutrophil–ymphocyte ratio; PLR, platelet–lymphocyte ratio; RDW, red cell distribution width.
Association of mutant IDH1 with inflammatory markers and other clinicopathological variables
The characteristics of patients stratified by IDH1 mutation status are presented in . The IDH1-mt patients were characterized by younger age (χ2=6.292, P<0.012), lower tumor grade (χ2=19.232, P<0.000), higher KPS (χ2=5.541, P<0.019), as well as reduced levels of NLR (χ2=4.583, P<0.032) and CRP (χ2=7.111, P<0.008). In addition, IDH1 mutation status was found to be in close association with PLR (χ2=2.807, P<0.094) and RDW (χ2=2.810, P<0.094). Similar results were obtained when the associations between IDH1-mt and inflammatory markers were analyzed using the Mann–Whitney U test. Namely, IDH1 mutation was strongly associated with NLR (P<0.024) and CRP (P<0.003). Also, it showed a tendency toward significance in relation to RDW (P<0.069) and PLR (P<0.094).
Table 1 Patient characteristics stratified by IDH1 mutation status
Moreover, Spearman correlation analysis was done to evaluate relationships between NLR and other inflammatory parameters. NLR was significantly correlated with PLR (ρ=0.633, P<0.000), CRP (ρ=0.280, P<0.000), and RDW (ρ=0.180, P<0.023). Thus, all the analyses showed that glioma patients with IDH1-mt are more likely to have lower inflammation levels compared to IDH1-wt individuals.
Prognostic role of IDH1 mutation and inflammatory markers on Kaplan–Meier analysis
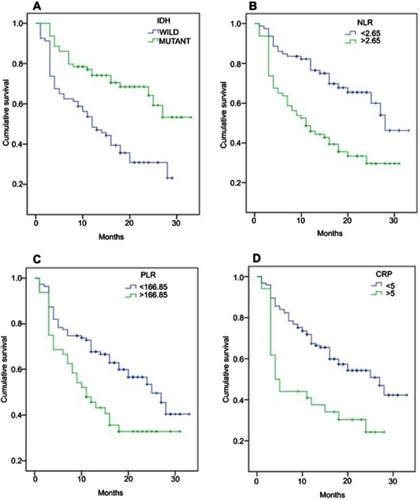
The OS curves stratified by IDH1 mutation status or by optimal cutoffs of NLR, PLR, and CRP are shown in . Seventy-nine (50%) IDH1-mt patients had median OS >33 months in comparison with 12 months for IDH1-wt patients (P<0.000) as shown in . NLR <2.65 was observed in 79 (50%) patients; presents the survival curves for patients with low and high NLR values (median OS of 28 vs 11 months, respectively; P<0.000). Low PLR levels (<166.85) were observed in 111 patients (70%) and were associated with better median OS of 25 months compared to 11 months for patients with high PLR (P<0.006) (). The OS curves for patients stratified by CRP of 5 mg/L show an increased median OS of 27 months in low CRP group (125 patients) vs 4 months in patients with higher CRP values (). Regarding RDW, Kaplan–Meier analysis failed to detect a significant difference in OS between patients with high and low levels (P<0.111).
Impact of mutant IDH1 and blood inflammatory markers on survival
The identification of factors associated with prognosis was performed using univariate and multivariate Cox regression analyses (). In univariate analysis, favorable prognostic factors were age<50.5 years (P<0.000), lower tumor grade (P<0.000), KPS ≥70 (P<0.000), tumor location in noneloquent area (P<0.030), IDH1 mutation (P<0.000), NLR<2.65 (P<0.000), PLR <166.85 (P<0.008), and CRP <5 mg/L (P<0.001). In a multivariate analysis including all variables, lower tumor grade (P<0.000), noneloquent location (P<0.015), IDH1-mt (P<0.044), and NLR <2.65 (P<0.022) remained associated with improved prognosis.
Table 2 Univariate and multivariate Cox regression analyses of factors affecting overall survival
Discussion
The main finding of the present study is that IDH1 mutation is significantly associated with reduced levels of inflammatory markers, in particular NLR (P<0.032). In addition, we showed that both IDH1-mt and low NLR were independent factors of better prognosis in multivariate analysis (P<0.044 and P<0.022, respectively).
NLR, reflecting the balance between neutrophilia and lymphopenia, has become a strong prognostic marker in human cancer. Citation29 There is growing evidence of the important contribution of neutrophils to chronic inflammation and thereby to cancer progression. Citation30–Citation32 Circulating neutrophils in cancer patients increase in number. Moreover, their population undergoes morphological and functional alterations and clearly differs from that of healthy donors’ neutrophils. Citation33 An increase in NLR level in cancer patients can be also caused by lymphopenia. Citation34 The mechanisms underlying lymphopenia in cancer patients are multifactorial and poorly understood. It is possible that the elevated myeloid cell content in IDH1-wt gliomas contributes to lymphocyte suppression via myeloid-derived suppressor cell activity. Citation35
Lower NLR values found in IDH1-mt patients in our study fit well into the proposed IDH1 mutation-driven inhibition of HIF1. Citation5 It is worth noting that IDH1 mutations exert numerous effects, in particular by inducing a glioma-CpG island methylator phenotype (G-CIMP) which results in altered patterns of gene expression and is associated with better outcome. Citation36,Citation37 Therefore, we cannot exclude that reduced levels of inflammatory markers in IDH1-mt patients could also potentially be triggered by changes in the expression profile.
The downregulation of HIF1 suggests a reduced expression of vascular endothelial growth factor (VEGF) and other HIF1-regulated inflammatory mediators, especially in lower grade gliomas. On the other hand, as the tumor grows, the level of hypoxia increases, leading to HIF1 upregulation through other hypoxia-induced signaling pathways. In line with this, we found that the levels of VEGF-A were significantly lower in the IDH1-mt low-grade glioma samples compared to the IDH1-wt ones; while in high-grade gliomas, this trend was not preserved (data not shown). The HIF1 inhibition by IDH1 mutation is supported by the results of Miroshnikova et al.Citation 38 Furthermore, Chesnelong et alCitation39 showed a marked reduction in expression of a large set of HIF-1α target genes in IDH1-mt gliomas. Also, an imaging study revealed that IDH1-mt glioblastomas have features of a less invasive tumor compared with IDH1-wt glioblastomas. Citation40
In this study, PLR and CRP were not independent factors associated with survival, though they were significantly related to survival in univariate analysis (P<0.008 and P<0.001, respectively). Besides, these parameters are strongly correlated with NLR (P<0.000 and P<0.000, respectively). There is increasing evidence that platelets, in addition to their fundamental role in hemostasis and thrombosis, have important functions in a broad range of immune responses. Citation41 Platelets can interact with other immune cells, contributing to various pathological states. Citation42,Citation43 A recent study identified a previously unrecognized role of platelets in leukocyte trafficking to the site of inflammation. Platelets were found to adhere preferentially to endothelial cell/cell junctions and to direct rolling and crawling neutrophils to these sites. Citation44 CRP is another well-known marker of systemic inflammation. The use of CRP as a tumor biomarker has gained interest; its prognostic role was demonstrated in various malignancies, including gliomas. Citation21,Citation45 As for RDW, although we found no significant impact of RDW on outcomes, a strong association was observed between RDW and NLR (P<0.023). RDW is a part of a standard blood count; it measures the size variability of circulating erythrocytes. Many studies demonstrated the prognostic significance of RDW in patients with different chronic inflammatory conditions,Citation46 and several studies showed its prognostic role in cancer. Citation22 The mechanisms of RDW fluctuations are little investigated.
This study has some limitations. First, our investigation was conducted in a single institution. Second, the most common IDH1R132H mutation was determined by immunochemistry, raising the possibility that for a given mutation, some cases were missed; also, we may have missed some of the rarer IDH1 and IDH2 mutations. Finally, there was a relatively small GII group of patients included in the analysis; larger cohorts should be followed in future studies.
Conclusion
Our results show that IDH1 mutation in gliomas is associated with lower levels of chronic inflammation which could account for the improved prognosis in this group of patients. These findings can be explained by the potential IDH mutation-driven inhibition of HIF1 and its downstream inflammatory mediators. Thus, the IDH1 mutation status at diagnosis can help with the therapeutic follow-up of patients, especially those not harboring this mutation. Accordingly, the need to develop potent anti-inflammatory drugs for IDH1-wt patients and for the treatment of cancer-related inflammation in general is becoming increasingly obvious. Citation47 As an example, one can cite the results of a Phase I/II trial which investigated the effect of siltuximab, an anti-IL-6 monoclonal antibody, in metastatic renal cell carcinoma patients; the drug was shown to stabilize disease in >50% of patients. Citation48 Also, a meta-analysis demonstrated that regular use of a nonsteroidal anti-inflammatory drug aspirin is inversely related to prostate cancer incidence and mortality. Citation49
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This study was supported by Ministry of Education and Science of the Republic of Kazakhstan (Grant No. 5377/GF4).
References
- Xia L, Wu B, Fu Z, et al. Prognostic role of IDH mutations in gliomas: a meta-analysis of 55 observational studies. Oncotarget. 2015;6(19):17354‒17365. doi:10.18632/oncotarget.v6i19
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 Mutations in Gliomas. N Engl J Med. 2009;360:765‒773. doi:10.1056/NEJMoa0808710
- Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469‒474. doi:10.1007/s00401-009-0561-9
- Dang L, White DW, Gross S, et al. Cancer associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739‒744. doi:10.1038/nature08617
- Molenaar RJ, Radivoyevitch T, Maciejewski JP, et al. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta. 2014;1846(2):326‒341.
- Zdzisińska B, Żurek A, Kandefer-Szerszeń M. Alpha-ketoglutarate as a molecule with pleiotropic activity: well-known and novel possibilities of therapeutic use. Arch Immunol Ther Exp. 2017;65:21‒36. doi:10.1007/s00005-016-0406-x
- Siddiq A. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32(4–5):931‒946. doi:10.1007/s11064-006-9136-5
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378‒389. doi:10.1016/j.apsb.2015.05.007
- Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1 alpha. Science. 2009;324:261‒265. doi:10.1126/science.1170944
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17‒30. doi:10.1016/j.ccr.2010.12.014
- Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2- hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484‒488. doi:10.1038/nature10843
- Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058‒1066. doi:10.1002/ijc.29519
- Noman MZ, Messai Y, Carré T, et al. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31:357‒377. doi:10.1615/CritRevImmunol.v31.i5
- Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349‒354. doi:10.1007/s004010051093
- Liang J, Piao Y, Holmes L, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20(1):187‒198. doi:10.1158/1078-0432.CCR-13-3045
- Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200‒207. doi:10.1016/j.semcancer.2013.02.001
- Menter DG, Tucker SC, Kopetz S, et al. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231‒269.
- Amankulor NM, Kim Y, Arora S, et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 2017;31:1‒13. doi:10.1101/gad.294991.116
- Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917‒930. doi:10.1007/s00401-016-1620-7
- Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119. doi:10.1371/journal.pone.010111924968121
- Wang CS, Sun CF. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J. 2009;32:471‒482.
- Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(9):16027‒16035.
- Auezova R, Ryskeldiev N, Doskaliev A, et al. Association of preoperative levels of selected blood inflammatory markers with prognosis in gliomas. OncoTargets Ther. 2016;9:6111‒6117. doi:10.2147/OTT
- Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Head Neck. 2018;40(11):2546‒2557. doi:10.1002/hed.25324
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi:10.1158/1055-9965.EPI-14-012924793958
- Zhang F, Chen Z, Wang P, Hu X, Gao Y, He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumor Biol. 2016;37:9323–9331. doi:10.1007/s13277-015-4774-3
- Sun Z, Ju Y, Han F, Sun X, Wang F. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer. J Clin Lab Anal. 2018;32(3):e22277. doi:10.1002/jcla.2018.32.issue-3
- Jin Y, Shi X, Shi L, et al. Prognostic value of circulating C-reactive protein levels in patients with non-small cell lung cancer: A systematic review with meta-analysis. J Cancer Res Ther. 2014;10(7):160‒166.
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju06124875653
- Kuang D, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948‒955. doi:10.1016/j.jhep.2010.08.041
- Trellakis S, Bruderek K, Dumitru C, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183‒2193. doi:10.1002/ijc.v129.9
- Wang J, Jia Y, Wang N, et al. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12(1):7. doi:10.1186/1479-5876-12-724397835
- Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562‒573. doi:10.1016/j.celrep.2014.12.039
- Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas and lymphomas. Cancer Res. 2009;69(13):5383‒5391. doi:10.1158/0008-5472.CAN-08-3660
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253‒268. doi:10.1038/nri3175
- Duncan CG, Barwick BG, Jin G, et al. A heterozygous IDH1R132H/WT mutation induces genome-wide alterations in DNA methylation. Genome Res. 2012;22:2339–2355. doi:10.1101/gr.123497.11122899282
- Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi:10.1038/nature1086622343889
- Miroshnikova YA, Mouw JK, Barnes JM, et al. Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol. 2016;18(12):1336–1345. doi:10.1038/ncb344327820599
- Chesnelong C, Chaumeil MM, Blough MD, et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol. 2014;16(5):686‒695. doi:10.1093/neuonc/nou085
- Price SJ, Allinson K, Liu H, et al. Less invasive phenotype found in isocitrate dehydrogenase-mutated glioblastomas than in isocitrate dehydrogenase wild-type glioblastomas: a diffusion-tensor imaging study. Radiology. 2017;283(1):215‒221. doi:10.1148/radiol.2016152679
- Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65‒76. doi:10.1016/j.thromres.2016.01.006
- Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5(3):1265‒1280.
- Pitchforda S, Pan D, Welch HCE. Platelets in neutrophil recruitment to sites of inflammation. Curr Opin Hematol. 2017;24:23‒31.
- Zuchtriegel G, Uhl B, Puhr-Westerheide D, et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 2016;14(5):e1002459. doi:10.1371/journal.pbio.100245927152726
- Strojnik T, Šmigoc T, Lah TT. Prognostic value of erythrocyte sedimentation rate and C-reactive protein in the blood of patients with glioma. Anticancer Res. 2014;34:339‒348.
- Su C, Liao LZ, Song Y, et al. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis. J Thorac Dis. 2014;6(10):1429‒1440.
- Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-related systemic inflammation: the challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther. 2017;102(4):599‒610. doi:10.1002/cpt.789
- Rossi JF, Négrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103(8):1154‒1162. doi:10.1038/sj.bjc.6605822
- Liu Y, Chen JQ, Xie L, et al. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. 2014;12:55. doi:10.1186/s12916-014-0141-224678716