Abstract
Background
Controversial conclusions had been reported in studies trying to confirm the impact of heart dose on overall survival (OS) reported in RTOG 0167 for non-small cell lung cancer (NSCLC) patients who underwent radiotherapy (RT). The purpose of this study is to investigate the association of lung and heart dosimetric parameters with OS in NSCLC patients treated by volumetric modulated arc therapy (VMAT).
Methods
Inoperable NSCLC patients treated by VMAT from March 2012 to December 2015 were retrospectively reviewed. OS and progression-free survival (PFS) were estimated with the Kaplan–Meier method. Univariate and multivariate analyses were conducted with Cox proportional hazards model. Multivariate model building was conducted using stepwise regression for variables with p-value smaller than 0.2 in the univariate analysis.
Results
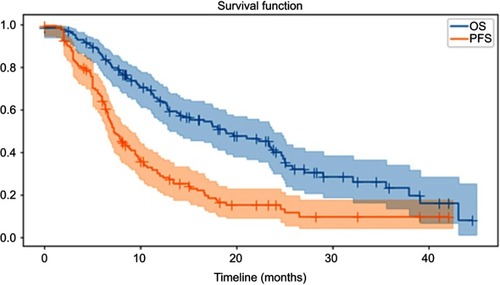
There were 130 NSCLC patients enrolled in this study with a median age of 63 years (range from 34 to 82 y). The median prescription dose for these patients was 56 Gy (range 40–70 Gy) with a mean heart and lung dose of 14.8±8.5 Gy and 13.6±4.4 Gy, respectively. The rates of patients with above grade III radiation pneumonitis (RP) and fibrosis were 8.5% and 8.5%, respectively. The 2-year PFS and OS of these patients were 15.2% and 39.8%, with a median PFS and OS of 7.2 and 18.8 months, respectively. RP was correlated with OS (p=0.048) and lung V20 was associated with PFS (p=0.04) according to the univariate analysis. Multivariate analysis demonstrated that RP (HR 1.39, 95%CI 1.010–1.909, p=0.043) and heart V15 (HR 1.02, 95%CI 1.006–1.025 p=0.002) were progression factors of OS, and no factor was associated with PFS.
Conclusions
RP and heart V15 were associated with OS for patients with stage III NSCLC who underwent VMAT. Heart and lung dosimetric parameters were highly correlated with each other, sparing of heart and lung should be considered equally during the treatment planning.
Introduction
The fact that high radiation dose to thoracic cancer can lead to heart toxicity has long been recognized with reported up to 20% of the patients with breast and lymphoma cancer elevated rates of heart disease typically 1 or more years after radiotherapy (RT).Citation1–Citation3 Due to few long-term survivors of patients with advanced non-small cell lung cancer (NSCLC), the clinical relevance of RT-associated heart disease for these patients is unclear. Recently, the landmark trial (radiation therapy oncology group (RTOG) 0617), which compared high-dose versus standard-dose thoracic radiation for patients with stage IIIA–IIIB NSCLC and closed early due to futility boundaries were crossed, reported that heart volume receiving 5 Gy (V5) or 30 Gy (V30) was a significant negative effect on survival.Citation4 However, the heart dose differences between two arms and specific cardiac toxicities were not reported.
Lots of studies were conducted to investigate whether the impact of heart dose on early overall survival (OS) reported in RTOG0617 could be confirmed in an independent cohort.Citation5–Citation9 Tucker et al retrospectively reviewed 465 patients with stage IIIA–IIIB NSCLC received 3D conformal radiotherapy (CRT), intensity-modulated radiotherapy (IMRT), or proton therapy with concurrent chemotherapy between 1999 and 2010 and reported that heart dose was not associated with early survival outcomes, but he suggested that dose to the heart should be minimized based on the known adverse effects of RT on vasculature and cardiac function.Citation5 On the other study, after reviewing and recontouring the normal tissues following RTOG 0617 guideline for 333 patients with locally advanced (LA) NSCLC, Speirs et al reported that heart V50 was associated with OS and cardiac toxicity for locally advanced NSCLC treated with chemoradiation.Citation6 Wang et al reviewed six trials including 127 patients with stage III NSCLC received 70–90 Gy (median 74 Gy) RT and reported that heart doses were not associated with OS, but he suggested that cardiac toxicity after RT may occur earlier than historically understood, and heart doses should be minimized.Citation7
These controversial conclusions indicated that the role of heart dose on the effect of OS was still unclear for patients with NSCLC who underwent RT. Studies suggested that although heart dose may be important, it may not be cardiac toxicity that is driving survival, heart dose may be a surrogate for other prognostic factors in stage III NSCLC rather than an independent predictor of outcome.Citation10 Oh et al retrospectively reviewed 453 patients with stage I–III esophageal cancer treated by definitive or preoperative chemoradiation and reported that cardiac dose was not independently predictive after adjusting for lung dose and other clinical factors of survival, but lung dose was a significant independent predictor of survival.Citation11
Meanwhile, advanced RT modalities, such as IMRT can lower heart doses and reduce their toxicities.Citation12,Citation13 As an extended form of IMRT, volumetric modulated arc therapy (VMAT) is able to achieve similar dosimetric distribution while decreasing machine unit (MU) and treatment delivery time compared with IMRT; however, the clinical outcomes and related toxicities of VMAT were rarely reported. The purpose of this study is to investigate the efficacy and safety of VMAT, as well as the association of lung and heart dosimetry with survival in patients with NSCLC treated by VMAT.
Materials and methods
Patients and treatment
Patients who underwent definitive RT for stage IIIA/IIIB NSCLC with or without concurrent chemotherapy from March 2012 to November 2015 were retrospectively reviewed in this study. Concurrent chemotherapy was administered with a combination of cisplatin 50 mg/m2 on days 1, 8, 29, etoposide 50 mg/m2 on days 3, 30; or carboplatin AUC 5 on day 1 and paclitaxel 500 mg/m2 on day 1, repeated every 21 days for 4 cycles. Gross tumor volume (GTV) was delineated on contrast CT images following the International Commission on Radiation Units and Measurements (ICRU) report 62 criteria.Citation14 Planning target volume (PTV) was considered to include the GTV plus a 10–15 mm margin. Normal lung volumes were contoured by excluding the GTVs. Heart was delineated according to the Atlas for organs at risk for thoracic radiation therapy. RT was delivered with VMAT technique for a prescription dose from 40 to 70 Gy at 2 Gy per fraction. Detailed VMAT optimization was reported in a previous paper.Citation15 This retrospective study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Wenzhou Medical University. The patient data confidentiality was maintained in this study and compliance with the Declaration of Helsinki. The need for written informed consent was waived by the IRB due to the retrospective nature of this study.
Dosimetric parameters
Dosimetric parameters of PTV and organs at risk (OARs) from final treated VMAT plans were extracted from Pinnacle (Phillips, version 9.8) treatment planning system (TPS) using home built MATLAB codes. For PTV, the maximum (Dmax), mean (Dmean) dose, and the volume covered by the prescription dose (V95%, V93%) and homogeneity index (HI) were extracted. The Dmax and D2% of the spinal cord, the V5, V15, V25, V30, and V50 (percent volume irradiated by X dose) of the heart, and the V5, V10, V13, V20, and V30 and Dmean of the lungs were also extracted.
Follow-up
Follow-up evaluation was conducted every 3 months for the first 2 years, and every 6 months thereafter. Patients were required to have an immediate examination or intervention if symptoms, such as fever, cough, or shortness of breath were observed during follow-up. Acute radiation pneumonitis (RP) and late fibrosis were diagnosed by at least two radiation oncologists with consensus according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.Citation16 RP was scored as greater than or equal to grade III side effects.
Statistical analysis
Progression-free survival (PFS) was calculated from the date of RT to the time of first local or distant recurrence, or death from any cause. OS was measured from the date of RT to death or the last follow-up visit. Survival curves were estimated using Kaplan–Meier method and comparisons were made using the log-rank test. Univariate and multivariate analyses were conducted with Cox proportional hazards model. Multivariate model building was conducted using stepwise regression (modification of the forward selection method) for variables with probability p-value smaller than 0.2 in the univariate analysis. Correlation analysis was done with Pearson’s method. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
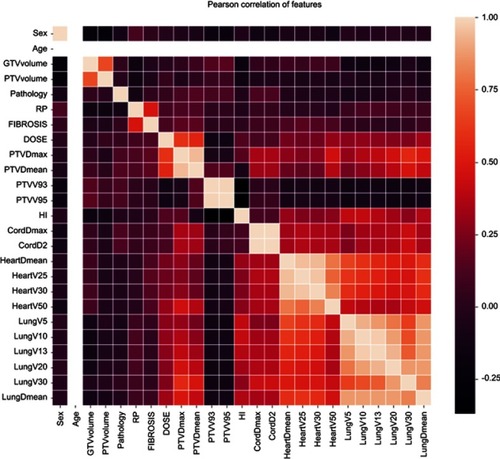
A total of 130 patients (Male 100, female 30) with stage III NSCLC who underwent VMAT were enrolled in this study with a median age of 63 years (range from 34 to 82 y). Detailed characteristics of these patients are presented in . The majority of patients had adenocarcinoma (36.9%) and squamous cell carcinoma (49.3%) with 66.9% patients had concurrent chemotherapy. The detailed dosimetric parameters are presented in . The median prescription dose for these patients was 56 Gy (range 40–70 Gy) with a mean heart and lung dose of 14.8±8.5 Gy and 13.6±4.4 Gy, respectively. The rates of patients with above grade III RP and fibrosis were 8.5% and 8.5%, respectively. presents the Pearson correlation heatmap between all the relative parameters. Lung V5, V10, V13, and lung mean dose were correlated with heart dosimetric parameters.
Table 1 Characteristics of patients with lung cancer who underwent VMAT treatment
Table 2 Dosimetric parameters of patients with lung cancer who underwent VMAT treatment
Figure 1 Heatmap of Pearson correlation between heart and lung dosimetric parameters.
Abbreviations: GTV, gross tumor volume; PTV, planning target volume; RP, radiation pneumonitis; HI, homogeneity index.
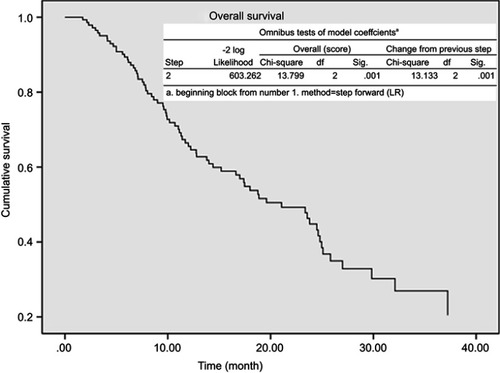
PFS and OS with 95% confidence interval (CI) of these patients are shown in with a median follow-up of 14 months. The 2-year PFS and OS of these patients were 15.2% and 39.8%, with a median PFS and OS of 7.2 and 18.8 months, respectively. Univariate and multivariate analysis on the factors that associated with OS and PFS is presented in . RP was correlated with OS (p=0.048) and lung V20 was associated with PFS (p=0.04) according to the univariate analysis. Multivariate analysis demonstrated that RP (HR 1.39, 95%CI 1.010–1.909, p=0.043) and heart V15 (HR 1.02, 95%CI 1.006–1.025 p=0.002) were progression factors of OS, and no factors were associated with PFS. The Kaplan–Meier curve and p-value (0.001) from stratified Cox proportional hazards model are presented in .
Table 3 Univariate and multivariate analysis of factors associated with overall survival and progression-free survival with Cox model
Discussion
The clinical outcome, impacts of heart and lung dosimetric parameters on the OS and PFS of patients with stage III NSCLC who underwent VMAT were investigated in this study. The RP and heart V15 were correlated with OS, while there was no heart and lung dosimetry clearly correlated with PFS.
Dosimetric studies had reported that VMAT and IMRT were able to achieve superior dose distributions compared with conformal RT technique in the treatment of lung cancer and resulted in better local control due to their dose sculpting abilities.Citation17 However, few studies had reported the clinical outcome of VMAT in the treatment of lung cancer. A 2-year PFS and OS of 15.2% and 39.8% were reported in this study for VMAT in the treatment of stage III NSCLC. Similarly, a 2-year OS of 58% for patients with stage Ib–IIIb (62% stage III) NSCLC and a 1-year OS of 57% for patients with NSCLC (85% stage III) were reported by Sura et al and Yom et al, respectively, for patients who underwent IMRT treatment.Citation18,Citation19 Liao et al also reported a 2-year PFS and OS of 38% and 46%, respectively, for patients with locally advanced NSCLC treated by IMRT.Citation20
The variations in PFS and OS for different studies mentioned earlier could be attributed to many factors, such as differences in histological type, adjuvant chemotherapy, comorbid conditions, RT techniques, etc.Citation21,Citation22 Treatment complications induced during RT also have a role in OS. RP is a common and lethal lung injury caused by the lung exposure during RT for patients with NSCLC.Citation22 RP is strongly correlated with the dose irradiated to normal lung and could affect 13–37% of the patients depending on the specific RT techniques.Citation23,Citation24 In this study, RP (HR 1.39, 95% CI 1.010–1.909, p=0.043) was correlated to OS in both univariate and multivariate analysis for NSCLC patients who underwent VMAT. Consistently, Inoue et al reported that severe RP was the only factor negatively associated with survival for patients who underwent definitive radiotherapy for lung cancer.Citation25 In another study, Minami-Shimmyo et al stated that deaths after concurrent chemoradiation therapy for patients with lung cancer were associated with RP or pulmonary fibrosis.Citation26 Oh et al also reported that lung dose is a significant independent predictor of survival for patients with esophageal cancer who underwent radiotherapy.Citation11
In this study, multivariate analysis indicated that heart V15 (HR 1.02) was a weak progression factor of OS for NSCLC patients who underwent VMAT. This weak correlation could not further support the statements reported in the previous studies that V5, V30, or V50 were associated with OS of patients with lung cancer who underwent radiotherapy.Citation4,Citation6 Concerns and reports regarding radiation-related cardiac and vascular toxicity were mainly raised in the era when two-dimensional RT was applied to treat the patients with breast cancer and Hodgkin’s lymphoma and severe complications were observed.Citation27,Citation28 Currently, with the wide application of IMRT and VMAT, heart exposure andthe risk of radiation-related events had been steadily declined.Citation29,Citation30 On the other hand, heart and lung dosimetric parameters tend to be highly correlated with each other, as demonstrated by the Pearson correlation results in in this study. Similarly, Tucker et al explained that the effect of heart dose on OS might be due to a correlation between heart and lung doses in their study and in the study of RTOG 0167.Citation5
Although we found that heart dose was weakly correlated with survival in this study, sparing of the heart should remain a priority during clinical practice in the RT of lung cancer, as many evidence indicated that cardiac exposure was associated with long-term cardiac disease and contribute to morbidity and even mortality.Citation31 One limitation of this study is that this is a retrospective study from one institution only; the impact of different modern RT techniques other than VMAT and dosimetric constraints of other OARs on survival needs further investigation.
Conclusions
In summary, RP and heart V15 were associated with OS for patients with stage III NSCLC who underwent VMAT. Heart and lung dosimetric parameters were highly correlated with each other, sparing of heart and lung should be considered equally during the treatment planning.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by funding of National Natural Science Foundation of China (11675122) and Wenzhou Municipal Science and Technology Bureau (2018ZY016).
References
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi:10.1093/jnci/djk06417341728
- van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi:10.1001/jamainternmed.2015.118025915855
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi:10.1200/JCO.2015.63.444426573075
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199.25601342
- Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119(3):495–500. doi:10.1016/j.radonc.2016.04.02527189523
- Speirs CK, DeWees TA, Rehman S, et al. Hart dose is an independent dosimetric predictor of OS in LA NSCLC. J Thorac Oncol. 2017;12(2):293–301. doi:10.1016/j.jtho.2016.09.13427743888
- Wang K, Eblan MJ, Deal AM, et al. Marks cardiac toxicity after radiotherapy for stage III non–small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. doi:10.1200/JCO.2016.70.022928113017
- Stam B, van der Bijl E, van Diessen J, et al. Heart dose associated with overall survival in locally advanced NSCLC patients treated with hypofractionated chemoradiotherapy. Radiother Oncol. 2017;125(1):62–65. doi:10.1016/j.radonc.2017.09.00428939179
- McWilliam A, Kennedy J, Hodgson C, et al. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106–113. doi:10.1016/j.ejca.2017.07.05328898766
- Cella L, D’Avino V, Palma G, et al. Modeling the risk of radiation-induced lung fibrosis: irradiated heart tissue is as important as irradiated lung. Radiother Oncol. 2015;117(1):36–43. doi:10.1016/j.radonc.2015.07.05126277435
- Oh P, Zhang M, Brady P, et al. Impact of lung and heart dose on survival after radiotherapy for esophageal cancer. J Clin Oncol. 2018;36(4 suppl):3. doi:10.1200/JCO.2018.36.4_suppl.3
- Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117(13):3004–3013. doi:10.1002/cncr.2584821264827
- Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087–1096. doi:10.1016/j.ijrobp.2006.01.05216682145
- International Commission on Radiation Units and Measurements. ICRU Reports 62 (Supplement to ICRU Report 50). Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda (MD): ICRU; 1999.
- Jin X, Xie C. Dosimetric and clinical benefits of conformal radiotherapy plus volumetric modulated arc therapy in the treatment of non-small cell lung cancer. Med Phys. 2015;42(6):3704–3705. doi:10.1118/1.4926135
- Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol. 2012;6(7):1025–1039. doi:10.1016/j.jaad.2012.02.010
- Bree I, van Hinsberg MG, van Veelen LR. High-dose radiotherapy in inoperable non-small cell lung cancer: comparison of volumetric modulated arc therapy, dynamic IMRT and 3D conformal radiotherapy. Med Dosim. 2012;37:353–357. doi:10.1016/j.meddos.2011.12.00222459649
- Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. doi:10.1016/j.radonc.2008.02.00518343515
- Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi:10.1016/j.ijrobp.2006.12.03117321067
- Liao ZX, Komaki RR, Thames HD Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi:10.1016/j.ijrobp.2009.02.03219515503
- Nalbantov G, Kietselaer B, Vandecasteele K, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol. 2013;109:100–106. doi:10.1016/j.radonc.2013.08.03524044794
- Goldstraw P, Ball D, Jett JR, et al. Non-small- cell lung cancer. Lancet. 2011;378:1727–1740. doi:10.1016/S0140-6736(11)60984-721565398
- Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three dimensional conformal radiotherapy (3-DCRT). Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi:10.1016/j.ijrobp.2006.07.133716997503
- Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–283.12865175
- Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655.11172945
- Minami-Shimmyo Y, Ohe Y, Yamamoto S, et al. Risk factors for treatment-related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thorac Oncol. 2012;7:177–182. doi:10.1097/JTO.0b013e31823c4c0722134071
- Cosset JM, Henry-Amar M, Girinski T, Malaise E, Dupouy N, Dutreix J. Late toxicity of radiotherapy in Hodgkin’s disease. The role of fraction size. Acta Oncol. 1988;26:123–129. doi:10.3109/02841868809090332
- Brenner DJ, Shuryah I, Jozsef G, DeWyngaert KJ, Formenti SC. Risk and risk reduction of major coronary events associated with contemporary breast RT. JAMA Intern Med. 2014;174:158–160. doi:10.1001/jamainternmed.2013.1179024166078
- Maraldo MV, Brodin P, Aznar MC, et al. Doses to carotid arteries after modern radiation therapy for Hodgkin lymphoma: is stroke still a late effect of treatment? Int J Radiat Oncol Biol Phys. 2013;87:297–303. doi:10.1016/j.ijrobp.2013.06.00423910709
- Maraldo MV, Brodin NP, Aznar MC, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol. 2013;24:2113–2118. doi:10.1093/annonc/mdt15623619032
- Hatakenaka M, Yonezawa M, Nonoshita T, et al. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83:e67–e73. doi:10.1016/j.ijrobp.2011.12.01822365626