Abstract
Background
Circulating tumor cells (CTCs) intravasate into the bloodstream throughout early cancer stages, promoting metastasis. The tumor microenvironment plays a crucial role in disease progression and outcome. The aim of this prospective study was to investigate the associations of intratumoral and stromal tumor-infiltrating lymphocytes (TILs) with CTCs among patients receiving neoadjuvant chemotherapy (NAC).
Methods
We analyzed CTCs in 30 patients with primary breast cancer before and after NAC. The numbers of intratumoral TILs (iTILs) and stromal TILs (sTILs) from pre-NAC formalin-fixed paraffin-embedded core biopsies and post-NAC surgical samples were analyzed. The associations of TILs with pathologic complete response (pCR) and outcome were also evaluated.
Results
Of the 30 patients, pCR was achieved in nine (30.0%) patients. A total of 25 (83.3%) patients were CTC-positive before NAC, and eight (26.7%) patients were CTC-positive after NAC. Neither CTC detection before NAC nor CTC after NAC was predictive of pCR. Nevertheless, the presence of CTCs after NAC was significantly associated with early metastatic relapse (P = 0.049) and worse disease-free survival (P = 0.009). After NAC, total sTILs, CD4+ T cells, and CD8+ T cells were significantly correlated with CTC detection. Increased infiltration of sTILs and CD4+ T cells was also an unfavorable prognostic factor as measured by the rate of metastatic relapse.
Conclusion
Detection of CTCs after NAC was positively associated with the metastatic relapse of breast cancer patients. Increased infiltration of sTILs after NAC was correlated with CTCs and was found to be an unfavorable prognostic factor.
Introduction
Breast cancer is the most prevalent malignant disease of women worldwide.Citation1 Metastasis, the main cause of breast cancer-related death, may be regarded as a progressive process from its inception in the primary tumor microenvironment to distant sites. Neoadjuvant chemotherapy (NAC) is being increasingly used for large operable or locally advanced primary breast cancer.Citation2 The effect of NAC has been attributed to the eradication of microdisseminated tumor cells that could potentially develop into distant metastases. Recently, the identification of surrogates for drug efficacy has become a major challenge. Pathologic complete response (pCR), including nodal involvement, has been acknowledged as a surrogate endpoint of NAC.Citation3,Citation4 However, this surrogate is suboptimal since the correlation between pCR and long-term outcome is not robust, and not all patients that achieve pCR are cured. Thus, other surrogate markers need to be identified.Citation5–Citation7
Multiple studies suggest that intravasation of circulating tumor cells (CTCs) can occur in the early stages of cancer, promoting the generation of micrometastatic reservoirs, some of which can progress to macrometastatic disease.Citation8,Citation9 CTCs can provide real-time liquid biopsy specimens for investigating the biologic behavior of tumor metastasis.Citation10 During the last decade, increasing evidence has verified that the detection of CTCs in the peripheral blood is a strong predictor of progression-free survival (PFS) and overall survival (OS) in patients with metastatic breast cancer.Citation11–Citation13 Several studies have also demonstrated a similar prognostic relevance for the detection of CTCs in patients with early-stage breast cancer.Citation9,Citation14,Citation15
However, the role of CTCs in patients receiving NAC remains unclear. Several reports have indicated that CTC counts decreased after NAC in some patients, but increased in other patients.Citation16,Citation17 A recent study observed an approximately two-fold increase in CTCs following paclitaxel treatment in all experimental models examined.Citation18 The REMAGUS 02 neoadjuvant Phase II study showed that CTC before therapy was significantly correlated with PFS and OS. However, tumor response to chemotherapy was not correlated with CTC detection before and after NAC.Citation19,Citation20 Moreover, no significant correlations were found for CTCs before and after NAC to PFS and OS in a study enrolling 115 breast cancer patients.Citation21 A decrease in CTC count has been reported to correlate with DFS, but not correlate with pCR.Citation16,Citation17 These studies yield discordant results concerning the possibility of monitoring therapeutic efficacy by detecting CTCs and suggest that CTCs may not display the same chemosensitivity as the primary tumor.
The metastatic process is generally considered to be a selective multistep process, and metastatic cancer cells may be derived from a few tumor subclones. CTCs usually show different chemosensitivity from the primary tumor, with different prognostic values. There may also be other explanations of the discrepancy observed between primary tumor and CTC characteristics. The tumor microenvironment, like breast cancer stroma, is considered to be involved in tumor progression and outcome.Citation22 Chemotherapy may evoke a host repair response, affecting the primary tumor microenvironment, increasing cancer cell dissemination, and inducing a more aggressive phenotype.Citation18,Citation23,Citation24 In addition, the immune system could mediate the antitumor activity of anticancer treatments. Tumor microenvironment immune balance has been suggested to be involved in breast cancer response and prognosis. Chemotherapy could trigger a tumor microenvironment immune response, which would affect treatment efficacy and outcome.Citation25,Citation26
Tumor-infiltrating lymphocytes (TILs) have been validated as important players in the treatment response against cancer cells and may be good surrogate markers of the immune balance affected by chemotherapy. However, conflicting results exist regarding the exact prognostic value of TILs, especially in post-treated residual tumors.Citation27 Studies that have evaluated TILs in breast cancer in both the intratumoral and stromal compartments separately with respect to the tumor response to NAC are limited.Citation28 The connection between CTCs and TILs, including intratumoral TILs (iTILs) and stromal TILs (sTILs), in breast cancer patients receiving NAC has not been examined. The aim of this study was to determine the prognostic effect of CTC detection prior to and after NAC in patients with large operable or locally advanced breast cancer. We also investigated the correlations between CTC detection and TILs in the intratumoral and stromal compartments separately.
Materials and Methods
Patients and Chemotherapy Regimen
This is an open-label, single-center, prospective study. The design of the study is shown in . Patients, who signed informed consent, diagnosed with large operable or locally advanced primary breast cancer from August 2013 to January 2014 were included. The present study was in compliance with the Helsinki Declaration. Eligibility criteria for the study were female patients aged >18 and <70 years with histologically proven invasive breast cancer, with no evidence of distant metastasis. Eligible patients had no history of previous malignancy. All patients received NAC, followed by surgery, hormone therapy, and radiotherapy if necessary. General information and disease-related characteristics (tumor phenotype, tumor size, lymph node status, and histological grade using the Nottingham combined histologic grading system) were collected from all participants. Estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 status were determined as described previously.Citation29,Citation30
Figure 1 Design of the study and time points of blood sampling and tissue sampling. #Surgery tissue samples were collected from patients and matched to healthy donors from whom biopsy tissue samples were sufficient for histologic evaluation.
Abbreviations: n, number; NAC, neoadjuvant chemotherapy; CTC, circulating tumor cell.
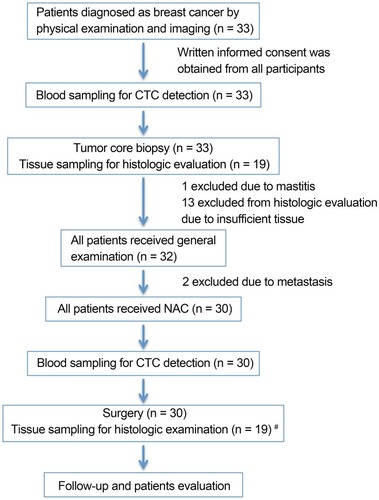
Some patients received four cycles of anthracycline-based regimen EC (with 80 mg/m2 epirubicin and 600 mg/m2 cyclophosphamide) once every 3 weeks followed by four cycles of taxane regimen T (with 80 mg/m2 docetaxel) once every 3 weeks. Other patients received six cycles of TEC (with 75 mg/m2 docetaxel, 80 mg/m2 epirubicin, and 600 mg/m2 cyclophosphamide) once every 3 weeks. HER2-positive patients received 6 mg/kg trastuzumab (loading dose, 8 mg/kg) once every 3 weeks for 1 year. All patients with hormone receptor (HR)-positive breast cancer received adjuvant hormone therapy. pCR was defined as the absence of residual invasive or noninvasive tumor cells in both breast and lymph nodes.
The patients were followed at the study sites at 3-month intervals for the first 2 years and at 6-month intervals thereafter. The median follow-up period was 40 months (range, 34–47 months). Each follow-up included a clinical examination, breast ultrasound, and chest computed tomography; abdominal ultrasound every 6 months; and bone scan every year.
Collection of Blood and Tissue Samples
Pre- and post-NAC blood samples were collected from all patients included in the study. A total of 30 mL of peripheral blood (PB) in heparin was collected from each patient before the initiation of NAC and 1 week after the completion of the final cycles of NAC. The first 5 mL of blood was discarded to reduce blood contamination by epithelial cells from the skin. PB was also collected from 20 healthy donors (HDs). Blood specimens were processed no later than 2 h after collection. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation. Pre-NAC formalin-fixed paraffin-embedded core biopsies and post-NAC surgical samples were also collected. Samples were obtained with the patients’ written informed consent after approval by the ethics committee of The First Affiliated Hospital with Nanjing Medical University. Neither patients nor clinicians were informed of the results.
Circulating Tumor Cell Detection
RNA Extraction and cDNA Synthesis
Total RNA of PBMCs from patients and HDs was isolation using TRIzol reagent (TaKaRa, China) according to the manufacturer’s instructions. All RNA preparation steps were performed under RNase-free coding. RNA concentration was determined using a NanoDrop 2000 spectrophotometer and RNA quality was verified by 1.5% non-denaturing agarose gel electrophoresis. Reverse transcription of RNA was carried out with the PrimeScript RT Master Mix system (TaKaRa).
Identification of Gene Transcripts in PBMCs
Real-time qPCR for GAPDH and marker genes (CK19, SBEM, and hMAM) was performed with cDNA samples prepared using PBMCs from patients and HDs. Gene expression was quantified with SYBR green assay using the ABI StepOnePlus system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The relative expression of each gene was calculated using the equation 2−∆Ct (∆Ct = Ctgene – CtGAPDH). Real-time qPCR positivity was defined as gene expression beyond the cut-off threshold, which was set at three standard deviations from the mean expression in CD45+ PBMCs from HDs. Samples positive for at least one of the markers were considered to contain CTCs.
Intratumoral and Tumor-Associated Stromal TIL Analysis
Formalin-fixed paraffin-embedded serial tissue sections from both the diagnostic core biopsies and the surgical samples were selected to include tumor tissue and tissue surrounding the tumor. We evaluated the amount of iTILs and sTILs based on criteria published by Denkert et al.Citation31 iTILs are defined as lymphocytes in direct cell-to-cell contact with tumor cells without intervening stroma and were reported as the percentage of tumor epithelial nests that contain infiltrating lymphocytes. sTILs were defined as the percentage of stroma area that contains a lymphocytic infiltrate without directly interacting with tumor cells.Citation32 Two experienced pathologists performed the histopathologic analysis independently and the mean was used for analysis.
Immunohistochemistry for total TILs, CD4+ T cells, and CD8+ T cells was performed in the intratumoral and stromal compartments separately, according to standard procedures.
Statistical Methods
In the present study, median and percentiles were analyzed for continuous variables and frequency (percentage) was used for categorical variables. Normality of distribution was tested by the Kolmogorov–Smirnov test. The Student’s t-test was used if data were normally distributed. Otherwise, the Wilcoxon–Mann–Whitney test was used. The χ2 test was used to analyze and compare frequencies for categorical variables. Statistical analyses were performed using STATA version 14.0 (Computer Resource Center, USA). P < 0.05 was considered significant in two-sided tests.
Results
Baseline Clinical Data
In the present study, one patient was excluded due to mammitis disease determined by core biopsy; two patients diagnosed with metastatic disease were also excluded. From August 2013 to January 2014, 30 invasive breast cancer patients were enrolled. Patient characteristics are shown in . The median patient age was 44.5 years (range, 29–68 years). Of these 30 patients, 15 were diagnosed as ER- and/or PR-positive and 14 exhibited HER2 overexpression. Pre- and post-NAC blood samples were obtained from all 30 patients. Pre-NAC formalin-fixed paraffin-embedded core biopsies and post-NAC surgical samples for TILs were collected from 19 patients.
Table 1 Patient Characteristics and Correlation with CTC Detection Prior to and After Neoadjuvant Chemotherapy
Association of pCR with Metastatic Relapse
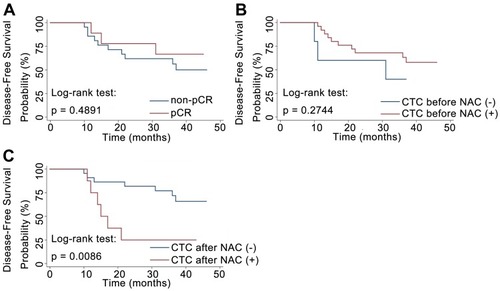
First, we found that pCR was associated with good prognosis only in a subgroup of patients. Among the 30 patients, nine patients achieved pCR. After a median follow-up period of 40 months (range, 34–47 months), 13 patients developed distant metastasis. pCR was associated with a lower rate of metastatic relapse in a subgroup of HR-negative breast cancer patients (P = 0.002), but no significant association was observed in the entire study population (see Supplementary Table 1). For all patients, we did not observe improved DFS in the cases with pCR after NAC (P = 0.489, ).
Figure 2 (A) DFS of patients with or without pCR after NAC. (B) DFS of patients with or without detectable CTCs before the initiation of NAC. (C) DFS of patients with or without detectable CTCs after NAC.
Abbreviations: DFS, disease-free survival; NAC, neoadjuvant chemotherapy; CTC, circulating tumor cell.
Association of CTC Detection with Metastatic Relapse and pCR
As pCR does not have a strong prognostic value in all breast cancer patients, we wondered whether CTC detection had a significant effect on metastatic relapse. Of the 30 patients, 25 patients were CTC-positive before NAC, and eight patients were CTC-positive after NAC. The rate of detection of CTCs was significantly higher in HER2-negative breast cancer patients than HER2-positive patients before NAC (P = 0.014, ). The disease-free probability was 25% for patients with CTCs and 68.2% for patients without CTCs after NAC. The presence of CTCs after NAC was significantly associated with early metastatic relapse (P = 0.049, ). Patients with CTCs after NAC also had a significantly worse DFS than that of patients without detectable CTCs (P = 0.009, ). By contrast, no significant correlation between CTC detection prior to NAC and relapse was observed (). In a subgroup analysis, patients were stratified according to HR status. No statistically significant predictor of metastatic relapse was observed for HR-positive or HR-negative breast cancer patients (all P > 0.05, ). Finally, we found that neither CTC detection before NAC nor CTC detection after NAC was predictive of pCR, indicating the discrepancy between primary tumor and CTC chemosensitivity.
Table 2 Association Between CTCs Detection and Recurrence in Breast Cancer Patients with Neoadjuvant Chemotherapy
Associations of TILs with pCR and CTCs Prior to and After NAC
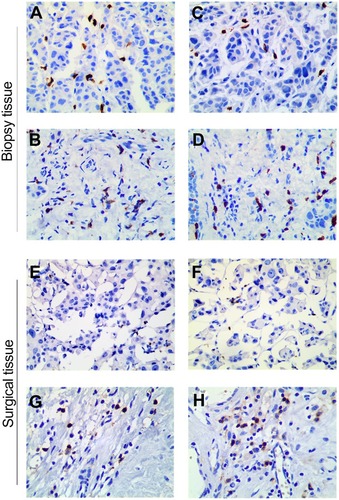
To investigate whether TILs were involved in primary tumor or CTC chemosensitivity, we detected iTILs and sTILs in core biopsies before NAC and surgical tissues after NAC. Representative images of a case are shown in . In the present study, TIL levels did not show statistical differences with respect to age, tumor size, tumor grade, Ki-67, HR status, or HER2 status (all P > 0.05, data not shown).
Figure 3 Histologic patterns of lymphocyte infiltrate in a breast cancer case included in this study (400×). (A, C) iCD4+ and sCD4+ in core biopsies prior to NAC; (B, D) iCD8+ and sCD8+ in core biopsies prior to NAC; (E, G) iCD4+ and sCD4+ in surgical tissues after NAC; and (F, H) iCD8+ and sCD8+ in surgical tissues after NAC.
Abbreviations: iCD4+, intratumoral CD4+ tumor-infiltrating lymphocyte; iCD8+, intratumoral CD8+ tumor-infiltrating lymphocyte; sCD4+, stromal CD4+ tumor-infiltrating lymphocyte; sCD8+, stromal CD8+ tumor-infiltrating lymphocyte.
First, we investigated the prediction of response to NAC by TILs detected in biopsy tissues prior to NAC. The overall median and range of iTILs and sTILs were 10% (0–250%) and 10% (1–80%), respectively. A significant correlation was not obtained between TILs and pCR or between TILs and CTC detection (data not shown).
In contrast with the detection of TILs prior to NAC, we suspected that the effector function of TILs might be affected by chemotherapy after NAC. After NAC, the median and range was 10% (2–70%) for total sTILs, 5% (1–50%) for CD4+ T cells, and 5% (1–20%) for CD8+ T cells in the stroma. No significant correlations were found between sTILs (including total sTILs, CD4+ T cells, and CD8+ T cells) and pCR (all P > 0.05, ). Of note, among the patients who received TIL evaluation, iTILs after NAC could not be detected for five patients who achieved pCR; thus, the association of iTILs with pCR could not be analyzed.
Table 3 Correlation of pCR or CTCs and Infiltrating Lymphocytes After Neoadjuvant Chemotherapy
Since chemotherapy could evoke a host repair response, affecting the primary tumor microenvironment thus facilitating tumor metastasis, we asked whether the contribution of TILs would be different between patients with CTCs and patients without CTCs after NAC. For patients who did not achieve pCR, iTILs, including total TILs, CD4+ T cells, and CD8+ T cells, were not significantly different between patients with and without CTCs after NAC (all P > 0.05, ). However, sTILs were significantly correlated with CTC detection after NAC (). The median and range of total sTILs in cases with and without CTC detection were 25% (10–70%) versus 10% (2–60%), respectively (P = 0.023). The median and range of CD4+ T cells in stroma in cases with and without CTC detection was 17.5% (5–50%) versus 4% (1–40%), respectively (P = 0.010). The median and range of CD8+ T cells in stroma in cases with and without CTC detection were 9% (3–20%) versus 2% (1–20%), respectively (P = 0.040).
Association of TILs with Metastatic Relapse
As sTILs were significantly correlated with CTCs after NAC, we wondered whether TILs could play a role in the prognosis of patients who received NAC; however, no significant correlation with metastatic relapse was observed for iTILs or sTILs detected in biopsy tissues prior to NAC (data not shown).
Similar to the relationship between TILs and CTCs, we focused on the prognostic significance of TILs in surgical tissues after NAC (). Increased infiltration of sTILs was found to be an unfavorable prognostic factor measured by the rate of metastatic relapse (P = 0.028). Patients with high CD4+ T cells in the stroma had a significantly higher rate of metastatic relapse (P = 0.011). By contrast, CD8+ T cells in the stroma were not significantly associated with patient relapse.
Discussion
NAC was introduced in the early 1970s to treat inoperable locally advanced breast cancer. pCR is currently the main endpoint of NAC treatment.Citation3,Citation4 However, the correlation between pCR and long-term outcome is not robust.Citation5 Our study found that pCR was associated with a lower rate of metastatic relapse only in a subgroup of HR-negative breast cancer patients; no correlation was found in the total population as reported previously.Citation33 Other surrogate markers with significant prognostic value are therefore needed.
As reported, CTCs are considered to be “seeds” of fatal metastasis. Much work has been conducted in the detection and characterization of CTCs in recent years, yielding discordant results concerning the possibility of monitoring therapeutic efficacy by detecting CTCs.Citation19–Citation21 Our study found that CTC detection was not correlated with pCR before or after NAC, indicating the discrepancy between primary tumor and CTC chemosensitivity. Of note, we found that the detection of CTCs before NAC was not correlated with metastatic relapse, whereas the detection of CTCs after NAC was significantly associated with a higher rate of metastatic relapse. In contrast with our results, previous studies have demonstrated that the detection of CTCs at the primary diagnosis of breast cancer was also associated with worse prognosis based on a large cohort.Citation15 We supposed that the prognostic relevance of CTCs after NAC could be more valuable than before NAC because NAC allowed for the identification of patients with CTCs evading standard chemotherapy and capable of generating metastases. Thus, a significant predictive prognosis value of CTCs after NAC, but not before NAC, was gained in our small sample size study.
CTCs are continuously under attack in the tumor microenvironment, which contains the immune surveillance system. Mego et al demonstrated that abnormalities in T-cell-mediated immunity could be found in patients with CTCs, which could potentially initiate and impact the dissemination of tumor cells.Citation34 Stromal gene expression analysis revealed a strong prognostic capacity of differential immune responses, highlighting the importance of stromal biology in tumor progression.Citation35 To our knowledge, no data to date are available regarding the role of TIL location in CTCs prior to/after NAC. In the present study, we investigated the correlation of TILs (sTILs and iTILs) with pCR and CTCs separately. A previous meta-analysis including 12 studies showed that higher TIL levels in pre-treatment biopsy tissues indicated higher pCR rates for NAC in breast cancer.Citation28 Given the small sample size, no significant correlations prior to NAC were found between TILs and pCR.
In contrast to TILs in tumors prior to NAC, the contribution of TILs in residual tumors after NAC to response and prognosis is still unclear. Injury from chemotherapy may lead to the formation of a new tumor immune microenvironment and changes in TIL function, possibly contributing to a different predictive value. We found that increased infiltration of sTILs after NAC was an unfavorable prognostic factor, significantly correlated with CTCs and metastatic relapse, which is consistent with a previous study indicating an increased number of TILs in patients with significantly worse DFS.Citation27 By contrast, no association was validated between iTILs after NAC and breast cancer prognosis. Another study also found that sTILs are superior to iTILs in predicting response to therapy.Citation32 We hypothesized that the location of TILs in breast cancer contributes differently to disease outcome, with biological differences between lymphocytes located close to tumor cells compared with those located distant from tumor cells and embedded in the stroma.
The contribution of different TIL subpopulations to biological and clinical tumor behavior remains unclear. CD4+ T cells play a critical role in tumor immunity, and the results of previous studies have been controversial. Each subset of CD4+ T cells has a unique role in immune response during tumor development.Citation36–Citation38 A previous study reported that CD4+ TILs have negative prognostic effects on breast cancer patient outcomes. CD4+ TILs could significantly increase in numbers and its dominant subsets changed to Treg and Th17 cells, which may contribute to tumor promotion.Citation39 Similarly, the present study suggested that increased CD4+ TILs in the stroma after NAC predicted early metastatic relapse, but no efficacy of the CD4+ T subset has been reported.
We found that high levels of CD8+ T cells in the stroma after NAC were associated with the presence of CTCs, which is in contrast to that of CD8+ TILs, which can effectively kill cancer cells.Citation40 However, prolonged exposure of CD8+ TILs to cancer cells can lead to complete or partial loss of their effector function, producing a state of exhaustion,Citation41 which may have contributed to the results of our study. The potential of TILs to predict the presence of CTCs and risk of relapse may depend on lymphocyte location, subpopulation, and optimal timing for sampling.
The present study has several limitations. First, the sample size was relatively small; additional studies are needed to further confirm our findings. Second, we did not detect CTCs by CellSearch; thus, the contribution of changes in CTC count and number in the tumor response and prognosis is lacking. Third, interactions among subgroups of TILs, interactions of TILs in the tumor site compared with those in the stroma, and interactions of TILs with tumor cells are complicated and we did not investigate the functional status of TILs in the present study. More functional assays will be required to determine the prognostic and predictive role of TILs and CTCs.
Conclusions
The detection of CTCs at different time points before, after, and during systemic therapy might serve as a tool to predict response and disease progression or guide treatment in breast cancer patients. Despite the above limitations, our findings suggest that the detection of CTCs was not correlated with pCR before or after NAC, indicating the discrepancy between primary tumor and CTC chemosensitivity. Given the small sample size, we did not identify a significant predictive role of TILs in the rate of pCR. However, detection of CTCs after NAC was positively associated with metastatic relapse of breast cancer patients. Although our data cannot confirm causality, it suggests that the locations of TILs at different time points have different associations with CTCs and disease progression. Increased infiltration of sTILs after NAC was significantly correlated with the detection of CTCs and was found to be an unfavorable prognostic factor. This may be because the tumor microenvironment and tumor–stromal interactions impact tumor cell dissemination and initiation of the metastatic cascade and thus play a prominent role in determining breast cancer outcome.
Abbreviations
NAC, neoadjuvant chemotherapy; CTC, circulating tumor cell; ER, estrogen receptor; PR, progesterone receptor; HR, hormone receptor; pCR, pathologic complete response; TIL, tumor-infiltrating lymphocyte; iTIL, intratumoral tumor-infiltrating lymphocyte; sTIL, stromal tumor-infiltrating lymphocyte; iCD4+, intratumoral CD4+ tumor-infiltrating lymphocyte; iCD8+, intratumoral CD8+ tumor-infiltrating lymphocyte; sCD4+, stromal CD4+ tumor-infiltrating lymphocyte; sCD8+, stromal CD8+ tumor-infiltrating lymphocyte; DFS, disease-free survival.
Disclosure
The authors have declared that no competing interests exist in this work.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (81502286, 81502299, 81572607, 81602321, and 81771953), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.2125425559415
- Jones RL, Smith IE. Neoadjuvant treatment for early-stage breast cancer: opportunities to assess tumour response. Lancet Oncol. 2006;7(10):869–874. doi:10.1016/S1470-2045(06)70906-817012049
- Pierga JY, Mouret E, Dieras V, et al. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer. 2000;83(11):1480–1487. doi:10.1054/bjoc.2000.146111076657
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi:10.1200/JCO.2007.10.682317785706
- Fontanella C, Loibl S, von Minckwitz G. Clinical usefulness and relevance of intermediate endpoints for cytotoxic neoadjuvant therapy. Breast. 2015;24(Suppl 2):S84–S87. doi:10.1016/j.breast.2015.07.02026279131
- Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23(36):9304–9311. doi:10.1200/JCO.2005.02.502316361629
- Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–1044. doi:10.1200/JCO.2005.02.691416505422
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi:10.1016/j.cell.2011.11.02522265420
- Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106:5. doi:10.1093/jnci/dju066
- Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384–6388. doi:10.1158/0008-5472.CAN-13-203024145355
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224. doi:10.1158/1078-0432.CCR-05-282116857794
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi:10.1056/NEJMoa04076615317891
- Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol. 2008;19(3):496–500. doi:10.1093/annonc/mdm50718187488
- Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009;27(13):2177–2184. doi:10.1200/JCO.2008.18.049719332733
- Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–695. doi:10.1016/S1470-2045(12)70209-722677156
- Onstenk W, Kraan J, Mostert B, et al. Improved circulating tumor cell detection by a combined EpCAM and MCAM cell search enrichment approach in patients with breast cancer undergoing neoadjuvant chemotherapy. Mol Cancer Ther. 2015;14(3):821–827. doi:10.1158/1535-7163.MCT-14-065325552367
- Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–7010. doi:10.1158/1078-0432.CCR-08-003018980996
- Karagiannis GS, Pastoriza JM, Wang Y, et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med. 2017;9:397. doi:10.1126/scitranslmed.aan0026
- Bidard FC, Belin L, Delaloge S, et al. Time-dependent prognostic impact of circulating tumor cells detection in non-metastatic breast cancer: 70-month analysis of the REMAGUS02 study. Int J Breast Cancer. 2013;2013:130470. doi:10.1155/2013/13047023762567
- Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645. doi:10.1158/1078-0432.CCR-09-204220406831
- Kasimir-Bauer S, Bittner AK, Konig L, et al. Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res. 2016;18(1):20. doi:10.1186/s13058-016-0679-326868521
- Horimoto Y, Polanska UM, Takahashi Y, Orimo A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adh Migr. 2012;6(3):193–202. doi:10.4161/cam.2063122568980
- Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. doi:10.1158/0008-5472.CAN-14-358726269531
- Roodhart JM, He H, Daenen LG, et al. Notch1 regulates angio-supportive bone marrow-derived cells in mice: relevance to chemoresistance. Blood. 2013;122(1):143–153. doi:10.1182/blood-2012-11-45934723690447
- Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19(1):28–33. doi:10.1158/1078-0432.CCR-11-270123258741
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–160. doi:10.1038/nrclinonc.2010.22321364688
- Garcia-Martinez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. doi:10.1186/s13058-014-0488-525432519
- Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115103. doi:10.1371/journal.pone.011510325501357
- Zhou W, He Z, Xue J, et al. Molecular subtype classification is a determinant of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph nodes. PLoS One. 2012;7(4):e35881. doi:10.1371/journal.pone.003588122563412
- Zhou W, Pan H, Xia T, et al. Up-regulation of S100A16 expression promotes epithelial-mesenchymal transition via Notch1 pathway in breast cancer. J Biomed Sci. 2014;21:97. doi:10.1186/s12929-014-0097-825287362
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi:10.1200/JCO.2009.23.737019917869
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi:10.1093/annonc/mdu45025214542
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi:10.1200/JCO.2003.12.00514559892
- Mego M, Gao H, Cohen EN, et al. Circulating Tumor Cells (CTC) are associated with defects in adaptive immunity in patients with inflammatory breast cancer. J Cancer. 2016;7(9):1095–1104. doi:10.7150/jca.1309827326253
- Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. doi:10.1038/nm176418438415
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19(2):203–208. doi:10.1016/j.coi.2007.02.00117292599
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.01715308095
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi:10.1126/science.120348621436444
- Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6(19):17462–17478. doi:10.18632/oncotarget.395825968569
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi:10.1038/nri319122437939
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi:10.1038/ni.203521739672
