Abstract
Purpose
To assess the overall survival (OS) of early human epidermal growth factor receptor 2 (HER2)-enriched breast cancer patients after receiving neoadjuvant trastuzumab (NAT) compared to adjuvant trastuzumab (AT) treatment and the difference in local-regional relapse (LRR) rate with this tumor and treatment between women after mastectomy and women after breast-conserving therapy (BCT).
Methods
Articles were retrieved from PubMed, Embase, Web of Science, and Cochrane Library. A pooled odds ratio (OR) with a 95% confidential interval (CI) was calculated. The StataSE version 12.0 software was employed for meta-analysis.
Results
Twelve available clinical studies containing 2366 subjects were included. The OS of NAT compared with that of AT was not significantly different (pooled OR=1.04; 95% CI, 0.47–2.33). There was a significantly lower LRR rate for patients with mastectomy compared to those with BCT (pooled OR=0.58; 95% CI, 0.38–0.89); however, subgroup analysis revealed that the significant advantage of LRR for mastectomy compared to BCT was only represented in women without trastuzumab treatment (pooled OR=0.52; 95% CI, 0.31–0.88) compared to those who received trastuzumab treatment (pooled OR=0.71; 95% CI, 0.34–1.49).
Conclusion
Early stage HER2-overexpression breast cancer patients benefit with an equivalent OS from NAT treatment compared to AT. Patients who underwent mastectomy and BCT experienced a similar LRR rate if they received anti-HER2 targeted therapy of trastuzumab, but the LRR rate was discernibly reduced in patients who received mastectomy compared to BCT if they did not also receive trastuzumab treatment.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Neoadjuvant systematic chemotherapy in breast cancer is a preoperative treatment, which is initially applied locally to advanced tumors to downgrade primary tumor and regional lymph nodes and enable their removal by operation. Currently, neoadjuvant chemotherapy is widely used in early resectable disease, mainly to allow breast-conserving therapy (BCT).Citation1–Citation3 Adjuvant chemotherapy is postoperative and attenuates breast cancer mortality by at least 15%.Citation4 Many randomized clinical trials and meta-analyses confirm the equivalent overall survival (OS) of breast cancer women after receiving preoperative chemotherapy compared to those who undergo postoperative chemotherapy;Citation5–Citation9 however, these studies are imperfect, as they all failed to perform the subgroup analysis on different molecular subtypes of breast cancer.
In the period before 1980, the modified radical mastectomy was the standard surgical method for breast cancer. In the early 1980s, two published randomized trials reformed this standard modality and confirmed that the survival benefits from BCT were equivalent to those of mastectomy.Citation10,Citation11 The Early Breast Cancer Trialists’ Collaborative Group carried out a large-scale meta-analysis that further pooled the results from both randomized trials, finding the equality of OS and disease-free survival (DFS) between mastectomy and BCT.Citation12 Currently, instead of mastectomy, BCT has become the appropriate and preferred treatment for most early stage breast cancer patients,Citation13 and it prevents the physical and psychological burden of sacrificing breast on women as much as possible.Citation14
Human epidermal growth factor receptor 2 (HER2)-overexpression breast cancer accounts for approximately 15–25% of the primary breast tumors.Citation15 Trastuzumab is currently indispensable for treating HER2-positive breast cancer. Prior to treatment with the anti-HER2 targeted drug, the local-regional recurrence (LRR) of this cancer molecular subtype is significantly greater than that of other phenotypes of disease, specifically Luminal A.Citation16 Voduc et alCitation16 found that the LRR rate of HER2-enriched breast cancer patients without trastuzumab treatment who underwent BCT and those who received trastuzumab was 21% and 17% at 10 years, respectively. After receiving trastuzumab, there is discernible reduction in the high LRR rate of HER2-positive breast tumors; a study from Debled et alCitation17 showed that those women treated with BCT or mastectomy had a significantly reduced 4-year LRR rate of 2.9% or 0%, respectively. However, the LRR rates between these two surgical strategies in treating HER2-amplified breast carcinoma were not compared in these studies. Therefore, the aim of our article was to separately pool all clinical studies that concomitantly documented the OS outcomes of early HER2-overexpression breast cancer patients who received neoadjuvant trastuzumab (NAT) and adjuvant trastuzumab (AT) and that concurrently described the LRR rate of those women undergoing mastectomy and BCT. We aimed to identify the more applicable and preferably primary treatment strategy for this subtype of breast tumor. We included women treated with NAT or mastectomy as the study cohort and those with AT or BCT treatment as the control cohort.
Methods
Search strategy
Based on the PRISMA-IDP Statement,Citation18 electronic searches were performed in PubMed, Embase, Web of Science, and Cochrane Library using the following retrieval strategy: ((HER2 OR (Human epidermal growth factor receptor 2)) AND ((“Breast Neoplasms”[Mesh]) OR (Breast cancer) OR (Breast tumour) OR (Breast tumour) OR (Breast carcinoma) OR (Breast Neoplasm))) AND ((((Neoadjuvant therapy) OR (Neoadjuvant treatment)) AND ((Adjuvant therapy) OR (Adjuvant treatment)) AND (Trastuzumab OR Herceptin) AND (Overall Survival)) OR (Mastectomy AND ((Breast conservation surgery) OR (Breast conserving surgery) OR (Breast preservative surgery) OR (Breast preservation surgery) OR (Breast preserving surgery) OR (Breast preservative surgery) OR (Breast conservation therapy) OR (Breast conserving therapy) OR (Breast preservative therapy) OR (Breast preservation therapy) OR (Breast preserving therapy) OR (Breast preservative therapy) OR (Breast conservation treatment) OR (Breast conserving treatment) OR (Breast preservative treatment) OR (Breast preservation treatment) OR (Breast preserving treatment) OR (Breast preservative treatment)) AND ((Local-regional relapse) OR (Local-regional recurrence) OR (Local relapse) OR (Local recurrence)))). No restrictions were required during the retrieval. The searching of citation was terminated as of 28th May 2019.
Inclusion criteria
Early HER2-enriched breast cancer patients;
English publications which covered the number or the rate of event and the total sample size;
Clinical trials which concurrently documented the data of LRR after BCT and that after mastectomy or OS after NAT and that after AT. LRR referred to the first recurrence of tumor in the ipsilateral breast, the chest wall or regional lymphatics, without evidence of distant metastasis. The definition of BCT was a combination of radiotherapy and surgery strategy involving lumpectomy, segmental mastectomy, quadrantectomy, or wedge resection. The methods of mastectomy included simple mastectomy and modified radical mastectomy. OS ranged from the date of diagnosis to the date of death or loss to follow-up.
Exclusion criteria
Trials published in non–English;
Patients with moderate-to-advanced stage disease;
Male patients;
Reviews, case reports, and conference papers;
Other details that did not meet the inclusion criteria.
Two co-authors (Qian Wu and Jing Xiong) independently screened the retrieved citations and reserved the pertinent studies according to the titles, abstracts, and full-text articles. If some disagreements surfaced, they were resolved by the third reviewer (Zhumin Su).
Data abstraction
The following information was abstracted using Microsoft Excel version 2016 (Microsoft Corporation, Redmond, Washington, USA) by two authors (Jing Xiong and Zhumin Su): first author, publication year, study duration, original nation, median age, median follow-up, whether patients received trastuzumab treatment, the number of patients undergoing BCT or mastectomy and how many of them developed LRR, and the number of women who received NAT or AT and how many died from either treatment paradigms. Provided that there were any inconsistencies, they were resolved by discussion.
Statistical analysis
The included clinical studies were discussed by their differences of eligible criteria, chemotherapy strategy, and implementation of radiotherapy, as well as the risk of bias of study evaluation using the new version of the Cochrane tool. The crude odds ratio (OR) with its 95% confidence interval (CI) of LRR with respect to mastectomy compared to BCT or OS regarding NAT versus AT in each trial was calculated and then pooled together. If the event number was not shown in the publication, it was computed based on the end point percentages of LRR and OS or other information. A heterogeneity Chi2 test with a significance level of P<0.1 was used to estimate the heterogeneity among different studies;Citation19 when it was not significant (P>0.1), a fixed-effect Mantel-Haenszel model was utilized to pool the data, otherwise, a random-effect Mantel-Haenszel model was applied.Citation19 The publication bias was assessed by creating a Begg’s funnel plot with 95% CI and Egg’s test with a significance level of P<0.05. All statistical tests were performed in StataSE version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Search results
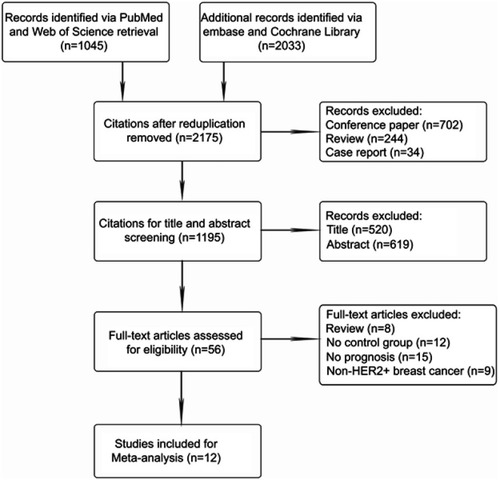
After systematic retrieval in the databases described earlier, 3078 citations were obtained. After deleting the duplicates (n=903), citations categorized as reviews (n=244), conference papers (n=702), and case reports (n=34), 1195 records were remained for the title and abstract screening. An additional 1139 citations were removed by this step, and 56 articles qualified for full-text article assessment; we excluded the nonmatched documents that were reviews (n=8) or covered non-HER2-overexpression breast tumors (n=9) as well as those with no valid control cohort (n=12) or no prognosis (n=15). Ultimately, 12 satisfactory studiesCitation16,Citation17,Citation20–Citation23 were involved in the meta-analysis. The PRISMA flow diagram of selecting qualified clinical trials is outlined in .
Characteristics of included studies
The original nations of the included articles were USA (n=2), France (n=1), Canada (n=3), the Netherlands (n=1), Spain (n=1), the UK (n=1), India (n=1), China (n=1), and Japan (n=1). The sample size ranged from 43 to 748 (median: 81.5), with a total number of 2366 subjects. The year range of included studies was 2008 to 2018. The other details including study duration, whether trastuzumab was received or absent, chemotherapy strategy, and which cohort (study cohort or control cohort) received radiotherapy are presented in .
Table 1 The details of eligible articles
Meta-analysis results
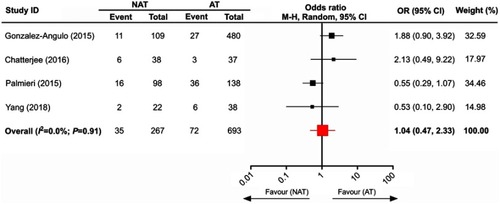
Four eligible trials with 960 subjects were included to analyze the OS between NAT and AT. As shown in , the pooled data indicated that there was no significant difference in the OS of HER2-overexpressing breast tumors after receiving NAT compared to AT (pooled OR=1.04; 95% CI, 0.47–2.33).
Figure 2 The comparison of overall survival between neoadjuvant trastuzumab and adjuvant trastuzumab.
Abbreviations: NAT, neoadjuvant trastuzumab; AT, adjuvant trastuzumab.
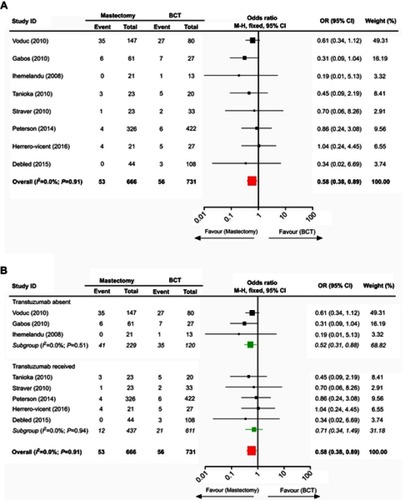
There were 8 valid studies with 1406 patients who were collected to investigate the comparison of the LRR rate between mastectomy and BCT in treating HER2-amplified breast cancer. The data analysis suggested that women with this tumor undergoing mastectomy benefited from a lower LRR rate than those in the treatment of BCT (pooled OR=0.58; 95% CI, 0.38–0.89) (). We further divided the included trials into subgroups based upon whether trastuzumab administration to patients was documented. Interestingly, the subgroup with absent trastuzumab still showed a lower LRR rate with mastectomy than with BCT (pooled OR=0.52; 95% CI, 0.31–0.88), while the LRR rate between these two surgical interventions was not significantly different in the subgroup that received trastuzumab (pooled OR=0.71; 95% CI, 0.34–1.49) ().
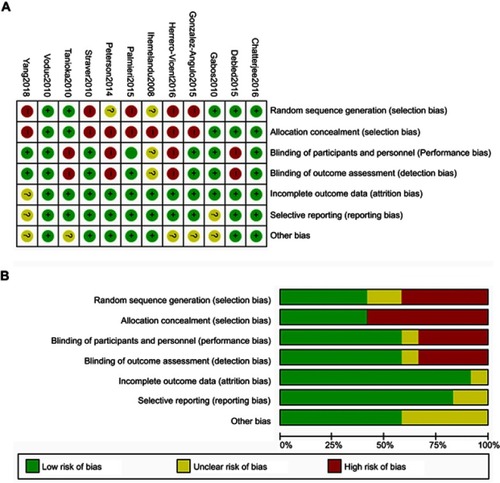
Risk of bias in analyzed studies
All included trials referencing the OS of NAT versus AT and the LRR of mastectomy versus BCT were combined to judge each risk of bias domain. The risk of bias summary and the risk of bias graph are presented in and , respectively.
Publication bias
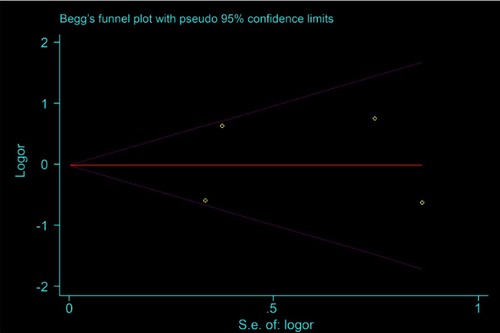
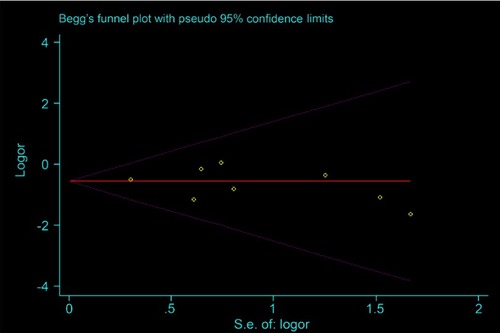
In the Begg’s funnel plots of the OS of NAT versus AT and the LRR of mastectomy versus BCT, data were uniformly arranged above and below the axis. Consistently, their publication bias in light of Egg’s test was not statistically significant (p=0.844 and 0.515, respectively), suggesting that the moderate heterogeneity in the OS of NAT versus AT was not by virtue of bias ( and in Supplementary, page 1 and 2).
Discussion
HER2-positive breast cancer necessitates at least 1 year of continuous trastuzumab treatment to achieve optimal therapeutic efficacy. Our results demonstrate that the early stage HER2-enriched breast tumor patients who receive NAT have a comparative OS to those who undergo AT. Additionally, greater local-regional control is attained in those who receive mastectomy compared to those who receive BCT before the era of trastuzumab administration; nevertheless, women with this disease who receive trastuzumab treatment derive an equivalent LRR rate with the two surgical approaches.
In agreement with our results, some trials ascertain no differences between patients with early HER2-positive breast cancer following treatment with NAT compared to AT in the local relapse-free survival, event-free survival, relapse-free survival as well as breast cancer-specific survival.Citation24–Citation26 However, the study of Chatterjee et alCitation24 indicated that the DFS of patients in AT cohort was significantly longer than that in NAT cohort. Trastuzumab emtansine (T-DM1) is an antibody-cytotoxic drug conjugate of trastuzumab and the cytotoxic agent emtansine; it has efficacious antitumor effects in trastuzumab-sensitive and trastuzumab-resistant HER2-amplified breast tumors and was initially approved for advanced HER2-positive breast cancer patients who have previously received trastuzumab treatment.Citation27,Citation28 The articles of HurvitzCitation29 and Minckwitz,Citation28 respectively, assessed the tumor response and the survival prognosis of early stage HER2-enriched subtype of breast tumors after treatment with T-DM1 and trastuzumab. Their findings demonstrated that women with this disease subset who received T-DM1 had significantly superior DFS and distant relapse-free survival and reduced pathological complete response (pCR) rates compared to those who received trastuzumab, but the OS was not significantly different. The mechanisms of action of T-DM1 eradicating HER2-positive breast tumor foci contrast to those of trastuzumab. T-DM1 activates caspase-3/caspase-7 to induce apoptotic cell death and releases the intracellular enzyme adenylate kinase which contributes to the cellular lysis. Trastuzumab antagonizes the constitutive growth-signaling properties of the HER2 system, mobilizes immune cells to kill the tumor target, and reinforces chemotherapy-induced cytotoxicity.Citation27,Citation30
For breast cancer patients with the HER2-enriched subtype, there are two distinct eras determined by the usage of trastuzumab in the adjuvant setting. In the period preceding the use of anti-HER2 targeted therapy, the LRR rate of this tumor ranges from 4% to 15%.Citation16,Citation31 The natural process of this phenotype of breast cancer has been positively influenced by trastuzumab.Citation32 Yin et alCitation33 analyzed six relevant studies and indicated that trastuzumab reduced the LRR rate of HER2-positive breast cancer by 50%. This conclusion was supported by a retrospective study performed by Panoff and colleagues,Citation34 which found that the LRR rate of women with this disease who underwent mastectomy plus trastuzumab was 1.7%. Our article finds the different LRR rates between HER2-amplified breast tumors undergoing mastectomy and those undergoing BCT before the application of trastuzumab coupled with the identical LRR rate among both surgical scenarios after its administration, which mirrors the trastuzumab-adjusted alteration of the natural course of this disease.
There is a viewpoint suggesting that the prognosis of HER2-enriched breast cancers primarily depends on the biological characteristics of the disease rather than the content of surgical approach.Citation35 Nevertheless, it may be somewhat confined and not comprehensive. A study enrolled 618 breast cancer patients that underwent either BCT or mastectomy, 92 of whom were classified as the HER2 subtype.Citation21 In the BCT cohort, HER2 subtype of breast cancer and lymph node positivity were independent prognostic factors associated with high-risk of LRR. This cancer subset was not associated with increased risk of LRR in the mastectomy cohort. Similarly, our results reaffirm that the different extents of surgery may be related to the prognosis of HER2-overexpression breast carcinoma devoid of trastuzumab treatment.
There are some limitations of this article that deserve mention. First, despite the absence of publication bias, only English literature was included which might give rise to selection bias. Second, the included studies in partial meta-analyses were limited, which might lead to result bias. More importantly, we did not investigate other factors that might affect the outcome of LRR in women because of insufficient information provided by the publications, such as chemotherapy regimen, fractionated mode, and dose of radiotherapy.
Conclusion
The OS of HER2-amplified breast tumor patients treated with NAT is equivalent to those with AT treatment. The LRR rate of those women who undergo mastectomy compared to BCT is identical in the absence of trastuzumab treatment, but mastectomy reduces the LRR rate compared to BCT in women who receive trastuzumab treatment.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
References
- Herrero-Vicent C, Guerrero-Zotano A, Gavila-Gregori J, et al.A prognostic index for locoregional recurrence after neoadjuvant chemotherapy. Ecancermedicalscience. 2016;10:647.27433280
- Ihemelandu CU, Naab TJ, Mezghebe HM, et al. Treatment and survival outcome for molecular breast cancer subtypes in black women. Ann Surg. 2008;247(3):463–469.18376191
- Gabos Z, Thoms J, Ghosh S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124(1):187–194.20814819
- Debled M, MacGrogan G, Breton-Callu C, et al. Surgery following neoadjuvant chemotherapy for HER2-positive locally advanced breast cancer. Time to reconsider the standard attitude. Eur J Cancer. 2015;51(6):697–704.25704790
- Straver ME, Rutgers EJT, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–2418.20373039
- Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691.20194857
- Tanioka M, Shimizu C, Yonemori K, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010;103(3):297–302.20606681
- Peterson DJ, Truong PT, Sadek BT, et al. Locoregional recurrence and survival outcomes by type of local therapy and trastuzumab use among women with node-negative, HER2-positive breast cancer. Ann Surg Oncol. 2014;21(11):3490–3496.24841346
- Chatterjee S, Arunsingh M, Agrawal S, et al. Outcomes following a moderately hypofractionated adjuvant radiation (START B Type) schedule for breast cancer in an unscreened non-caucasian population. Clin Oncol. 2016;28(10):e165–e172.
- Gonzalez-Angulo AM, Parinyanitikul N, Lei X, et al Effect of adjuvant trastuzumab among patients treated with anti-HER2-based neoadjuvant therapy. Br J Cancer. 2015;112(4):630–635.25584488
- Yang H, Zhou L, Wang S, et al. Retrospective analysis of concurrent docetaxel and epirubicin neoadjuvant versus adjuvant chemotherapy: Which leads to better outcomes for different subtype breast cancer patients? Medicine. 2018;97(40):e12690.30290661
- Palmieri C, Macpherson IR, Yan K, et al. Neoadjuvant chemotherapy and trastuzumab versus neoadjuvant chemotherapy followed by post-operative trastuzumab for patients with HER2-positive breast cancer. Oncotarget. 2016;7(11):13209–13220.26334099
References
- Clough KB, Acosta-Marin V, Nos C, et al. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: a french national survey. Ann Surg Oncol. 2015;22(11):3504–3511. doi:10.1245/s10434-015-4378-625665949
- Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121(15):2544–2552. doi:10.1002/cncr.2934825902916
- Vugts G, Maaskant-Braat AJ, Nieuwenhuijzen GA, Roumen RM, Luiten EJ, Voogd AC. Patterns of care in the administration of neo-adjuvant chemotherapy for breast cancer. A population-based study. Breast J. 2016;22(3):316–321. doi:10.1111/tbj.1256826945566
- Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi:10.1016/S0140-6736(11)61625-522152853
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi:10.1200/JCO.1998.16.8.26729704717
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi:10.1200/JCO.2007.15.023518258986
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. doi:10.1200/JCO.2001.19.22.422411709566
- Early Breast Cancer Trialist' Collaborative Group (EBCTG); Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. doi:10.1016/S1470-2045(17)30777-529242041
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi:10.1093/jnci/dji02115687361
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. doi:10.1056/NEJM1985031431211013883167
- Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. doi:10.1056/NEJM1981070230501027015141
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi:10.1016/S0140-6736(05)67887-716360786
- CC N. Treatment of early breast cancer. JAMA. 1991;265:391–395.1984541
- Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360(1):63–70. doi:10.1056/NEJMct080352519118305
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. doi:10.5858/arpa.2013-0953-SA24099077
- Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi:10.1200/JCO.2009.24.928420194857
- Debled M, MacGrogan G, Breton-Callu C, et al. Surgery following neoadjuvant chemotherapy for HER2-positive locally advanced breast cancer. Time to reconsider the standard attitude. Eur j cancer. 2015;51(6):697–704. doi:10.1016/j.ejca.2015.01.06325704790
- Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi:10.1001/jama.2015.365625919529
- Sutton AJ, Abrams KR, Jones DR. Methods for Meta-analysis in Medical Research. Wiley Series in Probability and Statistics-applied Probability and Statistics Section. Hoboken: Wiley; 2008.
- Ihemelandu CU, Naab TJ, Mezghebe HM, et al. Treatment and survival outcome for molecular breast cancer subtypes in black women. Ann Surg. 2008;247(3):463–469. doi:10.1097/SLA.0b013e31815d744a18376191
- Gabos Z, Thoms J, Ghosh S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124(1):187–194. doi:10.1007/s10549-010-1135-120814819
- Herrero-Vicent C, Guerrero-Zotano A, Gavila-Gregori J, et al. A prognostic index for locoregional recurrence after neoadjuvant chemotherapy. Ecancermedicalscience. 2016;10:647. doi:10.3332/ecancer.2016.64727433280
- Straver ME, Rutgers EJ, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–2418. doi:10.1245/s10434-010-1008-120373039
- Chatterjee S, Arunsingh M, Agrawal S, et al. Outcomes following a moderately hypofractionated adjuvant radiation (START B Type) schedule for breast cancer in an unscreened non-caucasian population. Clin Oncol (R Coll Radiol). 2016;28(10):e165–e172. doi:10.1016/j.clon.2016.05.00827369459
- Gonzalez-Angulo AM, Parinyanitikul N, Lei X, et al. Effect of adjuvant trastuzumab among patients treated with anti-HER2-based neoadjuvant therapy. Br J Cancer. 2015;112(4):630–635. doi:10.1038/bjc.2014.64725584488
- Palmieri C, Macpherson IR, Yan K, et al. Neoadjuvant chemotherapy and trastuzumab versus neoadjuvant chemotherapy followed by post-operative trastuzumab for patients with HER2-positive breast cancer. Oncotarget. 2016;7(11):13209–13220. doi:10.18632/oncotarget.480126334099
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi:10.1158/0008-5472.CAN-08-177619010901
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi:10.1056/NEJMoa181401730516102
- Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115–126. doi:10.1016/S1470-2045(17)30716-729175149
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol. 1999;26(4 Suppl 12):60–70.
- Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–2378. doi:10.1200/JCO.2007.14.428718413639
- Tsoutsou PG, Vozenin MC, Durham AD, Bourhis J. How could breast cancer molecular features contribute to locoregional treatment decision making? Crit Rev Oncol Hematol. 2017;110:43–48. doi:10.1016/j.critrevonc.2016.12.00628109404
- Yin W, Jiang Y, Shen Z, Shao Z, Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS One. 2011;6(6):e21030. doi:10.1371/journal.pone.002103021695277
- Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. 2011;128(3):899–906. doi:10.1007/s10549-011-1495-121475999
- Horton JK, Jagsi R, Woodward WA, Ho A. Breast cancer biology: clinical implications for breast radiation therapy. Int J Radiat Oncol Biol Phys. 2018;100(1):23–37. doi:10.1016/j.ijrobp.2017.08.02529254776