Abstract
Purpose
Thoracic radiotherapy (TRT) is the recommended therapeutic regimen for extensive-stage small cell lung cancer (ES-SCLC). Little is known about TRT benefits in elderly populations. The aim of this study was to evaluate TRT effects on the prognosis of elderly patients with ES-SCLC.
Patients and methods
This retrospective analysis reviewed the records of patients over 65 years of age with metastatic ES-SCLC treated between 2010 and 2016. Enrolled patients received standard chemotherapy regimens (etoposide plus cisplatin or carboplatin). A total of 93 eligible patients were subjected to propensity score matching, which led to 40 patients being assigned to the TRT group and 40 to the no thoracic radiotherapy (noTRT) group. The cohort of 80 patients (67 males) had the median age of 69 years (range, 65–85 years), with a median of 4 chemotherapy cycle (range, 1–8 cycles), and a median chest irradiation dose of 50 Gy (range, 30–60 Gy). We analyzed overall survival (OS), progression-free survival (PFS), and local recurrence-free survival (LRFS) as endpoints; survival rates were determined by the Kaplan–Meier method and compared across groups with log-rank tests. Multivariate prognostic analysis was performed with Cox regression modeling, and categorical variables were analyzed with Chi-square tests.
Results
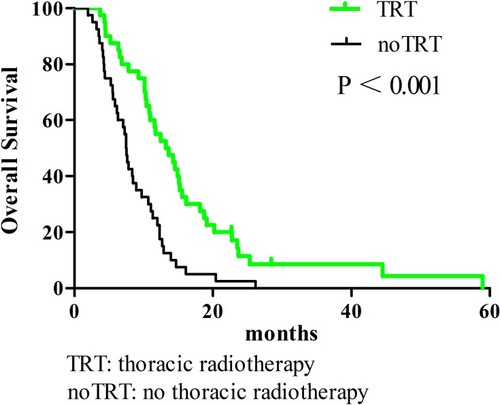
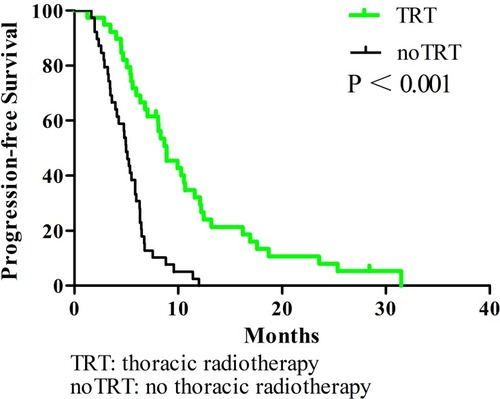
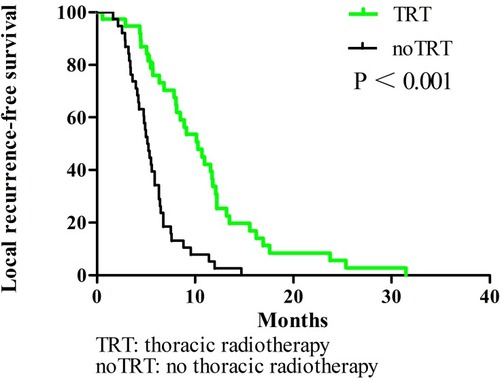
In all patients, the 1-year OS, PFS, and LRFS rates were 38.3%, 16%, and 17.9%, respectively. The TRT group had superior survival outcomes compared to the noTRT group: their 1-year OS, PFS, and LRFS rates were 55% vs. 25% (P < 0.001), 32.1% vs. 0% (P < 0.001), and 31% vs. 2.6% (P < 0.001), respectively. TRT did not increase the incidence of adverse reactions (P = 0.431).
Conclusion
TRT can improve chest tumor control and survival time in elderly ES-SCLC patients. Large-scale studies to further assess the benefits of TRT are warranted.
Introduction
Over the last several years, lung cancer has had the highest incidence and mortality rates of all malignant neoplasms.Citation1 The most recent data from Surveillance Epidemiology and End Results indicate that lung carcinoma occurs most frequently in patients 65 to 74 years of age. Of lung carcinoma patients, 69.4% are diagnosed after the age of 65, and 72% of patients who die from lung carcinoma are above 65 years of age.Citation2 Small cell lung cancer (SCLC) accounts for about 15–20% of lung cancer cases, and approximately 70% of SCLC cases have an initial diagnosis with extensive invasion. Extensive-stage SCLC (ES-SCLC) has a natural disease course of 2–4 months and a 1-year overall survival (OS) of only 2%.Citation3,Citation4 Although 60–70% of ES-SCLC patients respond to 4–6 cycles of platinum-based chemotherapy, improving the median survival time to 7–12 months, 80–90% of such patients suffer an intrathoracic recurrence within 1 year,Citation5 leading to a 2-year OS rate less than 5%, and long-term disease-free survival is rare.Citation6,Citation7 ES-SCLC patients are recommended to receive systemic chemotherapy as the standard therapeutic regimen. However, a European multicenter randomized clinical trial confirmed that the 2-year OS rate could improve from 3% to 13%, and intrathoracic recurrence could be reduced to 50% for ES-SCLC patients who had chemotherapy with subsequent chest irradiation.Citation8,Citation9 Consequently, the National Comprehensive Cancer Network guidelines recommended that ES-SCLC patients who respond to chemotherapy also receive thoracic radiotherapy (TRT). Because the majority of ES-SCLC patients are over 65-years-old, and few elderly patients are included in clinical trials, it is not known whether TRT can improve survival in elderly ES-SCLC patients.Citation10 Previous research provides evidence that in elderly patients with non-small cell lung cancer, TRT can decrease rates of chest recurrence and increase survival time.Citation11 However, much less work has been done investigating TRT in elderly patients with ES-SCLC. Therefore, the objective of this study was to evaluate the effectiveness of TRT in elderly ES-SCLC patients.
Materials and Methods
Patient Population
We completed a retrospective review of patients seen in our hospital with ES-SCLC between January 2010 and January 2018. The inclusion criteria were: (1) histopathologically confirmed SCLC; (2) distant metastasis (e.g., brain, bone, liver, lung, adrenal gland, abdominal cavity, etc.) in addition to a primary SCLC; (3) standard chemotherapy treatment (etoposide with cisplatin or carboplatin); and (4) pretreatment examinations with a complete medical history, physical examination, blood test, liver and kidney function tests, chest X-ray, cervical and thoracic enhanced computed tomography (CT) or positron emission computed tomography (PET-CT), and cranial enhanced magnetic resonance imaging (MRI). Patients diagnosed due to contralateral hilar or supraclavicular lymph node metastasis were excluded. In total, 93 patients were eligible for this study. The prognosis of ES-SCLC patients over 65 years of age has been reported to be related to the independent clinical factors of age, gender, TRT, and prophylactic cranial irradiation.Citation12 Propensity score matching consisting of the following items was applied: gender, Karnofsky Performance Status (KPS) score, weight loss, chemotherapy efficacy, presence of more than one metastasis.
Treatment
40 patients received chemotherapy consisting of etoposide (100 mg) on days 1–5 with cisplatin (30 mg/m2) on days 1–3 (EP), and 40 patients received etoposide (100 mg) on days 1–5 with carboplatin (500 mg) on day 1 (EC). With respect to radiotherapy. 33 patients received intensity-modulated radiation therapy, 5 received three-dimensional conformal radiation therapy, and 2 received conventional radiation therapy. Radiotherapies were administered with a PinnacleCitation3 8.0-m planning system to delineate gross tumor volume on enhanced computed tomography images, defined as radiographically visible tumor residual lesions and positive lymph nodes. The clinical target volume (CTV) mainly included 0.5–0.8 cm of the primary lesion and the draining area of positive lymph nodes. The planned target volume (PTV) was defined as the CTV plus a 0.5–1.0 cm margin and PGTV was expanded from GTV with a 0.5–1.0 cm margin. The prescription dose was 30–60 Gy in fractions of 1.8–3 Gy.Citation13 Doses in organs at risk were generally within normal limits; 9 patients received concurrent chemotherapy during thoracic radiotherapy.
Efficacy Evaluation and Follow-Up
Chemotherapy efficacy was evaluated 1 month after inductive chemotherapy according to the Response Evaluation Criteria in Solid Tumors 1.1. Radiation-related toxicities (i.e., hematologic decline, gastrointestinal reactions, radiation esophagitis, and radiation pneumonitis) were assessed 3 months after TRT on the basis of Common Terminology Criteria for Adverse Events 3.0. Acute adverse events greater than or equal to grade 2 were considered treatment-related side effects. Ultrasonography, enhanced CT and MRI, or PET-CT (performed every 3 months during the first 2 years after treatment, every 6 months during the next 2 years, and annually thereafter) was used to evaluate treatment efficacy. The primary end-points were OS (defined as the time from diagnosis to death or lost follow-up) and progression-free survival (PFS: defined as the time from diagnosis to disease progression or lost follow-up). The secondary end-point was local recurrence-free survival (LRFS: defined as the time from diagnosis to local recurrence or lost follow-up).
Statistical Analysis
All data analyses were performed using the SPSS 24.0 statistical software (IBM Corporation, Armonk, NY, United States). OS, PFS, and LRFS were calculated by the Kaplan–Meier method and compared across groups with log-rank tests. Multivariate prognostic analysis with a backward-forward stepwise method was performed with Cox regression modeling, and categorical variables were analyzed with Chi-square tests. P-value <0.05 of the two-sided test was considered significantly different statistically.
Results
Patient Characteristics
After propensity score matching, 40 patients each were enrolled in the TRT and noTRT groups. Patients with brain (13 TRT; 7 noTRT), lung (8 TRT; 9 noTRT), liver (5 TRT; 11 noTRT), bone (16 TRT; 19 noTRT), adrenal gland (7 TRT; 6 noTRT), or other cancers (10 TRT; 15 noTRT) were included in the study. Overall, there were 67 males; the median age of the cohort was 69 years (range = 65–85 years), the median chemotherapy cycle number was 4 cycles (range = 1–8 cycles), the median chest irradiation dose was 50 Gy (range = 30–60 Gy) (). The prescription dose of each enrolled elderly ES-SCLC patients in the TRT group after PSM could be seen in . For TRT group, the median volume, D50 and D95 of PTV were 378.78 cm3 (range = 60.43–932.72 cm3), 52.69 Gy (range = 31.32–58.98 Gy), 48.47 Gy (range = 30.01–65.17 Gy), respectively; and the median mean lung dose, V5 and V20 of lung were 13.24 Gy (range = 2.88–18.79 Gy), 48 Gy (range = 6.01–77 Gy) and 26 Gy (range = 3–32 Gy), respectively; the median maximum dose of spinal cord and mean esophageal dose were 40.56 Gy (range = 16–48.57 Gy) and 29.18 Gy (range = 11.50–66.59 Gy). Besides, the median duration of TRT was 38 days (range = 11–74 days), the median time between the start of chemotherapy and the end of radiotherapy was 4.7 months (range = 1.64–18.33 months), and the median time between the last of chemotherapy and the start of radiotherapy was 26.5 days (range = 14–75 days).
Table 1 The General Clinical Data of Elderly Patients with ES-SCLC Before and After PSM
Table 2 The Prescription Dose of Each Elderly ES-SCLC Patients in TRT Group After PSM
Survival Outcome
All enrolled patients were contacted regularly after medical treatment, and the median follow-up length was 37 months. The median OS, PFS, and LRFS times were 10.3 months, 5.9 months, and 6.4 months, respectively; the 1-year OS rates, PFS, and LRFS rates were 38.3%, 16%, and 17.9%, respectively; the 2-year OS, PFS, and LRFS rates were 7%, 4%, and 2.7%, respectively. Patients undergoing TRT had better survival outcomes than the noTRT group (, –). In TRT group, the patients received more than or equal to 50Gy had a similar survival outcome than those who did not, the median OS and PFS were 14.9 vs. 10.9 months (P = 0.282), 8.9 vs. 9.9 months (P = 0.778), respectively; the 1-year OS and PFS were 66.7% vs. 45.5%, 22.2% vs. 36.1%, respectively; the 2-year OS and PFS were 16.7% vs. 5.7%, 5.6% vs. 5.2%, respectively.
Table 3 Survival Comparison of Elderly ES-SCLC Patients with TRT or noTRT After PSM
Prognostic Analysis of Clinical Factors
Univariate analysis indicated that OS was related to number of metastases (P < 0.001), number of chemotherapy cycles (P = 0.014), chemotherapy efficacy (P < 0.001), and TRT (P < 0.001), time between the beginning of chemotherapy and the end of radiotherapy more than 4.7 months (P = 0.050). Multivariate analysis of factors with P values <0.1 in our univariate analysis indicated that elderly ES-SCLC patients with less than one metastasis, with more than four cycles of chemotherapy, who had effective chemotherapy, or who received of TRT had better prognoses than those without TRT ().
Table 4 Univariate and Multivariate Prognostic Analysis of Elderly ES-SCLC Patients After PSM
Chemotherapy Efficacy and Prognostic
Patients who received effective chemotherapy had better survival outcomes than those who did not. Specifically, the median OS, PFS, and LRFS times were 14.0 versus 7.9 months (P < 0.001), 8.0 versus 4.9 months (P < 0.001), and 10.2 versus 5.5 months (P < 0.001), respectively; the 1-year OS rates, PFS, and LRFS rates were 57.6% versus 25.5%, 30.3% versus 4.9%, and 33.6% versus 7%, respectively; the 2-year OS rates, PFS, and LRFS rates were 15.2% versus 2.1%, 9.1% versus 0%, and 6.7% versus 0%, respectively. TRT increased survival times in both effective and ineffective chemotherapy groups ().
Table 5 The Comparison of Survival in Different Induction Chemotherapy Efficacy Groups of Elderly ES-SCLC Patients After PSM
Adverse Reactions
In the noTRT and TRT groups, there were 32 and 29 cases of adverse effects (P = 0.431), respectively, and the incidences of myelosuppression and gastrointestinal reactions (grade 2 or worse) were 29 versus 26 cases (P = 0.899) and 16 versus 14 cases (P = 0.701), respectively. Of the 40 patients who underwent TRT, 2 suffered radiation esophagitis and 2 patients suffered radiation pneumonitis; All 4 patients recovered after active treatment.
Patterns of Failure
A total of 74 patients suffered from chest recurrence or distant metastasis after treatment. Patients who had chest recurrence, including local and regional, accounted for 45% (18/40) of cases in the TRT group and 87.5% (35/40) in the noTRT group. Local recurrence, distant metastasis, and both local recurrence and distant metastasis occurred in 7 (20%) versus 13 cases (33.3%), 18 (51.4%) versus 5 cases (12.8%), and 10 (28.6%) versus 21 cases (53.8%) in the TRT and noTRT groups, respectively.
Discussion
The findings of the present retrospective study suggest that the addition of TRT to treatment plans of elderly patients with ES-SCLC could improve OS and PFS, similar to what has been seen in young patients. Furthermore, induction chemotherapy effectiveness did not influence TRT effects on survival, and the addition of TRT appeared to provide survival benefits, decrease local recurrence rates, and improve quality of life for elderly ES-SCLC patients.
Though platinum-based chemotherapy, which has high response rates, has been the standard treatment regimen for ES-SCLC patients since the 1970s, thoracic recurrence is the most common cause of ultimate treatment failure and subsequent short-term patient death. Researchers have attempted unsuccessfully to improve survival time by increasing chemotherapy cyclesCitation14–Citation16 and optimizing first- and second-line chemotherapy regimens.Citation17–Citation21 The recent introduction of immunotherapy has brought new hope for many cancer patients.Citation22–Citation25 Immunotherapy clinical studies have suggested that treatment with checkpoint inhibitors may be viable options for ES-SCLC patients.Citation26–Citation28 The IMpower133 study—a double-blind, placebo-controlled, Phase 3 trial that evaluated atezolizumab plus carboplatin and etoposide in patients with ES-SCLC who had not previously received treatment (N = 202)—showed that the addition of atezolizumab extended OS (10.3 months to 12.3 months) improved the 1-year survival rate (38.2% to 51.7%), and increased the complete response rate (1.0% to 2.5%) compared to the placebo group (N = 202). Moreover, the survival benefit was most pronounced in patients older than 65 years, who had an Eastern Cooperative Oncology Group score of 1 and no brain or liver metastases.Citation26 Although immunotherapy prolongs survival in some cancer patients, the majority of patients do not benefit from immune checkpoint inhibitors.Citation29
Thoracic radiation therapy has been studied extensively in ES-SCLC. For example, a phase 3 randomized, controlled clinical trial demonstrated that in ES-SCLC, the addition of chest irradiation to effective chemotherapy improved 2-year OS from 3% to 13% and decreased intrathoracic recurrence by 50%.Citation8,Citation9 However, in many clinical studies, TRT was used as palliative care in elderly patients to relieve symptoms of local or distant metastasis sites, rather than the initial clinical recommendation as a combination therapy regimen. This treatment bias in the elderly may be due to high complication rates, low bone marrow reserve capacity, and pressure to maximize survival time from clinicians and families.Citation30 Our findings showing that TRT can extend survival of elderly ES-SCLC patients are consistent with the findings of a prior study of 118 elderly patients with metastatic ES-SCLC wherein the median OS times with or without TRT were 17 months and 11.7 months (P = 0.014), the median PFS times were 9.9 months and 6.5 months (P = 0.004), and the 2-year OS rates were 38.1% and 14.9%, respectively.Citation31 However, whether the efficacy of induction chemotherapy affects the prognosis of elderly ES-SCLC patients receiving TRT is still not known. In addition, elderly patients show great physiological heterogeneity. In the current study, we accounted for the general health and clinical factors of patients by propensity score matching, thereby minimizing the therapeutic influence of confounding factors.
Our research provides evidence suggesting that patients over 65 years with ES-SCLC may benefit from TRT. In patients who received TRT, the 2-year OS rate increased from 5.7% to 14.2%, the intrathoracic recurrence rate decreased by 48.2%, and the incidence of adverse reactions was unaffected by TRT. Though the prognosis of patients with effective induction chemotherapy was better than in those with inefficient chemotherapy [2-year OS rates of 22.3% and 0%, respectively (P < 0.001)], the addition of TRT significantly improved the OS, PFS and LRFS of patients with effective chemotherapy. What’s more, patients received TRT more than or equal to 50 Gy had a similar survival outcome than those who did not [2-year OS rates of 16.7% and 5.7%, respectively (P = 0.282)]. Therefore, for patients over 65 with ES-SCLC who respond to induction chemotherapy, it may be necessary to deliver TRT as an initial combination therapy regimen. For patients with ineffective induction chemotherapy, although PFS and LRFS increased after TRT, OS was not affected by TRT treatment statistically. This non-effect may be due to the robust invasiveness of partial tumor cells and the small number of enrolled patients in our study.
In summary, though chemotherapy is still the leading treatment for ES-SCLC, chest irradiation may improve treatment efficiency and survival time of patients over 65 years of age significantly. Our findings suggest that, regardless of the efficacy of induced chemotherapy, the addition of TRT may improve ES-SCLC patients’ outcomes. Furthermore, our findings suggest that TRT can reduce local recurrence and improve the quality of life in elderly patients.
This study had three noteworthy limitations: (1) Propensity score matching and multivariate prognostic analysis cannot completely avoid retrospective selection bias (e.g., patients’ cardiopulmonary function, etc.); (2) our small sample size may not fully represent all elderly ES-SCLC patients; and (3) other chronic diseases may affect patient survival. Optimal radiotherapy fields and doses for elderly patients with metastatic ES-SCLC have yet to be established. Large prospective studies with uniform chemotherapy and radiotherapy schemes will be needed to better understand treatment in this population.
Conclusion
The addition of chest irradiation for ES-SCLC patients over 65 years of age could significantly improve the treatment efficiency and the survival period of patients regardless of the effect of induced chemotherapy.
Abbreviations
TRT, thoracic radiotherapy; ES-SCLC, extensive-stage small cell lung cancer; noTRT, no thoracic radiotherapy; OS, overall survival; PFS, progression-free survival; LRFS, local recurrence-free survival; SCLC, small cell lung cancer; CT, computed tomography; PET-CT, positron emission computed tomography; MRI, magnetic resonance imaging; KPS, Karnofsky Performance Status; PSM: propensity score matching.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki. Additionally, this study has been approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital, and all patients have signed the informed consent.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank all the staff of Tianjin Medical University Cancer Institute and Hospital and all patients that were associated with this study.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA-Cancer J Clin. 2013;63(1):11. doi:10.3322/caac.2133223335087
- Rossi A, Maione P, Colantuoni G, et al. Treatment of small cell lung cancer in the elderly. Oncologist. 2005;42(1):399–411. doi:10.1634/theoncologist.10-6-399
- Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer. 2000;83(1):8–15. doi:10.1054/bjoc.2000.116410883661
- Eckert F, Muller AC. SCLC extensive disease–treatment guidance by extent or/and biology of response? Radiat Oncol. 2008;3:33. doi:10.1186/1748-717X-3-3318831728
- Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized Phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20(24):4665–4672. doi:10.1200/JCO.2002.12.11112488411
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?-a review. Transl Lung Cancer Res. 2016;5(1):26–38. doi:10.3978/j.issn.2218-6751.2016.01.1326958491
- Hurwitz JL, McCoy F, Scullin P, Fennell DA. New advances in the second-line treatment of small cell lung cancer. Oncologist. 2009;14(10):986–994. doi:10.1634/theoncologist.2009-002619819917
- Slotman BJ, Faivre-Finn C, Tinteren HV, et al. International multicenter randomized study on thoracic radiation therapy (RT) in extensive stage small cell lung cancer (ES-SCLC): patterns of disease recurrence. Int J Radiat Oncol Bio Phys. 2014;90(1):S3–S4. doi:10.1016/j.ijrobp.2014.06.023
- Slotman BJ, Faivrefinn C, Van Tinteren H, et al. Randomized trial on thoracic radiotherapy (TRT) in extensive-stage small cell lung cancer. J Clin Oncol. 2014;32(Suppl):abstr 7502. doi:10.1200/jco.2014.32.15_suppl.7502
- Eskandar A, Ahmed A, Daughtey M, Kenderian S, Mahdi F, Khan A. Racial and sex differences in presentation and outcomes of small cell lung cancer in the United States: 1973 to 2010. Chest. 2015;147(4):e164–e165. doi:10.1378/chest.14-305125846546
- Liu TX, Dong M, Zhang YM. Therapeutic effect of three dimensional conformal radiotherapy in patients aged 65 years and older with non-small cell lung cancer. J Chin Lung. 2006;9(5):462–464. doi:10.3779/j.issn.1009-3419.2006.05.16
- Chen X, Fang J, Nie J, et al. Multivariate analysis of prognostic factors in the elderly patients with small cell lung cancer a study of 160 patients. Clin J of Lung Cancer. 2014;17(1):15–23. doi:10.3779/j.issn.1009-3419.2014.01.03
- Xu LM, Cheng C, Kang M, et al. Thoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancer. Sci Rep. 2017;7(1):9255. doi:10.1038/s41598-017-09775-028835666
- Schiller JH, Adak S, Cella D, DeVore RF, Johnson DH. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593–a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(8):2114–2122. doi:10.1200/JCO.2001.19.8.211411304763
- Giaccone G, Dalesio O, McVie GJ, et al. Maintenance chemotherapy in small-cell lung cancer: long-term results of a randomized trial. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1993;11(7):1230–1240. doi:10.1200/JCO.1993.11.7.12308391065
- Sculier JP, Paesmans M, Bureau G, et al. Randomized trial comparing induction chemotherapy versus induction chemotherapy followed by maintenance chemotherapy in small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol. 1996;14(8):2337–2344. doi:10.1200/JCO.1996.14.8.23378708726
- Lara PJ, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–2535. doi:10.1200/JCO.2008.20.106119349543
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85–91. doi:10.1056/NEJMoa00303411784874
- Niell HB, Herndon JN, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23(16):3752–3759. doi:10.1200/JCO.2005.09.07115923572
- Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18(4):1138–1145. doi:10.1158/1078-0432.CCR-11-205922228633
- Von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. doi:10.1200/JCO.2013.54.539225385727
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa180100529658856
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X27979383
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa150764326412456
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-726712084
- Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa180906430280641
- Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol. 2018;13(9):1393–1399. doi:10.1016/j.jtho.2018.05.00229775808
- Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi:10.1200/JCO.2016.67.660127458307
- Lecis D, Sangaletti S, Colombo MP, Chiodoni C. Immune checkpoint ligand reverse signaling: looking back to go forward in cancer therapy. Cancers (Basel). 2019;11:5. doi:10.3390/cancers11050624
- Ershler WB, Longo DL. Aging and cancer: issues of basic and clinical science. J Natl Cancer Inst. 1997;89(20):1489–1497. doi:10.1093/jnci/89.20.14899337345
- An C, Jing W, Zhang Y, et al. Thoracic radiation therapy could give survival benefit to elderly patients with extensive-stage small-cell lung cancer. Future Oncol. 2017;13(13):1149–1158. doi:10.2217/fon-2016-046728326834