Abstract
Purpose
Sentinel lymph node biopsy (SLNB) is the standard management for clinically node-negative cutaneous melanoma patients. This study aimed to evaluate the role of SLNB in Taiwanese melanoma patients and in particular, patients with acral lentiginous melanoma (ALM).
Patients and methods
We retrospectively analyzed the clinicopathological characteristics and survival outcomes of the patients who underwent primary surgery followed by either SLNB or nodal observation at the Linkou Chang Gung Memorial Hospital from January 2000 to December 2011.
Results
Among the total of 209 patients, 127 underwent SLNB and 51 underwent nodal observation only after primary surgery. There were no significant differences in clinicopathological features between the two groups except that patients who underwent SLNB were older and had a higher rate of ALM than those under nodal observation. The median follow-up time was 43.5 months until July 2013. The patients who underwent SLNB had significantly better disease-free survival (DFS) (57.1 vs 18.7 months, p < 0.01) and melanoma-specific survival (MSS) (112.4 vs 45.2 months, p < 0.01) than those under observation. Improvement in DFS (HR: 0.51, p < 0.01) and MSS (HR: 0.60, p = 0.03) was observed even after adjusting for age and disease pathology by multivariate analysis. This benefit of clinical outcomes persisted in patients with ALM, Breslow thickness ≤2 mm, or no ulceration, but not in patients with non-ALM, Breslow thickness >2 mm, or ulceration.
Conclusion
SLNB was associated with favorable outcomes in patients with clinically node-negative cutaneous melanoma, particularly in Taiwanese patients with ALM, Breslow thickness ≤2 mm, and nonulcerated melanoma.
Introduction
Cutaneous melanoma is the malignancy arising from melanocytes of the skin. It accounts for less than 5% of skin cancers but is responsible for 80% of the deaths from skin cancers; therefore, it is the most serious and aggressive skin cancer and one of the leading causes of death in the USA.Citation1,Citation2 However, it occurs infrequently in Asians, in whom acral lentiginous melanoma (ALM) is the most common subtype of melanoma.Citation3–Citation8 In contrast to non-ALM, physical stress rather than sun exposure is the major risk factor and possible cause for ALM.Citation9,Citation10 Previous studies showed ALM has a deeper Breslow thickness than non-ALM, and Asian ALM patients have more Breslow thickness >4 mm than Caucasians and black individuals,Citation11 indicating that Asian ALM is a distinct subtype of cutaneous melanoma.Citation12 The difference in pathophysiology between ALM and non-ALM could be the reasons for distinct disease natures; therefore, the therapeutic options for Western melanoma might not be applied in Asian melanoma completely.
Morton et alCitation13 demonstrated sentinel lymph node biopsy (SLNB) in 1992, which can accurately identify patients with occult nodal metastasis which was not found clinically before surgery.Citation13,Citation14 Nowadays, SLNB has become an alternative option of elective lymph node dissection (ELND) and the standard of care in the treatment of clinically node-negative melanomaCitation15 based on one randomized phase III study, Multicenter Selective Lymphadenectomy Trial (MSLT)-1 trial, demonstrating the survival of SLNB-identified node-positive melanoma patients possibly could be improved by immediate lymph node dissection (LND).Citation16,Citation17 However, this study failed to demonstrate the survival benefit in the SLNB group compared to the nodal observation group. Some clinicians criticized the role of SLNB because of false-positive results, cost-effectiveness issue, no benefit of overall survival, and so on.Citation18–Citation20
The reason why the MSLT-1 trial demonstrated no difference for overall survival might be the lower nodal positive rate <20% in all patients leading to underestimating the overall efficacy of SLNB.Citation19 One of our retrospective studies reported 127 Taiwanese patients who underwent SLNB, whose positive rate was found to be 34.6%, which was higher than the MSLT-1 study.Citation7 As there are differences in the prevalence and pathophysiology of pathologic subtypes between Western and Asian countries, we aimed to retrospectively analyze the contribution of SLNB to the outcomes in patients with clinically node-negative cutaneous melanoma in Taiwanese patients with ALM predominant melanoma and higher positive rate of SLNB.
Materials And Methods
Patients
A total of 465 melanoma patients were treated at Chang Gung Memorial Hospital (CGMH) at Linkou branch in Taiwan from January 2000 to December 2011, and medical records of melanoma patients were retrospectively reviewed. We aimed to analyze the role of SLBN in cutaneous melanoma so the patients with operable cutaneous melanoma were enrolled in the current study; therefore, patients with mucosal melanoma, ocular melanoma, melanoma in situ, and metastatic/inoperable melanoma were excluded. A total of 209 patients with operable cutaneous melanoma underwent curative intent surgery including wide excision of the primary melanoma tumor with or without management of regional lymph nodes. The clinicopathological features and clinical survival outcomes of melanoma patients who underwent SLNB or had nodal observation were included for further analysis. Neither of these patients underwent adjuvant therapy with high-dose interferon-α, chemotherapy, targeted therapyCitation21 or immune checkpoint inhibitorsCitation22,Citation23 which showed their efficacy in adjuvant setting. Melanoma patients were staged according to the 7th American Joint Committee on Cancer (AJCC) staging system published in 2010.Citation24 Patients who were diagnosed before 2010 were restaged using the AJCC 7th edition. This study was approved by the institutional review board (IRB) of CGMH (101-0757B). The patients’ consent to review their medical records was not required by the IRB of CGMH as this is a retrospective study and the investigators have the responsibility to keep patient data confidentiality and compliance with the Declaration of Helsinki.
Sentinel Lymph Node Biopsy
All patients underwent wide excision of the primary melanoma with or without SLNB. The detailed protocol of SLNB performed in the current study was published in our previous reports.Citation7,Citation25 The patients who had a positive SLN underwent complete lymph node dissection (CLND). Lymph node sampling indicates random biopsy of regional lymph node without SLN mapping.
Statistical Considerations
Continuous data are presented as mean (range) and categorical data are presented as number (percentage). Both t-test and chi-square were used to examine the difference in the clinicopathological features between SLNB and nodal observation groups. Melanoma-specific survival (MSS) time was defined as the date from tumor excision to the death of melanoma, last follow-up, or data cutoff. Patients who died of causes other than melanoma were censored in the MSS analysis. Disease-free survival (DFS) time was defined as the date from tumor excision to the first evidence of recurrence, death, last follow-up, or data cutoff. Survival was analyzed using the Kaplan–Meier method with the log-rank p-value. The survival outcomes were adjusted for imbalanced factors using multivariate analysis. Subgroup analysis with univariate analysis was performed using the Cox proportional hazard model. Statistical analyses were performed using SPSS software (version 21.0, SPSS Inc, Chicago, IL). The p-value <0.05 was considered statistical significance in the current study.
Results
Patient Characteristics
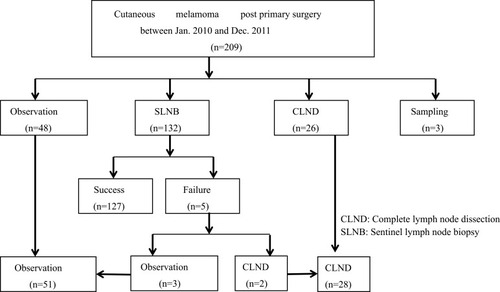
Among the 209 patients who underwent curative intent surgery, 127 patients underwent successful SLNB, 51 patients had nodal observation only (including 3 patients whose SLNB failed), 28 patients underwent CLND (including 2 patients whose SLNB failed), and 3 patients underwent lymph node sampling ().
Between the SLNB group and nodal observation group, there were no significant differences in clinicopathological features except that patients who underwent SLNB were older (mean age, 63.7 vs 58.1 years, p = 0.03) and had a higher rate of ALM (70.9% vs 47.1%, p = 0.02) than those under nodal observation. Most melanomas located over the lower extremity had Breslow thickness >2 cm and Clark level of IV or V ().
Table 1 Patients’ Characteristics In SLNB And Nodal Observation Group
DFS And MSS Between SLNB And Observation Groups
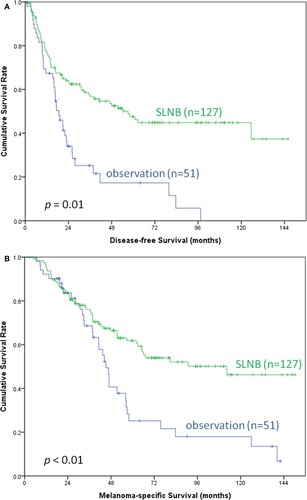
By July 2013, the median follow-up time was 43.5 months in all cohorts. The patients in the observation group had shorter follow-up time than in the SLNB group (median 37.6 vs 47.6 months, p =0.04), possibly resulting from shorter survivals for patients in the observation group. The patients who underwent SLNB had significantly better DFS (median, 57.1 vs 18.7 months, p < 0.01) and MSS (median, 112.4 vs 45.2 months, p < 0.01) than those under nodal observation (). Improved DFS (HR: 0.46, p < 0.01) and MSS (HR: 0.56, p = 0.01) were observed even after adjusting for age and disease pathology ().
Table 2 Multivariate Analysis Including Unbalanced Factors
Figure 2 Kaplan–Meier survival curves of DFS (A) and MSS (B) in melanoma patients with SLNB and nodal observation. The patients who underwent SLNB (n = 127) had significantly better DFS (median, 57.1 vs 18.7 months, p < 0.01) and MSS (median, 112.4 vs 45.2 months, p < 0.01) than those under nodal observation (n = 51).
Abbreviations: DFS, disease-free survival; MMS, melanoma-specific survival; SLNB, sentinel lymph node biopsy.
Subgroup Analysis In Patients With ALM Or Non-ALM
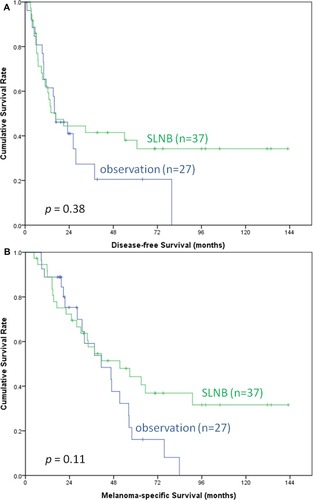
To understand the benefit of SLNB in patients with ALM or non-ALM as ALM is the most common subtype in Asian patients, subgroup (ALM or non-ALM) analysis was performed. Among the patients with ALM, those who underwent SLNB (n = 90) had a significantly better DFS (median, 62.6 vs 19.3 months, p < 0.01) and a favorable trend of MSS (median, not reached vs 45.2 months, p = 0.05) compared to those under nodal observation (n = 24) (). However, among the patients with non-ALM, DFS (median, 16.7 vs 16 months, p = 0.38) and MSS (median, 51.4 vs 41.3 months, p = 0.11) did not differ between the patients who underwent SLNB (n = 37) and those under nodal observation (n = 27) ().
Figure 3 Kaplan–Meier survival curves of DFS (A) and MSS (B) in ALM patients with SLNB and nodal observation. Those who underwent SLNB (n = 90) had a significantly better DFS (median, 62.6 vs 19.3 months, p < 0.01) and a favorable trend of MSS (median, not reached vs 45.2 months, p = 0.05) compared to those under nodal observation (n = 24).
Abbreviations: DFS, disease-free survival; MMS, melanoma-specific survival; ALM, acral lentiginous melanoma; SLNB, sentinel lymph node biopsy.
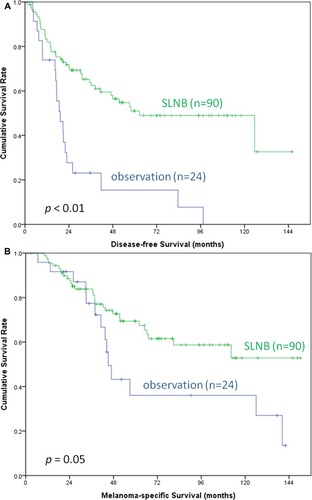
Figure 4 Kaplan–Meier survival curves of DFS (A) and MSS (B) in non-ALM patients with SLNB and nodal observation. Among the patients with non-ALM, DFS (median, 16.7 vs 16 months, p = 0.38) and MSS (median, 51.4 vs 41.3 months, p = 0.11) did not differ between the patients who underwent SLNB (n = 37) and those under nodal observation (n = 27).
Abbreviations: DFS, disease-free survival; MMS, melanoma-specific survival; ALM, acral lentiginous melanoma; SLNB, sentinel lymph node biopsy.
Subgroup Analyses Of DFS And MSS
To understand the benefit of SLNB in certain subgroups of all patients, additional subgroup analysis was performed. We further performed subgroup analyses to identify patients who had better DFS and MSS after SLNB. Patients in the SLNB group with ALM (HR = 0.32, 95% CI=0.19–0.56, p<0.01), Breslow thickness ≤2 mm (HR = 0.12, 95% CI = 0.04–0.41, p < 0.01), no ulceration (HR = 0.34, 95% CI = 0.16–0.72, p < 0.01) had a significantly better DFS than those in the observation group. Patients in the SLNB group without ulceration had a significantly better MSS than those in the observation group as well (HR = 0.31, 95% CI = 0.13–0.78, p = 0.01) ().
Table 3 Subgroup Analyses Of Disease-Free Survival And Melanoma-Specific Survival (SLNB Vs Nodal Observation)
Discussion
This retrospective study compared 127 patients receiving successful SLNB to 51 patients having only nodal observation after primary surgery in a single institute in Taiwan. In the current study, SLNB followed by immediate LND was associated with favorable DFS and MSS in clinically node-negative cutaneous melanoma patients. This benefit of clinical outcomes persisted in patients with ALM, Breslow thickness ≤2 mm, or no ulceration but not in patients with non-ALM, Breslow thickness >2 mm, or ulceration. To the best of our knowledge, this is the first report about the benefit of SLNB in Asia, where a higher SLNB positive rate was found than in Western countries.Citation7,Citation26–Citation29 Besides, this study is also the first report concerning the predictive role of ALM.
In an early prospective study comparing ELND with clinical observation of the lymph nodes in 740 patients with intermediate-thickness melanoma (1.0–4.0 mm), ELND only benefits patients with a Breslow thickness of 1.0–2.0 mm, nonulcerated melanoma, and limb melanoma.Citation30 Thereafter, SLNB becomes the alternative option of ELND due to the procedure being minimally invasive and having reliable staging and limited morbidity.Citation13 These results were consistent with our findings that SLNB benefitted patients with Breslow thickness ≤2 mm, nonulcerated melanoma, and ALM. In addition, although previous studies considered that thin melanoma (Breslow thickness ≤1.0 mm) had a very low risk of regional metastases and would not have benefitted from SLNB,Citation31 we still routinely performed SLNB in patients with thin melanoma because the positive rate of thin melanoma was 25% in our previous study.Citation7
Previous studies failed to demonstrate the survival impact of SLNB in overall patients.Citation16,Citation17,Citation30 The MSLT-1 trial indicated that the SLNB only benefits the survival in patients with nodal metastases because of the lower rates of node metastases and fewer events of recurrence and death than in Asian melanoma patients, who predominantly have ALM.Citation16,Citation17 In contrast, the current study demonstrated the survival benefit in patients undergoing SLNB comparing to nodal observation in Asian melanoma, particularly in ALM which has more aggressive disease nature than non-ALM. Therefore, we highly suggested SLNB should be performed in clinically node-negative Asian melanoma patients.
The data in the current study were collected before the era of targeted therapy and immune checkpoint inhibitors which have been approved to be the standard of care in advancedCitation32-34 or resectableCitation22,Citation35 melanoma; therefore, the survival outcomes after surgery in current study have not been confounded by such effective treatment nowadays. SLNB was associated with favorable survival outcomes in terms of DFS and MSS in Asian melanoma without the interference of targeted therapy and immune checkpoint inhibitors.
There were several limitations to the current study. There was a bias in this retrospectively single-institutional study. The numbers of patients in SLNB and nodal observation were imbalanced even though we tried to analyze the survival outcomes by adjusting imbalanced factors. In addition, some pathologic factors were missing because the main tumors were removed by excisional biopsy at local hospitals and the residual tumors from further wide excision at CGMH were insufficient for measurement of thickness or ulceration. Furthermore, the numbers in some subgroups were limited to perform subgroup analyses; therefore, our study could merely provide additional information in subgroup analyses. A larger study is still warranted to validate these findings.
Conclusion
SLNB was associated with favorable survival outcomes in patients with clinically node-negative cutaneous melanoma, particularly in Taiwanese patients with ALM, Breslow thickness ≤2 mm, and nonulcerated melanoma. Therefore, this retrospective study should motivate further study in similar patient populations, particularly in a prospective manner.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
The abstract of this paper was presented at the XXII International Pigment Cell Conference (IPCC) as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the Pigment Cell & Melanoma Research: Hyperlink with DOI: https://doi.org/10.1111/pcmr.12292.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1 ):11–30. doi:10.3322/caac.2116623335087
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355(1 ):51–65. doi:10.1056/NEJMra05216616822996
- Ishihara K, Saida T, Yamamoto A. Updated statistical data for malignant melanoma in Japan. Int J Clin Oncol. 2001;6(3 ):109–116. doi:10.1007/PL0001209111706778
- Lee MW, Koh JK, Kwon KS, et al. Clinical and histopathological study of cutaneous melanoma in Korea. Korean J Dermatol. 2003;41(1 ):43–47.
- Chang JWC, Yeh KY, Wang CH, et al. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma Res. 2004;14(6 ):537–541. doi:10.1097/00008390-200412000-0001615577327
- Luk N, Ho L, Choi C, Wong K, Yu K, Yeung W. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol. 2004;29(6 ):600–604. doi:10.1111/ced.2004.29.issue-615550131
- Wu CE, Hsieh CH, Chang CJ, et al. Prognostic factors for Taiwanese patients with cutaneous melanoma undergoing sentinel lymph node biopsy. J Formos Med Assoc. 2013;114:415–421.23969039
- Liu XK, Li J. Acral lentiginous melanoma. Lancet. 2018;391(10137 ):e21. doi:10.1016/S0140-6736(18)31071-7
- Jung HJ, Kweon -S-S, Lee J-B, Lee S-C, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in koreans: relevance of spreading pattern and physical stress. JAMA Dermatol. 2013;149(11 ):1281–1288. doi:10.1001/jamadermatol.2013.585324067997
- Sheen YS, Liao YH, Lin MH, et al. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep. 2017;7(1 ):5564.28717212
- Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145(4 ):427–434. doi:10.1001/archdermatol.2008.60919380664
- Chang JW, Guo J, Hung CY, et al. Sunrise in melanoma management: time to focus on melanoma burden in Asia. Asia Pac J Clin Oncol. 2017;13(6 ):423–427. doi:10.1111/ajco.1267028198155
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4 ):392–399. doi:10.1001/archsurg.1992.014200400340051558490
- Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220(6 ):759–767. doi:10.1097/00000658-199412000-000097986143
- Balch CM, Morton DL, Gershenwald JE, et al. Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60(5 ):872–875. doi:10.1016/j.jaad.2008.09.06719389531
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7 ):599–609. doi:10.1056/NEJMoa131046024521106
- Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13 ):1307–1317. doi:10.1056/NEJMoa06099217005948
- Thomas JM. Sentinel-node biopsy in melanoma. N Engl J Med. 2007;356(4 ):418;author reply 419–421.
- Gershenwald JE, Ross MI. Is sentinel-node biopsy superior to nodal observation in melanoma? Nat Clin Pract Oncol. 2007;4(5 ):278–279. doi:10.1038/ncponc079117389881
- Torjesen I. Sentinel node biopsy for melanoma: unnecessary treatment? BMJ. 2013;346:e8645. doi:10.1136/bmj.e864523299845
- Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19 ):1813–1823. doi:10.1056/NEJMoa170853928891408
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19 ):1824–1835. doi:10.1056/NEJMoa170903028891423
- Eggermont AMM, Robert C, Suciu S. Adjuvant pembrolizumab in resected stage III melanoma. N Engl J Med. 2018;379(6 ):593–595.
- Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol. 2018;25(8 ):2105–2110. doi:10.1245/s10434-018-6513-729850954
- Liu SH, Chang WC, Kao PF, et al. Lymphoscintigraphy and intraoperative gamma probe-directed sentinel lymph node mapping in patients with malignant melanoma. J Formos Med Assoc. 2004;103(1 ):41–46.15026857
- Uhara H, Takata M, Saida T. Sentinel lymph node biopsy in Japan. Int J Clin Oncol. 2009;14(6 ):490–496. doi:10.1007/s10147-009-0941-019967483
- Noro S, Yamazaki N, Nakanishi Y, Yamamoto A, Sasajima Y, Kawana S. Clinicopathological significance of sentinel node biopsy in Japanese patients with cutaneous malignant melanoma. J Dermatol. 2011;38(1 ):76–83. doi:10.1111/jde.2010.38.issue-121175760
- Ito T, Moroi Y, Oba J, et al. The prognostic value of a reverse transcriptase-PCR assay of sentinel lymph node biopsy for patients with cutaneous melanoma: a single-center analysis in Japan. Melanoma Res. 2012;22(1 ):38–44. doi:10.1097/CMR.0b013e32834dcfdf22108607
- Namikawa K, Yamazaki N, Nakai Y, et al. Prediction of additional lymph node positivity and clinical outcome of micrometastases in sentinel lymph nodes in cutaneous melanoma: a multi-institutional study of 450 patients in Japan. J Dermatol. 2012;39(2 ):130–137. doi:10.1111/jde.2012.39.issue-221950448
- Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7(2 ):87–97. doi:10.1007/s10434-000-0087-910761786
- Balch CM, Gershenwald JE. Clinical value of the sentinel-node biopsy in primary cutaneous melanoma. N Engl J Med. 2014;370(7 ):663–664. doi:10.1056/NEJMe131369024521113
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2017;377(14 ):1345–1356. doi:10.1056/NEJMoa170968428889792
- Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4 ):582–588. doi:10.1093/annonc/mdz01130715153
- Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–636. doi:10.1056/NEJMoa190405931166680
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19 ):1789–1801. doi:10.1056/NEJMoa180235729658430