Abstract
Despite advancements in diagnosis and therapy, relapse of rectal cancer after clinical complete remission (cCR) remains a frequent event. The key factors influencing the treatment strategy for the management of patients achieving cCR following neoadjuvant chemoradiotherapy (Neo-CRT) remain to be identified. We present the case of a 64-year-old man with rectal cancer. The patient was initially admitted to the hospital in September 2011 with a 3-month history of change in his stools. Following his re-hospitalization in November 2011, a biopsy specimen of the neoplasm suggested the presence of rectal adenocarcinoma; laboratory investigations also revealed elevated levels of carcinoembryonic antigens (CEA; carbohydrate antigen 199) in the serum. Subsequently, the patient received Neo-CRT, as well as symptomatic and supportive treatment. The level of serum CEA returned to normal, without signs of swollen lymph nodes in the pelvic cavity. The patient was diagnosed with rectal cancer based on the elevated level of serum CEA, colonoscopy, and contrast-enhanced magnetic resonance imaging. He relapsed 4 months after cCR following Neo-CRT and underwent laparoscopic Miles’ surgery in April 2013. The relapse may have been mainly attributed to residual tumor cells. This case report and literature review may contribute to the clinical recognition of treatment for patients with rectal cancer achieving cCR following Neo-CRT.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies in the United States.Citation1 The introduction of new and effective chemotherapeutic substances and biologics during the past decade has significantly improved the systemic treatment of CRC patients.Citation2 Neoadjuvant chemoradiotherapy (Neo-CRT) is standard treatment for stage II-III rectal cancer, which consists of chemotherapy and radiotherapy aiming at reducing locoregional relapse.Citation3
Evidence exists to support both surgical and nonoperative observational approaches to the management of patients with distal rectal cancer who achieve a complete response following Neo-CRT.Citation4 This report describes one rectal cancer patient, achieving clinical complete remission (cCR) following Neo-CRT, got relapse 4 months later. Approaches of surgery are also discussed.
Case Report
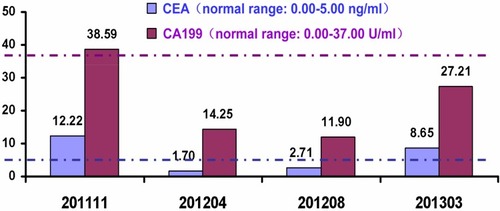
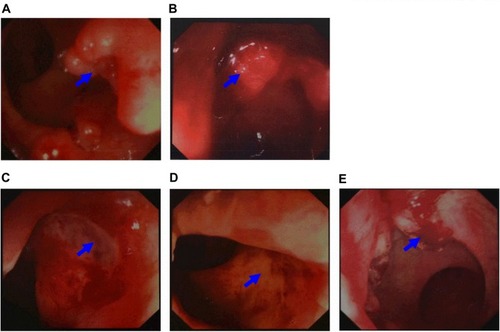
A 64-year-old male, without smoking, alcohol addiction, or any other specific underlying disease histories, was initially admitted to the hospital in September 2011 with a 3-month history of change in his stools. Colonoscopy showed an ulcer-like neoplasm situated 6 cm from the anal margin with a little bleeding, covering half of the lumen (). A biopsy specimen of the neoplasm suggested high-grade intraepithelial neoplasia in rectal mucosa. The patient was re-hospitalized in November 2011. The re-examination of colonoscopy showed an ulcer type neoplasm on the dentate line with erosion, which was brittle and subjected to hemorrhage, covering half of the lumen (). A biopsy specimen of the neoplasm suggested rectal adenocarcinoma. Laboratory investigations disclosed elevated levels of carcinoembryonic antigens (CEA; 12.2 ng/mL; normal range: 0.0–5.0 ng/mL) and CA199 (38.59 U/mL; normal range: 0.00–37.00 U/mL) in the serum Contrast-enhanced MRI showed uneven thickening in rectal wall which consisted heterogeneous enhancement in enhanced scanning. The patient was then given radiotherapy with conventional fractionation as follows (Treatment region A: rectum, 200cGy per day; five fractions per week, total dose, 5000cGy/25 fractions, and treatment region B: pelvic cavity, 180cGy per day; five fractions per week, total dose, 4500cGy/25 fractions). In February 2012, he had a re-examination of colonoscopy, which showed a 2 × 2 cm2 ulcer on distal rectal wall (near the dentate line) with white tongue coating (). A biopsy specimen of the ulcer suggested rectal mucosal erosion. Contrast-enhanced MRI showed slight incrassation on lower rectal wall, no significant abnormal enhancement was found. Afterward, the patient started chemotherapy which is given in 3 week cycles and consists of capecitabine 1250 mg/m2 twice a day, day 1–14 combined with oxaliplatin 130 mg/m2 once every 3 weeks, 5 cycles of chemotherapy in total. Treatment-related toxicity is monitored through the procedure and during which the patient was also receiving symptomatic and supportive treatment. The patient was re-examined in April 2012, his serum level of carcinoembryonic antigens came out to be CEA, 1.70 ng/mL; normal range: 0.0–5.0 ng/mL, CA199, 14.25 U/mL; normal range: 0.00–37.00 U/mL. In August 2012, a contrast-enhanced MRI showed a high T2 signal slight incrassation on lower rectal wall, CEA,2.71 ng/mL; normal range: 0.0–5.0 ng/mL, CA199, 11.90 U/mL; normal range: 0.00–37.00 U/mL, and the colonoscopy showed a scar with smooth surface on distal rectal wall (near the dentate line) (). In October 2012, another contrast-enhanced MRI showed a high T2 signal 1.2 × 1.2cm tubercle with enhanced edge on the right wall of lower rectum (near the anal canal), no signs of swollen lymph node was found in pelvic cavity. In March 2013, the laboratory investigation came out to be CEA, 8.65 ng/mL; normal range: 0.0–5.0 ng/mL, CA199, 27.21 U/mL; normal range: 0.00–37.00 U/mL, the colonoscopy showed a 2 cm × 4 cm neoplasm on dentate line with erosion, which was brittle and subjected to hemorrhage (). A biopsy of the resected specimen suggested villous adenoma, suspecting canceration, and contrast-enhanced MRI showed a high T2 signal 1.5 cm × 1.5 cm tubercle with an enhanced edge on the right wall of the lower rectum (near the anal canal), no signs of swollen lymph node found in the pelvic cavity.
Figure 1 Results of colonoscopy. (A) Colonoscopy showed an ulcer-like neoplasm situated 6 cm from the anal margin with a little bleeding, covering half of the lumen in September 2011 (arrows). (B) The re-examination of colonoscopy showed an ulcer type neoplasm on dentate line with erosion, which was brittle and subjected to hemorrhage, covering half of the lumen in November 2011 (arrows). (C) In February 2012, the re-examination of colonoscopy, which showed a 2 × 2 cm2 ulcer on distal rectal wall (near the dentate line) with white tongue coating (arrows). (D) In August 2012, the colonoscopy showed a scar with smooth surface on distal rectal wall (near the dentate line, arrows). (E) In March 2013, the colonoscopy showed a 2 cm × 4 cm neoplasm on the dentate line with erosion, which was brittle and subjected to hemorrhage (arrows).
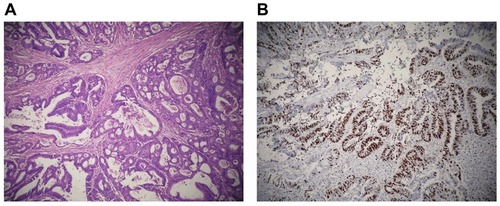
According to the history, the patient should be diagnosed as rectal cancer relapsed 4 months after cCR under Neo-CRT. There was no sign of cancer metastasis observed before the surgery. The patient received Laparoscopic Mile’s surgery in April 2013, during which no metastatic nodules was observed on abdominal viscera, and the tumor was located below the pelvic peritoneal reflex. The surgery dissociated the sigmoid colon and the rectum, then closed the incision with EC60 (made in Shanghai jmu medical device co. LTD, Shanghai, China) and made sigmoid flexure fistula at the left-side abdomen. The tumor specimen consisted of the anus and rectum (length: 13 cm). The tumor located in the rectum measured 1.5×1.2 cm, 5.5 cm from the upper band, and 2.5 cm from the dentate line. Gross morphological analysis showed ulcer infiltration type, growing around a quarter of the rectal wall. The tumor infiltrated peri-nerve tissue, but no sign of invasion of lymphatic vessel or blood vessel observed. No sign of tumor infiltration on both upper and lower bands. Lymph node metastases: all four mesenteric lymph nodes were clear, and the immune response (SH, PH, GH) of the lymph nodes was all positive. The precancerous interstitial reaction consisted of lymphocyte, plasmacyte, histocyte reaction and fibrosis proliferation reaction, and the non-tumor area reaction came out to be chronic mucosa tissue inflammation. Microscopic findings of the rectum mass showed tubular adenocarcinoma (stage II-III, ). The immunoenzyme labeled Ki-67 test showed 55% of the tumor tissue was positive (). The postoperative follow-up showed the patient was recovering very well. shows the patient’s disease stage.
Table 1 The Disease Stage of the Patient
Discussion
CRC is the third most commonly diagnosed cancer among both men and women, with over 135,400 new colorectal cancer cases and 50,000 deaths estimated to have occurred in the United States in 2017.Citation1,Citation5 The highest incidence rates are found in Australia and New Zealand, Europe, and North America, whereas the lowest rates are found in Africa and South-Central Asia. Rates are substantially higher in males than in females.Citation6 Neo-CRT is a standard treatment for stage II-III rectal cancer.Citation7 A proportion of patients, who receive preoperative chemoradiation for locally advanced (T3, T4, NX) rectal cancer, achieve a cCR and a pathologic complete remission (pCR) in the region of 15% to 30%;Citation8 digital rectal examination (DRE) is only able to identify a small proportion of patients who actually achieve a pCR, and only 25% to 50% of patients achieving a cCR are confirmed as a pCR at subsequent surgery.Citation9 Complete clinical response represents the patient is disease-free according to the colonoscopy biopsy after Neo-CRT, while a pathologic complete response patient is diagnosed by observing cancer cell in the specimen cut during the surgery after Neo-CRT. Patients with rectal cancer receiving preoperative chemoradiotherapy can experience late failures.Citation10,Citation11
The predicted risk of local recurrence in rectal cancer is dependent on the depth of tumor invasion, lymphovascular involvement, tumor differentiation and lymph node involvement. These factors also influence the risk of developing metastatic disease. A study showed that 17% of pCR patients with identifiable nodal involvement were predicted to be at high risk of developing locally recurrent disease, and approximately 30–50% of those were expected to develop simultaneous disseminated disease.Citation12 It suggests that, for cCR patients, there could be tumor cells remaining after Neo-CRT. And referring to a recent review about 502 rectal cancer cases, the mean lymph node yield for proctectomy specimens without Neo-CRT was 13, with a median of 10 lymph nodes. In contrast, specimens after neoadjuvant therapy (NAT) had a mean lymph node yield of 9, with a median of 7 lymph nodes.Citation13 Comparing to this case, considering the patient achieved cCR following Neo-CRT, and all four mesenteric lymph nodes were clear; the key to his relapse could be the remaining of the cancer cells. So it is worth taking into consideration how to treat patients who have already achieved cCR after Neo-CRT. Or, on the basis of the existing Smith,Citation14 Habr,Citation15 and MaasCitation16 standards, we may need to develop a better assessment so that the assessment of cCR is more accurate. The major view thinks it impossible to find out whether a cCR patient has achieved pCR, so cCR patients have to take the total mesorectal excision (TME). But according to studies from University of Sao Paulo, recurrence and cancer-related mortality rates showed no statistical difference between stage p0 and stage c0 (P = 0.2).Citation17 According to some studies, long-term oncological outcomes after a cCR and deferred strategy are favorable.Citation18,Citation19 Moreover, combined up-front chemoradiotherapy was associated with tolerable and acceptable side effects. A significant number of patients had complete disappearance of their tumors (30.5%) within a median follow-up of 36 months.Citation20 Therefore, it was suggested that patient achieving cCR after Neo-CRT should be subjected to long-term follow-up monitoring of the CEA and CA199 levels. It seems that CEA was more sensitive than CA199 in this case (). Ulrik Wallin et al have demonstrated an association between low pretreatment CEA levels, interruption in chemoradiation therapy, and pathologic complete response in nonsmoking patients treated with Neo-CRT for locally advanced rectal cancer.Citation21 In addition to CEA, focal adhesion kinase (FAK) and Ku70/Ku80 were also studied as predictors of tumor response and risk factor for recurrence after Neo-CRT in rectal cancer.Citation22,Citation23 In this setting, the selection of a single or a combination of multiple biological markers for the accurate prediction of cCR can determine the sensitive population and allow precise treatment of different individuals. There are also scholars honoring minimally invasive surgeries rather than ordinary ones. There is already a trend to cure cCR patient with rectal cancer using minimally invasive laparoscopic surgeries. It is also confirmed by the study about surgery versus open resection for mid-low rectal cancer. There was no statistical difference in local recurrence rate (9.1% vs 6.4%; log-rank = 0.432; P = 0.511) and distant recurrence rate (19.7% vs 15.5%; log-rank = 0.505; P = 0.477) between the laparoscopic and open groups in 5 years.Citation24 That is why laparoscopic surgery is becoming the standard procedure of the mid-low rectal cancer. Recently, transanal total mesorectal excision (TaTME) has emerged as a focused area of laparoscopic surgery that is becoming a safe and feasible approach in the rectal surgery.Citation25 In this case, the patient was unwilling to take the surgery by the time achieved cCR after Neo-CRT, but he changed his mind when the relapse was observed and confirmed during the follow-up, and eventually recovered well after the surgery. We can infer from this case that patients achieved cCR after Neo-CRT should keep follow-up constantly. More frequent follow-up visits may contribute to an early diagnosis of cancer.Citation26 Operation will be performed when the relapse is confirmed. However, minimally invasive surgery should be recommended for a patient receiving abdominoperineal resection.
Ethics Statement
This study was approved by the Research Ethics Committee of Shanghai Jiaotong University affiliated Sixth People’s Hospital, and informed consent was obtained from the patient. All specimens were collected in the operating room immediately (≤15 min) after tissue removal and were snap-frozen in liquid nitrogen and stored at −80◦C. All methods were performed in accordance with the relevant guidelines and regulations. Participants gave their written informed consent for the materials to appear in publications without limit on the duration of publication.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This study was supported by grants from the medical-engineering cross fund of Shanghai Jiao Tong University (No. YG2017MS13), the pre-research fund of Shanghai Sixth People’s Hospital (LYZY-0229), the International Foundation of Translational Medicine for Abroad Scholars and Students, US and China (No. UCTMP2015-03C001), and the National Natural Science Foundation of China (No. 81272401).
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3 ):177–193. doi:10.3322/caac.2139528248415
- Pohl M, Schmiegel W. Colorectal cancer – personalized, stage-adjusted tumour therapy. Dtsch Med Wochenschr. 2013;138(36 ):1790–1795. doi:10.1055/s-0033-134334324002881
- Xiao J, Tan Y, Li W, et al. Tumor volume reduction rate is superior to RECIST for predicting the pathological response of rectal cancer treated with neoadjuvant chemoradiation: results from a prospective study. Oncol Lett. 2015;9(6 ):2680–2686. doi:10.3892/ol.2015.310126137128
- Dedemadi G, Wexner SD. Complete response after neoadjuvant therapy in rectal cancer: to operate or not to operate? Digestive Dis. 2012;30(Suppl 2 ):109–117. doi:10.1159/000342039
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1 ):7–30. doi:10.3322/caac.2138728055103
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2 ):69–90. doi:10.3322/caac.2010721296855
- Martin LK, Bekaii-Saab T. Optimizing neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl Compr Cancer Net. 2013;11(3 ):298–307. doi:10.6004/jnccn.2013.0041
- De Felice F, Izzo L, Musio D, et al. Clinical predictive factors of pathologic complete response in locally advanced rectal cancer. Oncotarget. 2016;7(22 ):33374–33380. doi:10.18632/oncotarget.v7i2226992214
- Glynne-Jones R, Wallace M, Livingstone JI, Meyrick-Thomas J. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Dis Colon Rectum. 2008;51(1 ):10–19; discussion 19–20. doi:10.1007/s10350-007-9080-8
- Valentini V, Minsky BD. Tumor regression grading in rectal cancer: is it time to move forward? J Clin Oncol. 2014;32(15 ):1534–1536. doi:10.1200/JCO.2014.55.476624752055
- Tan Y, Fu D, Li D, et al. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: a population-based analysis. Front Oncol. 2019;9:497. doi:10.3389/fonc.2019.0049731263674
- Hughes R, Glynne-Jones R, Grainger J, et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21(1 ):11–17. doi:10.1007/s00384-005-0749-y15864605
- Amajoyi R, Lee Y, Recio PJ, Kondylis PD. Neoadjuvant therapy for rectal cancer decreases the number of lymph nodes harvested in operative specimens. Am J Surg. 2013;205(3 ):289–292. doi:10.1016/j.amjsurg.2012.10.02023351510
- Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6 ):965–972. doi:10.1097/SLA.0b013e3182759f1c23154394
- Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12 ):1692–1698. doi:10.1007/DCR.0b013e3181f42b8921178866
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35 ):4633–4640. doi:10.1200/JCO.2011.37.717622067400
- Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9(1 ):90–99. doi:10.1016/j.gassur.2004.10.01015623449
- Gani C, Kirschniak A, Zips D. Watchful waiting after radiochemotherapy in rectal cancer: when is it feasible? Visc Med. 2019;35(2 ):119–123. doi:10.1159/00049916731192245
- Couch DG, Hemingway DM. Complete radiotherapy response in rectal cancer: a review of the evidence. World J Gastroenterol. 2016;22(2 ):467–470. doi:10.3748/wjg.v22.i2.46726811600
- Habr-Gama A, de Souza PM, Ribeiro U Jr., et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41(9 ):1087–1096. doi:10.1007/BF022394299749491
- Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA – a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum. 2013;56(7 ):859–868. doi:10.1097/DCR.0b013e31828e5a7223739192
- Gomez Del Pulgar T, Cebrian A, Fernandez-Acenero MJ, et al. Focal adhesion kinase: predictor of tumour response and risk factor for recurrence after neoadjuvant chemoradiation in rectal cancer. J Cell Mol Med. 2016;20(9 ):1729–1736. doi:10.1111/jcmm.1287927171907
- Pucci S, Polidoro C, Joubert A, et al. Ku70, Ku80, sClusterin: a “cluster” of predicting factors for neoadjuvant chemoradiotherapy treatment response in patients affected by locally advanced rectal cancer. Int J Radiat Oncol. 2016.
- Li S, Chi P, Lin H, Lu X, Huang Y. Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc. 2011;25(10 ):3175–3182. doi:10.1007/s00464-011-1683-421487864
- Chen CC, Lai YL, Jiang JK, et al. Transanal total mesorectal excision versus laparoscopic surgery for rectal cancer receiving neoadjuvant chemoradiation: a matched case-control study. Ann Surg Oncol. 2016;23(4 ):1169–1176. doi:10.1245/s10434-015-4997-y26597369
- Cai Y, Li Z, Gu X, Fang Y, Xiang J, Chen Z. Prognostic factors associated with locally recurrent rectal cancer following primary surgery (Review). Oncol Lett. 2014;7(1 ):10–16. doi:10.3892/ol.2013.164024348812