Abstract
Purpose
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck cancers and is thought to be related to the mucosal immune system. Radiation therapy (RT) is the primary treatment for NPC due to the high radiosensitivity of cancer cells. However, little is known about the impact of RT on the mucosal immune system.
Patients and methods
In this study, the expression of immune markers CD19, CD24, CD27, CD8, and IgA before and after RT, were analyzed using flow cytometry. Cytokines were assessed using the enzyme-linked immunosorbent assay. Reactive oxygen species (ROS) was assayed by flow cytometry and fluorescence staining using 2ʹ,7ʹ -dichlorofluorescein diacetate.
Results
We found that primary NPC patients had a significant increase in CD19+CD138−IgA+ B cells, which was then decreased after RT. Interestingly, the changes in CD19+CD138−IgA+ B cell frequency was accompanied by corresponding frequency changes in cytotoxic T cells (CTL), which are powerful anti-tumor lymphocytes. Mechanistically, we found that ROS release during RT specifically eliminated CD19+CD138−IgA+ B cells.
Conclusion
These findings suggest that RT may regulate the immune system and opens up new avenues for the utilization of immune-radiotherapy in NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is associated with the Epstein-Barr virus (EBV) and is characterized by epistaxis, nasal obstruction and discharge, and headache.Citation1 NPC has a marked geographic distribution, where it is endemic to North Africa, Southern Asia and southern China.Citation2 Due to its anatomic characteristics and highly sensitivity to radiation therapy (RT), NPC is generally treated with RT but rather than with surgery.Citation3,Citation4 The mechanism of RT for tumor treatment is complex. Studies have demonstrated that high dose irradiation directly breaks double-stranded DNA in tumor cells triggering apoptosis.Citation5,Citation6 On the contrary, low dose irradiation leads to the release of damage-associated molecular pattern molecules, cytokines and reactive oxygen species (ROS) from tumor cells which may interact with immune cells.Citation7–Citation9 However, the specific immune responses elicited after RT treatment in patient with NPC as well as its relationship with prognosis remains unknown.
Nasopharyngeal-associated lymph reticular tissue is a major mucosal inductive site, and has mucosal immune characteristics, such as high expression levels of secreted IgA (sIgA), which protects against EBV in NPC patients.Citation10 However, the function of IgA+ B cells in the cancer microenvironment remains controversial. Several studies revealed that in addition to sIgA producing CD19−IgA+ plasma cells, there is another subset of CD19+CD138−IgA+ B cells that serve as regulatory B cells (Breg) of the mucosal immune system during cancer progression.Citation11,Citation12 Human Breg cells are usually identified as CD19 positive, CD24 high and CD27 positive (CD19+CD24hiCD27+).High numbers of these cells are found in the peripheral blood mononuclear cells (PBMC) of patients with autoimmune diseases, where they play a suppressive role.Citation13,Citation14 Thus, CD19+CD138−IgA+ Bcells, CD19+CD24hiCD27+ Breg, along with other cell subsets, including regulatory T cells (Tregs) may act as suppressive immune cells that influence the NPC prognosis. To date, the role of Bregs in NPC is poorly understood.
ROS is an electrophilic chemical species with a short half-life and is generated by incomplete oxidation. It has long been implicated as a secondary messenger involved in cell signaling activation and cell fate decisions.Citation15,Citation16 ROS can control B cell development, activation and apoptosis through activation of the B cell receptor or caspases signaling pathways.Citation17–Citation19 In the present study, we demonstrated increased percentages of CD19+CD138−IgA+ B cells in the PBMC of NPC patients where they played a suppressive function. Additionally, we showed that RT triggered the release of ROS into the tumor microenvironment, which led to the elimination of IgA+ B cells and increased cytotoxic T cell (CTL) populations, potentially augmenting the anti-tumor effect.
Materials and methods
Patients and controls
Peripheral blood was obtained from 38 primary NPC patients (mean, 47.8years; range, 15–64 years). Of those, 24 patients had blood recollected after RT. All patients had pathologically confirmed NPC and had not received prior treatment. summarizes the characteristics of NPC patients. All patients were staged using the 7th edition of the Staging System of the American Joint Committee of Cancer. The study was approved by the medical ethics council of Fudan University Shanghai Cancer Center. Informed consent was obtained from all patients and healthy volunteers recruited in this study.
Table 1 Clinical characteristics in 38 patients with NPC
Cell isolation and flow cytometry
PBMCs were isolated using discontinuous Lymphoprep (Axis-ShieldPoCAs, Oslo, Norway) gradient centrifugation and were re-suspended in phosphate-buffered saline (PBS) containing 2% fetal bovine serum. Isolated PBMCs were collected by centrifugation, incubated with Fc-blocking antibody (purified anti-human CD16/32, BioLegend, San Diego, CA, USA) to prevent nonspecific binding and then incubated with the following antibodies (CD19: CF506236, BD Biosciences, CA, USA, 1:50; CD24: BDB563371, BD Biosciences, 1:50; CD27: BDB340424, BD Biosciences, 1:50; CD8: BDB564805, BD Biosciences, 1:50; IgA: ab193189, Abcam, CA, USA, 1:50) for 30 min on ice. After staining, cells were analyzed using CyAn-ADP (Beckman Coulter). Intracellular cytokine staining was performed by using the stimulation cocktail (eBioscience, San Diego, CA, USA) pre-stimulated for three hours as the manufacturer’s recommended protocol. Samples were analyzed by CyAn-ADP (Beckman Coulter) and data were processed using Flow Jo software (TreeStar, Ashland, OR).
Enzyme-linked immunosorbent assay (ELISA)
The serum and supernatant of radiated PBMCs were obtained, and the level of IgA, IL-10, TGF-β, TNF-α, IFN-γ and granzyme B were measured using Human Ready-SET-Go (eBioscience) according to the manufacturer’s protocol. A microplate reader (Tecan Infinite 200 PRO) was used to measure optical density at 450 nm.
In vitro ionizing radiation
Isolated PBMCs were further purified using the human pan B cell kit(StemCell Technologies, Vancouver, BC, Canada). Briefly, purified B cells were placed into 6cm diameter petri-dishes, and exposured to 4-Gy of ionizing radiation. In the diphenyleneiodonium chloride (DPI) group, cells were pretreated with 1 mM DPI30 min prior to exposure to ionizing radiation. After 18 hrs, the supernatant and cells were harvested for measurement of Cell viability using trypan blue staining.
Flow cytometry analysis of ROS
Intracellular accumulation of ROS was examined by flow cytometry after staining with the fluorescent probe, DCFH-DA (2ʹ,7ʹ-dichlorodihydro-fluorescein diacetate, 10 μM; Sigma-Aldrich). Briefly, monocytes were seeded in 24-well plates (1x106 cells/ml) and pretreated with 10 μM DCFH-DA for 30 min at 37 °C. Cells were then washed, re-suspended in PBS and prepared for further CD19 and IgA staining.
Statistical analysis
Two group comparisons were performed using Student’s t-test. Multiple group comparisons were performed using one-way analysis of variance, followed by Bonferroni correction or Mann-Whitney U testing to compare two individual groups. Statistical analysis was performed using Prism 6.0 (La Jolla, CA). The P-values of 0.05 were considered statistically significant.
Results
Enumeration and function of CD19+CD138−IgA+ B cells in healthy controls and NPC patients pre- and post-RT
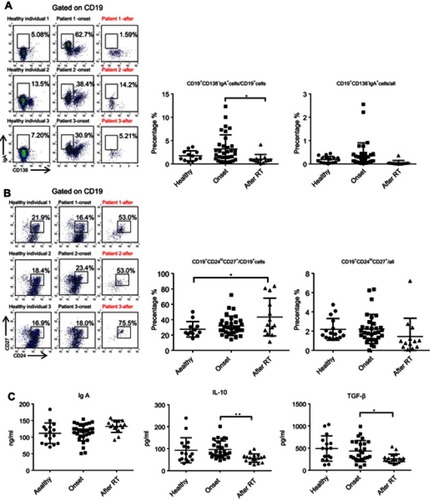
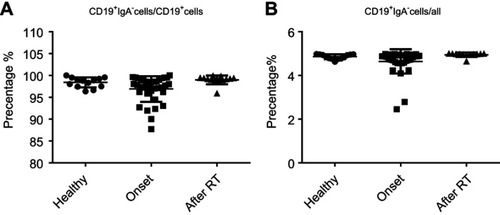
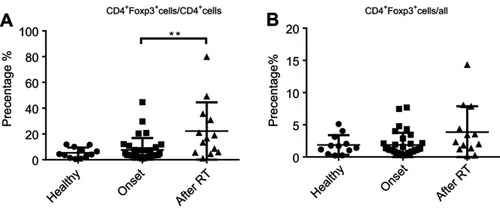
We previously demonstrated that CD19+CD138−IgA+ B cells (Plasma cells) have a suppressive role in colorectal tumors and are associated with poor prognosis.Citation12 Due to the relation of NPC to the mucosal immune system, we assessed the CD19+IgA+ B cells in the PBMCs of patients. As shown in , CD19+CD138−IgA+ B cells were significantly increased in primary NPC blood cells (onset), and were subsequently decreased to a level below the control after RT. We then assessed serum cytokine expressions and found that the sIgA levels did not change with RT treatment; however, the production of TGF-β and IL-10 increased before RT and subsequently decreased to the control level after RT (), This trend is consistent with changes in cytokines. The percentage of CD19+IgA− cells were not affected on the other hand (). Conventionally, Bregs (refers to CD19+CD24hiCD27+ B cells) were assessed after treatment; thus, we detected this B cell subset in our cell populations. Intriguingly, we found that CD19+CD24hiCD27+ B cells were remarkably increased after RT, in some cases resulting in an 80% CD19+CD24hiCD27+B cell population ().Conventional CD19+CD24hiCD27+ Breg cells do not share similar features with IgA+ B cells within the mucosal immune system and likely have inconspicuous functions in NPC. We also detected other suppressive cytokine producing cells such as Tregs. As with Bregs, the change of Tregs surprisingly increased after RT (); however, this trend is not consistent with changes in cytokines. Collectively, we found that the PMBC CD19+CD138−IgA+B cells, rather than conventional Bregs and Tregs, may play a suppressive role in NPC onset and are specifically targeted by RT.
Figure 1 CD19+CD138−IgA+B cells wereincreased inprimary NPC patients and decreased following radiotherapy. (A) CD19+CD138−IgA+ B cells were detected by FACS in healthy individual and inpatients before (onset) and after radiotherapy. (B) CD19+CD24hiCD27+ B cells were detected by FACS in healthy individual, patients before radiotherapy and post-radiotherapy. (C) Levels of sIgA, TGF-β, and IL-10 were detected by ELISA. *P<0.05 and **P<0.01. Columns and error bars represent mean ± SEM.
Changes in the percentage changes of CTLs and other immune effector cells
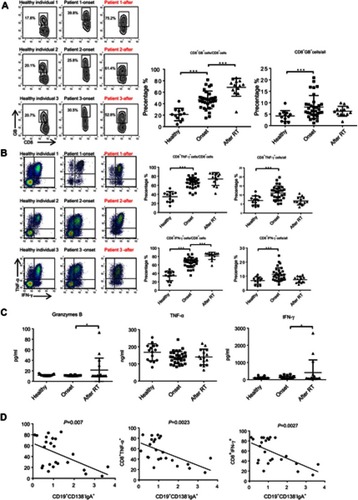
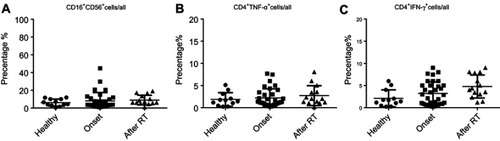
Next, we assessed the changes in the percentage of other immune effector cells. As shown in and , the proportion of CD8+ Granzyme B (GB)+/CD8, CD8+IFN-ɣ+/CD8 and CD8+TNF-α+/CD8 were significantly increased in primary NPC (before), indicating that CD8 cells differentiate to anti-tumor CTLs after cancer onset. Importantly, the percent of CTL/CD8 cells increased to a greater extent after RT and was negatively correlated with the percentage of CD19+CD138−IgA+ B cells (). Secreted levels of Granzyme B, IFN-ɣ and TNF-α were detected by ELISA, and results demonstrate upregulation of IFN-ɣ and Granzyme B after RT (). We also detected minimal changes in the percentage of other anti-tumor effector cells, such as natural killer cells and helper T cells (). Based on these findings, we assumed that serum cytokine changes might be caused by changes in the expression of CD19+CD138−IgA+B cell.
Figure 2 The function of CTL was augmented by RT. (A and B) The proportion of CD8+ Granzyme B (GB)+/CD8, CD8+IFN-ɣ+/CD8 and CD8+TNF-α+/CD8 was detected by FACS in healthy individuals and in patients before and after radiotherapy. (C) Secreted levels of Granzyme B, IFN-ɣ, and TNF-α were detected by ELISA. (D) The percent of CTL/CD8 cells was negatively correlated with the percentage of CD19+CD138-IgA+ B cells. *P<0.05 and ***P<0.001. Columns and error bars represent mean ± SEM.
RT induced ROS eradicates CD19+CD138−IgA+ B cells in vitro
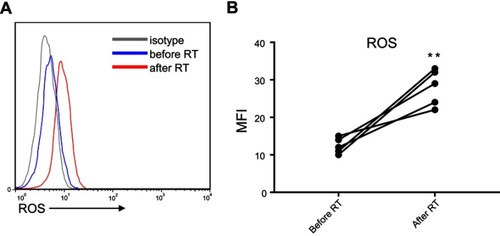
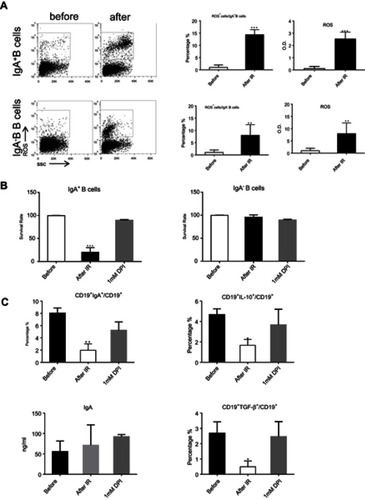
We investigated the mechanism responsible for CD19+CD138−IgA+ B cell elimination following RT. As ROS were reported to be released by the ionizing radiation (IR) used in RT, we evaluated ROS production in the peripheral blood of patients before and after RT and found increased levels following radiation therapy (). Next, we exposed petri-dishes containing PMBC to 4Gy ionizing radiation, and detected ROS expression inside B cells and in the microenvironment. After in vitro ionizing radiation, the immune cells and their supernatants were harvested to assess ROS levels. As shown in , ROS was found in the supernatant and the cytoplasm of CD19+CD138−IgA+ B cells (IgA+ B cells). To confirm this result, cells were pretreated with ROS scavenging agent DPI and subsequently irradiated with 4 Gy of ionizing radiation. After 18 hrs, B cells were analyzed for apoptosis and function. As expected,CD19+CD138−IgA+ B cells were more prone to apoptosis after ionizing radiation, while most of the CD19+IgA−B cells (IgA− B cells) remained alive. Treatment with DPI reversed this phenomenon (). The number of CD19+CD138−IgA+ B cells decreased after exposure to IR; however, no changes were observed when cells were pretreated DPI. Intriguingly, levels of IL-10 and TGF-β produced from IgA+ cells were down-regulated after irradiation, indicating an impaired function of CD19+CD138−IgA+ B cells following exposure to ionizing radiation. However, the functions of these cells were unaffected when pretreated with DPI (). Levels of sIgA did not change following radiation treatment, indicating that production of sIgA is not the main function of IgA+ B cells in cancer (). Taken together, our data demonstrate that RT-induced ROS eliminates CD19+CD138−IgA+ B cells and results in an increased anti-tumor immune response in NPC.
Figure 3 ROS released by RT eradicated CD19+CD138−IgA+ B cells. (A) Generation of ROS was detected by FACS and ELISA after in vitro ionizing radiation. (B) The survival of IgA+ B cells and IgA− B cells were detected after radiation. (C) Levels of sIgA, IL-10, and TGF-β were detected by ELISA after IR. *P<0.05, **P<0.01and ***P<0.001. Columns and error bars represent mean ± SEM.
Discussion
In this study, we illustrated the suppressive function of immune cells in NPC patients and determined the changes in the immune cell profile before and after RT. We found that during the onset of NPC, the percentage of CD19+CD138−IgA+ B cells and its cytokines was increased, and these numbers were subsequently decreased after RT. The percentage of other suppressive immune cells remained unchanged. Meanwhile, CTLs were slightly up-regulated during disease onset and were further increased by RT. Moreover, we observed that irradiation-induced ROS release specifically targeted CD19+CD138−IgA+ B cells.
B cells play an important suppressive role in mucosal-associated lymphoid tissues, particularly in NPC, as it is a mucosal immune-related cancer. The IgA+ plasma cells suppress inflammation by secreting anti-microbial antibodies, whereas CD19+CD138−IgA+ cells negatively regulate anti-tumor effectors by expressing PD-1 and producing TGF-β.Citation12,Citation20 Thus, we first detected CD19+CD138−IgA+ cells and other efficiency regulatory cells such as Tregs in patients’ PMBC. We have previously indicated that CD138− plasma cells, rather than CD138+ antibody secreting cells, play a role in mucosal immunity. Thus, we used CD138 to distinguish the subsets. The results are consistent with our previous findings, whereby CD19+CD138−IgA+ B cells increased in primary cancer microenvironment. Moreover, the anti-NPC treatment effectively eliminated this subset, indicating a possible innovative mechanism for RT used in NPC treatment. Notably, this trend was consistent with changes in cytokine expression. Thus, it can be suggested that the serum cytokine changes may be caused by changes in the expression of CD19+CD138−IgA+B cells. Furthermore, we excluded the CD19+CD24hiCD27+ Breg subset, which was involved in defining populations of B cells in this study.Citation14
Interestingly, our current results showed that Breg cells are not involved in the RT process, despite being the only B cell subset that survived after RT. We are currently working on possible explanations of this phenomenon. Conversely, Tregs are important for suppression of anti-tumor immunity, thus were considered for analysis in this study. Results indicate that the expression of Tregs was similar to that of Bregs. In addition, we found that the expression of myeloid-derived suppressor cells remained unchanged before and after RT treatment (data not shown), indicating that they are not related to the mechanisms of radiotherapy in nasopharyngeal cancer.
CTLs are the critical effector cells in tumor immunity, and we have previously demonstrated that they can be utilized as an efficient means to treat tumors. Therefore, we sought to determine whether the decrease of CD19+CD138−IgA+ B cells after RT was accompanied by an increase of CTL cells. It was found that the proportion of CTL cells increased, along with the secretion of cytokines and particles. Moreover, the expression of CTL was inversely proportional to the expression of CD19+CD138−IgA+ Bcells, suggesting a possible correlation between the two, which will require further investigation.
RT is used to directly or indirectly eliminate cancer cells. One of the ways in which cancer can be targeted indirectly is through the release of ROS.Citation7 Tominaga et al found that gene instability in tumor cells caused by RT could be reduced using various anti-ROS methods.Citation21 Studies by Wang et al have shown that cancer cells can utilize changes in ROS metabolism activity to resist radiation therapy. In addition, Danhier et al outlined that cancer cells can perform a series of actions to achieve the following goals: produce sufficient energy to promote cell proliferation (biological energetics), generate debris (biosynthesis), and generate a reduction of molecular pool from oxidative stress.Citation22–Citation24 Most importantly, many studies have found that ROS generated by RT can lead to changes in the immune microenvironment.Citation25 We observed that after ionizing radiation, ROS could enter the cytoplasm of all PBMCs; however, only CD19+CD138−IgA+ B cells were targeted and eliminated through decreases in TGF-β and IL-10. This is a novel and meaningful finding, as it suggests that CD19+CD138−IgA+ B cells are likely the target of RT during NPC treatment, and that these cells play a critical role in the suppression of anti-tumor immunity. Collectively, these results confirm that RT-induced ROS eliminates CD19+CD138−IgA+ B cells and results in an increased anti-tumor immune response in NPC, potentially providing a novel approach to tumor therapy.
Patients and controls
All participants, and a parent or legal guardian of participants under the age of 18 years, had written informed consent for donating their samples to Fudan University Shanghai Cancer Center.
Author contributions
L.W. and W.L. designed and performed the experiments, analyzed the data, and wrote the manuscript. H.C. and C.Y. designed the experiment and wrote the manuscript. S.C. and X.T. performed the experiments and analyzed the data. L.W. and W.L. contributed equally to this work. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accounted for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the General Program of National Natural Science Founds of China (81771736) and the Young Elite Scientists Sponsorship Program by CAST (YESS20160094). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplementary materials
References
- Wu L, Li C, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med. 2018;15:3687–3692. doi:10.3892/etm.2018.587829556258
- Peng H, Tang -L-L, Liu X, et al. Anti-EGFR therapy concurrently with induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Sci. 2018;109:1609–1616. doi:10.1111/cas.1358929575438
- Hu J, Kong L, Gao J, Hu W, Guan X, Lu JJ. Use of radiation therapy in metastatic nasopharyngeal cancer improves survival: a SEER analysis. Sci Rep. 2017;7:721. doi:10.1038/s41598-017-00655-128389658
- Toya R, Murakami R, Saito T, et al. Radiation therapy for nasopharyngeal carcinoma: the predictive value of interim survival assessment. J Radiat Res. 2016;57:541–547. doi:10.1093/jrr/rrw03827242338
- Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014;1:24. doi:10.3389/fmolb.2014.0002425988165
- Thoms J, Bristow RG. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol. 2010;20:217–222. doi:10.1016/j.semradonc.2010.06.00320832013
- Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi:10.1016/j.ejca.2004.02.03115315800
- Jeong H, Bok S, Hong B-J, Choi H-S, Ahn G-O. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 2016;51:157–163. doi:10.5045/br.2016.51.3.15727722125
- Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014;15:927–943. doi:10.3390/ijms1501092724434638
- Teow SY, Liew K, Khoo AS, Peh SC. Pathogenic role of exosomes in Epstein-Barr Virus (EBV)-associated cancers. Int J Biol Sci. 2017;13:1276–1286. doi:10.7150/ijbs.1953129104494
- Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi:10.1038/nature1439525924065
- Liu, R, Lu Z, Gu J, et al. MicroRNAs 15A and 16–1 activate signaling pathways that mediate chemotaxis of immune regulatory B cells to colorectal tumors. Gastroenterology. 2017. doi:10.1053/j.gastro.2017.09.045
- Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi:10.1182/blood-2010-07-294249blood-2010-07-29424920962324
- Zha B, Wang L, Liu X, et al. Decrease in proportion of CD19+ CD24(hi) CD27+ B cells and impairment of their suppressive function in Graves’ disease. PLoS One. 2012;7:e49835. doi:10.1371/journal.pone.0049835PONE-D-12-1813123189166
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi:10.1126/science.113048116809515
- Van Acker H, Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017;25:456–466. doi:doi:10.1016/j.tim.2016.12.00828089288
- Park GB, Choi Y, Kim YS, Lee H-K, Kim D, Hur DY. ROS-mediated JNK/p38-MAPK activation regulates bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int J Oncol. 2014;44:977–985. doi:10.3892/ijo.2014.225224402682
- Polikowsky HG, Wogsland CE, Diggins KE, Huse K, Irish JM. Cutting edge: redox signaling hypersensitivity distinguishes human germinal center B cells. J Immunol. 2015;195:1364–1367. doi:10.4049/jimmunol.150090426157177
- Richards SM, Clark EA. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur J Immunol. 2009;39:3395–3403. doi:10.1002/eji.20093958719877015
- Sun R, Wang X, Li X. Correlation analysis of nasopharyngeal carcinoma TNM staging with serum EA IgA and VCA IgA in EBV and VEGF-C and -D. Med Sci Monit. 2015;21:2105–2109. doi:doi:10.12659/MSM.89341526191775
- Tominaga H, Kodama S, Matsuda N, Suzuki K, Watanabe M. Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. J Radiat Res. 2004;45:181–188. doi:10.1269/jrr.45.18115304958
- Sonveaux P. ROS and radiotherapy: more we care. Oncotarget. 2017;8:35482–35483. doi:doi:10.18632/oncotarget.1661328415657
- Danhier P, Bański P, Payen VL, et al. Cancer metabolism in space and time: beyond the Warburg effect. Biochim Biophys Acta Bioenerg. 2017;1858:556–572. doi:10.1016/j.bbabio.2017.02.00128167100
- Wang, H., Bouzakoura S, De Mey S, et al. Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget. 2017;8:35728–35742. doi:10.18632/oncotarget.1611328415723
- Ozsoy HZ, Sivasubramanian N, Wieder ED, Pedersen S, Mann DL. Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. J Biol Chem. 2008;283:23419–23428. doi:10.1074/jbc.M80296720018544535