Abstract
Objectives:
In this study, we aim to investigate the correlations of quantitative parameters of contrast-enhanced ultrasonography (CEUS) and Spalt-Like Transcription Factor 4 (Sall4)/Wnt/β-catenin signaling pathway with clinicopathological features and prognosis of patients with hepatocellular carcinoma (HCC).
Methods:
The CEUS was performed to detect the liver function and the prognosis of patients. The expression of Sall4, WNT3a and β-catenin was evaluated using immunohistochemical staining. Sall4, WNT3a and β-catenin mRNA expression was measured by SYBR green qPCR assay.
Results:
We found that the mRNA and protein expression of Sall4, WNT3a and β-catenin in the HCC tissues were significantly upregulated compared with the adjacent normal tissues. Upregulation of these proteins was associated with tumor differentiation, TNM stage, tumor size, vascular invasion and liver cirrhosis of HCC patients. In addition, we found that decreased time to peak and washout time and increased peak intensity and area under the curve of CEUS in the HCC were also correlated with TNM stage, tumor size and vascular invasion. Moreover, Sall4, WNT3a and β-catenin protein were significantly associated with the TTP, PI, AUC, and WOT.
Conclusion:
This study suggests that quantitative parameters of CEUS and Sall4/Wnt/β-catenin signaling may be helpful for early diagnosis and prognosis prediction of HCC patients.
Introduction
Hepatocellular carcinoma (HCC) accounting for 70–85% of the primary tumors of the liver is one of the highest mortality tumors all over the world.Citation1 The improvement in HCC treatment including liver resection, chemotherapy and transplantation has improved 5-year survival rates.Citation2 However, survival rates of the HCC patients diagnosed in late stage remain extremely low.Citation3 Therefore, development of novel and helpful early detection approaches is urgently needed for improving the therapeutic efficiency for HCC.
The Spalt-Like Transcription Factor 4 (Sall4) oncogene plays a central function in embryo-fetal development and is absent in differentiated tissues.Citation4 Reexpression of Sall4 in HCC tissues involved in demethylation of its CpGs is associated with poor prognosis.Citation5,Citation6 In addition, 1 week after treatment, higher levels of Sall4 protein in serum were observed in HCC patients, and these patients had poor prognosis evidenced by high tumor recurrence and low overall survival rate.Citation7 Thus, reexpression of Sall4 is relevant to the poor prognosis of HCC patients. Activation of WNT/β-catenin signaling that was positively correlated with Sall4 has been described as a crucial regulator in tumorgenesis.Citation8 Constitutive expression of Sall4 causes myelodysplastic syndrome)/acute myeloid leukemia through the Wnt/beta-catenin pathway.Citation9 In esophageal squamous cell carcinoma (ESCC), silencing of Sall4 inhibited tumor formation in vivo via Wnt/β-catenin signaling pathway.Citation10 And Sall4 promotes intrahepatic cholangiocarcinoma cell proliferation through Wnt/β-catenin signaling and enhancing epithelial–mesenchymal transition (EMT) process.Citation11 Thus, these studies proposed that Sall4/Wnt/β-catenin signaling may be a potentially promising molecular signaling for guiding HCC diagnosis and treatment.
Contrast-enhanced ultrasonography (CEUS), a typical nontraumatic examination method providing blood perfusion parameters, has been widely applied for diagnosis of many human cancers, including breast cancer and liver cancer.Citation12 Abundant new vessel generates around the tumors. CEUS is able to accurately and clearly show the characteristics of angiogenesis and neovessels in liver tumors and parenchyma.Citation13
Thus, in this study, we aim to investigate the association between the quantitative parameters of CEUS and Sall4/Wnt/β-catenin pathway in early diagnosis of HCC.
Patients and methods
Clinical patients recruitment
One hundred and sixty-eight patients diagnosed as HCC without diabetes, cardiovascular disease and other tumors from February 2011 to December 2011 in the General Hospital of Ningxia Medical University were included. The age ranged from 20 to 76 years (median is 52 years). The follow-up of all the patients was completed. The clinical information is shown in . The HCC tissues and adjacent tissues were obtained from surgical section and stored at liquid nitrogen until use. The tumor cells were confirmed by H&E staining. All subjects provided written informed consent. This study was approved by the Ethics Committee of General Hospital of Ningxia Medical University and was conducted in accordance with the Declaration of Helsinki.
Table 1 Clinicopathological features of 168 patients with hepatocellular carcinoma
Medical records of patients were collected. All patients who underwent tumor resection were followed up. The clinical features and postoperative symptoms were recorded. The CEUS was performed every 3 months after the surgery to evaluate the liver function. The follow-up started from the end of the surgery and lasted until December 2016. The survival time was defined as from the first day in hospital to the date of death with any reasons. The time of disease-free survival (DFS) was defined as until a tumor recurrence and/or metastatic lesions were observed. The DFS rates (1-, 3- or 5-year) were recorded.
CEUS
The patients were routinely examined with iU22 ultrasonic inspection instrument (Royal Philips Electronics NV, Eindhoven, the Netherlands) according to the standard operating procedure. All CEUS were performed and evaluated by two sonographers with more than 3 years’ experience in CEUS. Both low-frequency and high-frequency probe were used for CEUS. All patients received an intravenous injection of 2.4 mL of SonoVue, followed by 5 mL of saline flush. There was an interval of 10 mins between the two examinations in every patient. Four mins after contrast agent administration, CEUS process was recorded for 180 s to observe the lesions. The following CEUS patterns of nodules were considered: extent of enhancement during the arterial phase. The dynamic image was recorded on the hard disk of the sonography machine. Qlab software was used to analyze the microvessel image. The peak intensity (PI), area under the curve (AUC), blood perfusion time–intensity curve, time to peak (TTP) and washout time (WOT) were recorded for analysis.
Immunohistochemical staining assay
Immunohistochemical staining was used to determine the expression of Sall4, WNT3a and β-catenin. Briefly, the tissue sections cut at 4 μm were deparaffinized and hydrated, and then were retrieved with citrate buffer at boiling water for 15 mins. Then, the sections were incubated with primary antibodies (mouse monoclonal anti-Sall4, 1:200, mouse monoclonal anti- WNT3a, 1:300, rabbit polyclonal anti-β-catenin, 1:200, Cell Signal Technology, Danvers, MA, USA) overnight at 4°C. The sections were then incubated with secondary antibody for 60 mins at 37°C. The signaling was visualized by diaminobenzidine (DAB) and counterstained with hematoxylin.
For evaluating the expression of Sall4, WNT3a and β-catenin, the integral optical density (IOD) was obtained by ImageJ (National Institutes of Health, Bethesda, MA, USA). The IOD of Sall4, WNT3a and β-catenin expression was normalized to the average IOD of them in normal tissues.
RNA extraction and quantitative PCR analysis
Total RNA was extracted from cells using trizol reagent (Invitrogen, Carlsbad, CA, USA). Sall4, WNT3a and β-catenin mRNA expression was measured by SYBR green qPCR assay (Takara, Dalian, China) according to the recommended protocol. Expression of β-actin was used as an endogenous control. The primers were used as follows: Sall4, sense, CTAGACACGGCTCCCAATGC, antisense, AGACGTGA-ACTGCCCGAATC; WNT3a, sense, CTGGAGCTAGTGT-CTCCTCTCT, antisense, GGAAGAAGCCTCATCCAC-CA; β-catenin, sense, ATAAGAGCTCCTTGTGCGGC, antisense, GGCCATGTCCAACTCCATCA; β-actin, sense, AGGGGCCGGACTCGTCATACT, antisense, GGCGGCA-CCACCATGTACCCT. q-PCR was performed at the condition: 95.0°C for 3 mins and 39 circles of 95.0°C for 10 s and 60°C for 30 s. Data were processed using 2−ΔΔCT method.
Statistical analysis
SPSS17.0 statistical software (SPSS Inc. Chicago, IL, USA) was used to analyze the data. The data were presented as mean ± SD. The clinical association between protein expression, quantitative parameters of CEUS and clinicopathological variables in HCC patients was evaluated by Chi-square test. The difference among the groups was analyzed by Student’s t-test or one-way ANOVA depending on the conditions. And multivariate survival analysis was performed using Cox proportional hazards model. P-value <0.05 was considered to be statistically significant.
Results
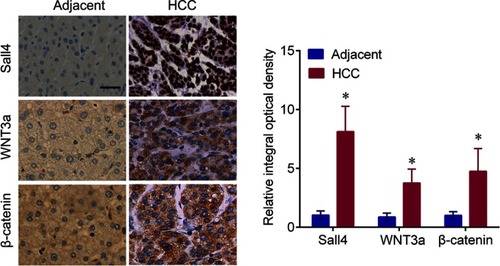
The expression of Sall4, WNT3a and β-catenin in HCC and adjacent tissues
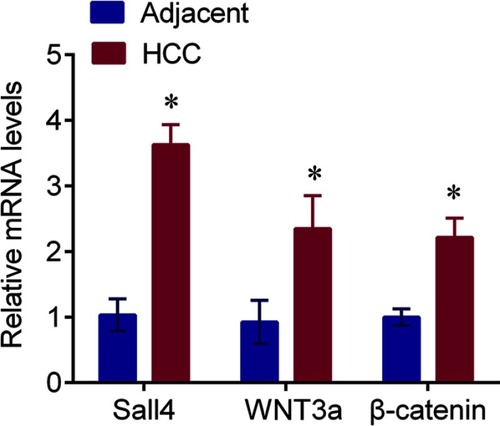
Sall4 was expressed in the nucleus, and WNT3a and β-catenin were expressed in the cytoplasm. The expressions of Sall4, WNT3a and β-catenin in the HCC tissues evaluated by IHC were significantly upregulated compared with the adjacent tissues (). The mRNA levels of Sall4, WNT3a and β-catenin were measured by q-PCR. In line with the findings of immunochemistry, the results revealed that Sall4, WNT3a and β-catenin mRNA levels were higher in the HCC tissues than those in the adjacent tissues (, all P<0.05).
Alterations of the parameters of CEUS in liver parenchyma and HCC tissues
Angiogenesis in HCC was enhanced compared with the liver parenchyma. We analyzed of the blood perfusion time–intensity curve and found that the TTP and WOT of CEUS in the HCC were significantly decreased, whereas PI and AUC of CEUS in HCC were dramatically increased than in the liver parenchyma (all P<0.05, ).
Table 2 Comparisons of quantitative parameters of CEUS between liver parenchyma and HCC lesions
Associations between Sall4, WNT3a and β-catenin expression and clinicopathological features of HCC patients
The expression of Sall4, WNT3a and β-catenin was not associated with age, gender and smoking history (P>0.05), but associated with tumor differentiation, TNM stage, tumor size, vascular invasion and liver cirrhosis of HCC patients (P<0.05). However, the positive rates of these proteins had no significant difference among viral cirrhosis, alcohol cirrhosis and other types of cirrhosis. In addition, Sall4 expression was significantly associated with HBV, HCV and alcohol history (P<0.05), whereas WNT3a and β-catenin expression was not associated with these features (P>0.05) ().
Table 3 Association of Sall4, WNT3a and β-catenin protein expressions with clinicopathological features of HCC patients
Association between the parameters of CEUS and clinical features of HCC patients
Based on positive expressions of Sall4, WNT3a and β-catenin protein in HCC tissues, the patients with HCC were divided into positive and negative groups. The results revealed that no significant association was observed between the patients’ age, gender and tumor differentiation and the TTP, PI, AUC and WOT (P>0.05), whereas Sall4, WNT3a and β-catenin expression, tumor size, vascular invasion and TNM stage were significantly associated with the TTP, PI, AUC and WOT (P<0.05, ).
Table 4 Association of quantitative parameters of CEUS with clinicopathological features of HCC patients
Association between Sall4, WNT3a and β-catenin expression, and the parameters of CEUS in DFS of HCC patients
The HCC patients were divided into 2 groups based on the median of TTP, PI, AUC and WOT (22 s, 60.3 dB, 593.4 dB*s and 72 s, respectively). We found that the DFS rates were not associated with age and gender, but associated with TNM stage, tumor size, tumor differentiation and vascular invasion, as well as Sall4, WNT3a and β-catenin expression (P<0.05). And the results showed that the HCC patients with positive Sall4, WNT3a and β-catenin expression had significantly low survival rates. In addition, the preoperative TTP, PI, AUC, and WOT were also associated with the DFS rates, suggesting reduced survival rates in patients with short TTP, strong PI, large AUC or short WOT (). Furthermore, we selected the factors including tumor differentiation, TNM stage, tumor size, vascular invasion, Sall4, WNT3a and β-catenin expression, the parameters of CEUS, including TTP, PI, AUC and WOT for multivariate survival analysis. We found that tumor differentiation, TNM stage, tumor size, tumor number, vascular invasion, Sall4, WNT3a and β-catenin expressions, and preoperative TTP and PI () were significantly correlated with HCC, indicating that Sall4, WNT3a and β-catenin expressions and preoperative TTP and PI are the independent prognostic factors for HCC patients.
Table 5 Univariate survival analysis of risk factors for the prognosis of HCC patients
Table 6 Multivariate survival analysis with Cox proportional hazards model of risk factors for the prognosis of HCC patients
Discussion
In this study, we found that the mRNA and protein expression of Sall4, WNT3a and β-catenin in the HCC tissues were significantly upregulated compared with the adjacent tissues. Upregulation of these proteins was associated with tumor differentiation, TNM stage, tumor size, vascular invasion and liver cirrhosis of HCC patients. In addition, we found that decreased TTP and WOT and increased PI and AUC of CEUS in the HCC were also correlated with TNM stage, tumor size and vascular invasion. In addition, Sall4, WNT3a and β-catenin protein were significantly associated with the TTP, PI, AUC and WOT. Collectively, our study supported that combination of Sall4/Wnt/β-catenin signaling with CEUS may become a useful supplementary method for HCC patients early diagnosis.
Sall4 is highly expressed in various cancers, including gastric cancer, breast cancer, lung cancer, colorectal cancer and HCC.Citation14–Citation16 Sall4 silencing by RNA interference or Sall4 peptide inhibitor treatment led to impaired lung cancer cell growth through EGFR and IGF1R signaling pathways.Citation17 Sall4 exhibits its oncogenic roles in gastric cancer progression through directly activating CD44 expression.Citation18 High Sall4 expression levels were associated with low overall survival, event-free survival and the presence of metastasis in hepatoblastoma, suggesting Sall4 is an independent prognostic predictor for overall survival.Citation19 In line with our results in this study, Hao et al found that Sall4 expression positively correlated with lymph node metastasis and TNM stages in colorectal cancer; and β-catenin was expressed significantly higher in colorectal cancer than normal tissue. The patients with colorectal cancer of co-expression of these two molecules showed advanced lymph node metastasis and TNM stage.Citation20 Co-location of Sall4 and β-catenin was found in the nucleus and cytoplasm, which indicated that the function of Sall4 in promoting lymph node metastasis and advanced clinical stage might partly be due to the interaction with β-catenin.Citation20 Sall4 modulates the stemness of ESCC cells via Wnt/β-catenin signaling pathway and in EMT.Citation10 And Sall4 promotes intrahepatic cholangiocarcinoma cell proliferation by activating Wnt/β-catenin signaling.Citation11
Interestingly, Sall4, WNT3a and β-catenin protein were significantly associated with the TTP, PI, AUC and WOT evaluated by CEUS. CEUS can be used to observe the neovessels in tumors. The parameters of CEUS reflect the proportions between the portal veins and hepatic arteries, and the vascular structures in tumors.Citation21 PI and AUC of CEUS can estimate enhancement intensity, and the TTP and WOT of CEUS are used to evaluate enhancement time.Citation22 PI and AUC of CEUS are applied to reveal the intratumoral vascularity of ovarian tumors as noninvasive parameters.Citation23 Tian and Wang showed that the PI and AUC of poor-differentiated HCC lesions were significantly lower than those of well-differentiated HCC lesions.Citation24 Our findings showed that decreased TTP and WOT and increased PI and AUC of CEUS in HCC lesions were correlated with TNM stage, tumor size and vascular invasion. Besides, the preoperative TTP, PI, AUC and WOT were associated with the DFS rates, suggesting reduced survival rates in patients with short TTP, strong PI, large AUC or short WOT. Additionally, this study first time found that Sall4/Wnt/β-catenin expression was associated with TTP, PI, AUC and WOT in patients with HCC. Thus, combination CEUS and Sall4/Wnt/β-catenin signaling can more accurately evaluate the intratumoral neovascular generation and prognosis of HCC patients.
Conclusion
This study suggests that combination quantitative parameters of CEUS and Sall4/Wnt/β-catenin signaling may be a useful supplementary method for early screening and diagnosis in HCC patients. And a larger cohort is needed in the future to confirm our results study and provide strong evidence for predicting prognosis of patients with HCC.
Ethics approval and informed consent
This study was approved by Ethics Committee of General Hospital of Ningxia Medical University.
Author contributions
Jianjun Wang and Guanghui Liu contributed to study design, data analysis and wrote this paper. Jiaxin Huang, Qianfeng Ma performed clinical sample collection. Jiaxin Huang and Jianjun Wang performed qPCR. Qianfeng Ma performed immunohistochemical staining assay. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. doi:10.1053/j.gastro.2016.11.04828043904
- Villanueva A, Llovet JM. Liver cancer in 2013: mutational landscape of HCC–the end of the beginning. Nat Rev Clin Oncol. 2014;11(2):73–74. doi:10.1038/nrclinonc.2013.24324395088
- Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239. doi:10.1053/j.gastro.2015.05.06126099527
- Jones B. Liver cancer: SALL4–a cancer marker and target. Nat Rev Clin Oncol. 2013;10(8):426. doi:10.1038/nrclinonc.2013.112
- Yin F, Han X, Yao SK, Wang XL, Yang HC. Importance of SALL4 in the development and prognosis of hepatocellular carcinoma. World J Gastroenterol. 2016;22(9):2837–2843. doi:10.3748/wjg.v22.i9.283726973422
- Fan H, Cui Z, Zhang H, et al. DNA demethylation induces SALL4 gene re-expression in subgroups of hepatocellular carcinoma associated with hepatitis B or C virus infection. Oncogene. 2016;36(17):2435–2445. doi:10.1038/onc.2016.399
- Han SX, Wang JL, Guo XJ, et al. Serum SALL4 is a novel prognosis biomarker with tumor recurrence and poor survival of patients in hepatocellular carcinoma. J Immunol Res. 2014;2014:262385. doi:10.1155/2014/39412724860834
- Shuai X, Zhou D, Shen T, et al. Overexpression of the novel oncogene SALL4 and activation of the Wnt/beta-catenin pathway in myelodysplastic syndromes. Cancer Genet Cytogenet. 2009;194(2):119–124. doi:10.1016/j.cancergencyto.2009.06.00619781444
- Ma Y, Cui W, Yang J, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108(8):2726–2735. doi:10.1182/blood-2006-02-00159416763212
- He J, Zhou M, Chen X, et al. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via Wnt/beta-catenin pathway in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35(1):98. doi:10.1186/s13046-016-0444-627329034
- Zhu L, Huang F, Deng G, et al. Knockdown of Sall4 inhibits intrahepatic cholangiocarcinoma cell migration and invasion in ICC-9810 cells. Onco Targets Ther. 2016;9:5297–5305. doi:10.2147/OTT.S10721427601921
- Bo XW, Xu HX, Wang D, et al. Fusion imaging of contrast-enhanced ultrasound and contrast-enhanced CT or MRI before radiofrequency ablation for liver cancers. Br J Radiol. 2016;89(1067):20160379. doi:10.1259/bjr.2016037927626506
- Takada H, Tsuchiya K, Yasui Y, et al. Irregular vascular pattern by contrast-enhanced ultrasonography and high serum Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein level predict poor outcome after successful radiofrequency ablation in patients with early-stage hepatocellular carcinoma. Cancer Med. 2016;5(11):3111–3120. doi:10.1002/cam4.93227748052
- Liu W, Xiao P, Wu H, et al. MicroRNA-98 plays a suppressive role in non-small cell lung cancer through inhibition of SALL4 protein expression. Oncol Res. 2016;25(6):975–988. doi:10.3727/096504016X14791726591124
- Tian Q, Xiao Y, Wu Y, et al. MicroRNA-33b suppresses the proliferation and metastasis of hepatocellular carcinoma cells through the inhibition of Sal-like protein 4 expression. Int J Mol Med. 2016;38(5):1587–1595. doi:10.3892/ijmm.2016.275428026002
- Dirican E, Akkiprik M. Functional and clinical significance of SALL4 in breast cancer. Tumour Biol. 2016;37(9):11701–11709. doi:10.1007/s13277-016-5150-727444278
- Yong KJ, Li A, Ou WB, et al. Targeting SALL4 by entinostat in lung cancer. Oncotarget. 2016;7(46):75425–75440. doi:10.18632/oncotarget.1225127705911
- Yuan X, Zhang X, Zhang W, et al. SALL4 promotes gastric cancer progression through activating CD44 expression. Oncogenesis. 2016;5(11):e268. doi:10.1038/oncsis.2016.6927819668
- Zhou S, Venkatramani R, Gomulia E, Shillingford N, Wang L. The diagnostic and prognostic value of SALL4 in hepatoblastoma. Histopathology. 2016;69(5):822–830. doi:10.1111/his.1300527252091
- Hao L, Zhao Y, Wang Z, et al. Expression and clinical significance of SALL4 and beta-catenin in colorectal cancer. J Mol Histol. 2016;47(2):117–128. doi:10.1007/s10735-016-9656-526779651
- Liu Y, Xu Y, Cheng W, Liu X. Quantitative contrast-enhanced ultrasonography for the differential diagnosis of endometrial hyperplasia and endometrial neoplasms. Oncol Lett. 2016;12(5):3763–3770. doi:10.3892/ol.2016.520627895728
- Zheng W, Xiong YH, Han J, et al. Contrast-enhanced ultrasonography of cervical carcinoma: perfusion pattern and relationship with tumour angiogenesis. Br J Radiol. 2016;89(1065):20150887. doi:10.1259/bjr.2015088727340932
- Wang J, Lv F, Fei X, et al. Study on the characteristics of contrast-enhanced ultrasound and its utility in assessing the microvessel density in ovarian tumors or tumor-like lesions. Int J Biol Sci. 2011;7(5):600–606.21614152
- Tian H, Wang Q. Quantitative analysis of microcirculation blood perfusion in patients with hepatocellular carcinoma before and after transcatheter arterial chemoembolisation using contrast-enhanced ultrasound. Eur J Cancer. 2016;68:82–89. doi:10.1016/j.ejca.2016.08.01627728840