Abstract
Purpose
LncRNA TP73-AS1 has been demonstrated to promote the developments of several types of human cancer. However, its role in colorectal cancer (CRC) is unknown.
Methods
All CRC patients (n=70, 40 males and 30 females, 38 to 66 years’ old, 52.1 ± 5.3 years’ old) in this study were enrolled in the Affiliated Hospital of Southwest Medical University from July 2012 to January 2014. Cells, vectors, and transient transfections, RT-qPCR, western-blotting, as well as measurements of cell migration and invasion abilities were carried out during the research.
Results
In the present study, we found that TP73-AS1 was upregulated in CRC tissues compared with adjacent non-CRC tissues in CRC patients. Upregulation of TP73-AS1 was closely correlated with poor prognosis. TGF-β1 was also upregulated in CRC tissues and positive correlated with TP73-AS1. TP73-AS1 overexpression caused upregulated TGF-β1 in CRC cells, while TGF-β1 overexpression showed no significant effect on TP73-AS1. TP73-AS1 and TGF-β1 overexpressions caused enhanced migration and invasion of CRC cells. TGF-β inhibitor treatment caused suppressed migration and invasion of CRC cells and attenuated effects of TP73-AS1 and TGF-β1 overexpression.
Conclusion
Therefore, TP73-AS1 may inactivate TGF-β1 to inhibit the migration and invasion of CRC cells.
Introduction
The early diagnosis of many types of human cancers is quite technically challengeable. As a result, many cancer patients are diagnosed at inoperable conditions and their survival rate is quite poor.Citation1 Therefore, accurate prediction of the prognosis of cancer patients at advanced stages is necessary to assist the development of individualized treatment strategies.Citation2 Colorectal cancer (CRC) is considered as a major cause of deaths in cancer patients, with aggressive nature and unacceptably high incidence rate of malignancy.Citation3 Treatment of CRC patients mainly relies on chemotherapeutic and radiation therapies, which may induce severe side effects.Citation4,Citation5 In addition, many CRC patients experience recurrence even after curative resection.Citation6 Therefore, novel biomarkers are urgently needed to guide the treatment of CRC.
Recently, researchers have characterized a considerable number of genetic alterations on the pathogenesis of CRC, which involved in the occurrence and development of CRC.Citation7 Besides, it was also well established that non-coding RNAs, such as long non-coding RNAs (lncRNAs, >200nt) have also been proved to be critical factors in cancer biology.Citation8 LncRNAs regulate cancer development mainly by regulating cancer-related genes.Citation9 Therefore, the regulation of lncRNA expression may have potential therapeutic value for cancer treatment.Citation10 Although LncRNA TP73-AS1 has been proved to promote several types of cancer including CRC,Citation11–Citation14 two recent studies reported opposite expression patterns and functionality of TP73-AS1 in CRC. Our study was therefore performed to further explore the role of TP73-AS1 in CRC.
Materials and Methods
Research Subjects and Specimens
All CRC patients (n=70, 40 males and 30 females, 38 to 66 years’ old, 52.1 ± 5.3 years’ old) included in this study were enrolled in the Affiliated Hospital of Southwest Medical University from July 2012 to January 2014. All patients were initially diagnosed as CRC by X-ray imaging and confirmed by histopathological examinations. Patients with other clinical disorders, with a history of malignancies, or received any therapies before admission were excluded from this study. Based on the staging criteria proposed by AJCC, 14, 17, 22 and 17 patients were classified into stages I–IV, respectively. The Affiliated Hospital of Southwest Medical University Ethics Committee approved this study before admission of patients. All patients signed informed consent.
During the diagnosis, a biopsy was performed on all patients to obtain cancer (CRC) tissues as well as non-cancer tissues. All tissue specimens were confirmed by 3 experienced pathologists. Specimens were stored in liquid nitrogen at the specimen library of the Affiliated Hospital of Southwest Medical University before use.
A 5-Year’s Follow-Up
All the 70 CRC patients were followed up for 5 years. Follow-up was performed through telephone or by an outpatient visit in some cases to monitor their survival. Patients who were lost during follow-up were not included. Patients died of other clinical disorders or accidences were also excluded.
Cells, Vectors and Transient Transfections
Human CRC cell lines CR4 (Sigma-Aldrich, USA) and RKO (ATCC, USA), as well as normal colon cell line CCD-18Co, were used in this study. Eagle’s Minimum Essential Medium (10% FBS) was used as cell culture medium. Cell culture conditions were 5% CO2 and 37°C.
TP73-AS1 and TGF-β1 expression vectors were constructed by inserting full-length TP73-AS1 or TGF-β1 cDNAs into pcDNA3.1 vectors (Sangon, Shanghai, China). CR4 and RKO cells were cultivated overnight to confluence of 70–80%, followed by transient transfections performed using lipofectamine™ 2000 (Thermo Fisher Scientific) with vector at dose of 10 nM. Subsequent experiments were performed at 24 hrs after transfections. Cells without transfections (control) and cells transfected with empty vectors (negative control) were included to serve as two controls.
Total RNA Extraction and RT-qPCR
Total RNA extractions from CR4, RKO and CCD-18Co cells, as well as tissues, were performed using Ribozol reagent (Sigma-Aldrich, USA). Following cDNA synthesis using AMV Reverse Transcriptase (Promega Corporation, USA), qPCR reaction systems were prepared using SYBR® Green master mix (Bio-Rad, USA) with 18S rRNA or GAPDH as endogenous controls to detect the expression of TP73-AS1 and TGF-β1, respectively. PCR reactions were repeated 3 times. All data were analyzed using 2−ΔΔCT method.
Western Blotting
Total protein extractions from CR4 and RKO cells (collected at 24 hrs after transfection) were performed using ReadyPrep™ Protein Extraction Kit (Bio-Rad). After denaturing, protein samples were subjected to gel electrophoresis using 10% SDS-PAGE. Following gel transfer to PVDF membranes, blocking was performed in 5% non-fat milk for 2 hrs at room temperature. After that, membranes were first incubated with rabbit polyclonal TGF-β1 (ab92486, 1:1200; Abcam) and GAPDH (ab9485, 1:1200, Abcam) primary antibodies, followed by incubation with an anti-rabbit IgG-HRP secondary antibody (1:1200, MBS435036, MyBioSource). Signals were developed using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific). Image J v.1.46 software was used to process all data.
Measurement of Cell Migration and Invasion Abilities
CR4 and RKO cells (collected at 24 hrs after transfection) were used to prepare single-cell suspensions using Eagle’s Minimum Essential Medium containing 1% FBS. In cases of TGF-β inhibitor treatment, SD 208, a potent ATP-competitive TGF-β receptor inhibitor (RI) inhibitor (10 ng/mL, R&D Systems, Inc., Minneapolis, MN, USA) was used to treat cells at 37°C for 24 hrs before use. Cell suspensions were transferred to upper Transwell chamber with 0.1 mL per well, and Eagle’s Minimum Essential Medium (20% FBS) was added into the lower Transwell chamber. It is worth noting that Matrigel (356234, Millipore, USA) was used to coat the upper chamber membrane before invasion assay to mimic in vivo cancer cell invasion. After cell culture for 2 hrs, 0.5% crystal violet (Sigma-Aldrich, USA) was used to stain the upper chamber membrane and stained cells were observed under an optical microscope.
Statistical Analysis
Experiments were repeated 3 times to obtain solid data. Differences between cancer and non-cancer tissues were analyzed by performed paired t-test. Differences among different cell transfection groups or among different clinical stages were analyzed by performed one-way ANOVA and Tukey’s test. Linear regression was used to analyze the correlation between TP73-AS1 and TGF-β1. For survival analysis, the 70 patients were first grouped into low (n=37) and high (n=33) TP73-AS1 groups (Youden’s index) using TP73-AS1 expression data in CRC tissues, followed by performing K-M method and log-rank test to plot and compare survival curves. Differences were statistically significant when p < 0.05.
Results
TP73-AS1 Was Upregulated in CRC
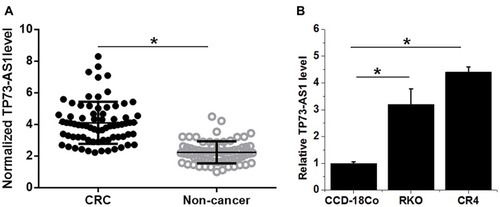
TP73-AS1 expression was detected by performing RT-qPCR. TP73-AS1 expression in CRC and non-cancer tissues were compared by performing a paired t-test. It was found that expression levels of TP73-AS1 were significantly higher in CRC tissues comparing to non-cancer tissues (, p<0.05). In addition, expression levels of TP73-AS1 were also higher in cells CRC cell line CR4 and RKO than in cells of normal colon cell line CCD-18Co (, p<0.05).
Figure 1 TP73-AS1 was upregulated in CRC tissues. Analysis of TP73-AS1 expression by paired t-test revealed that expression levels of TP73-AS1 were significantly higher in CRC tissues comparing to non-cancer tissues (A). In addition, ANOVA (one-way) and Tukey’s test analysis showed that expression levels of TP73-AS1 were also higher in CRC cell line CR4 and RKO than in cells of normal colon cell line CCD-18Co (B) (*p<0.05).
TP73-AS1 Is Correlated with the Survival of CRC Patients
Before survival analysis, TP73-AS1 expression in CRC tissues was first compared by performing ANOVA (one-way) and Tukey’s test. It was observed that TP73-AS1 expression levels were not significantly different among patients with different clinical stages (). Seventy patients were first grouped into low (n=37) and high (n=33) TP73-AS1 groups (Youden’s index) using TP73-AS1 expression data in CRC tissues, followed by performing K-M method and log-rank test to plot and compare survival curves. The results showed that patients with high levels of TP73-AS1 had significantly worse survival conditions ().
Figure 2 TP73-AS1 is correlated with the survival of CRC patients. Analysis of TP73-AS1 expression by performing ANOVA (one-way) and Tukey’s test showed that TP73-AS1 expression levels were not significantly different among patients with different clinical stages (A). Survival curve analysis showed that patients with high levels of TP73-AS1 had significantly worse survival conditions (B).
TP73-AS1 Promoted TGF-β1 Expression
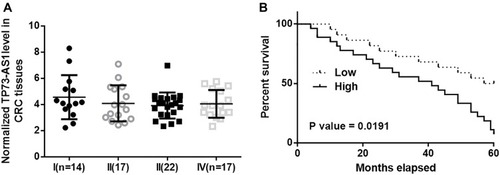
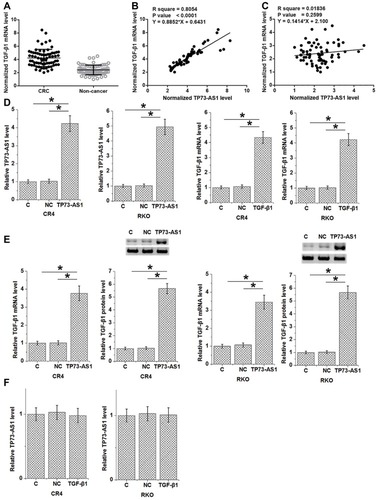
TGF-β1 mRNA was also detected by performing RT-qPCR. TGF-β1 mRNA expression in CRC and non-cancer tissues were compared by performing a paired t-test. It was found that expression levels of TGF-β1 mRNA were significantly higher in CRC tissues comparing to non-cancer tissues (, p<0.05). Linear regression was used to analyze the correlation between TP73-AS1 and TGF-β1. It was found that TP73-AS1 and TGF-β1 mRNA were significantly and positively correlated in CRC tissues (), but not in non-cancer tissues (). To further investigate the relationship between TP73-AS1 and TGF-β1, TP73-AS1 and TGF-β1 expression vectors were transfected into CR4 and RKO cells. Expression levels of TP73-AS1 and TGF-β1 mRNA were significantly increased at 24 hrs after transfections comparing to C and NC two controls (, p<0.05). In addition, TP73-AS1 overexpression caused upregulated TGF-β1 mRNA and protein in CRC cells (, p<0.05), while TGF-β1 overexpression showed no significant effect on TP73-AS1 ().
Figure 3 TP73-AS1 promoted TGF-β1 expression. Paired t-test analysis showed that expression levels of TGF-β1 mRNA were significantly higher in CRC tissues comparing to non-cancer tissues (A). Linear regression showed that TP73-AS1 and TGF-β1 mRNA were significantly and positively correlated in CRC tissues (B), but not in non-cancer tissues (C). Expression levels of TP73-AS1 and TGF-β1 mRNA were significantly increased at 24 hrs after transfections comparing to C and NC two controls (D). In addition, TP73-AS1 overexpression caused upregulated TGF-β1 mRNA and protein in CRC cells (E), while TGF-β1 overexpression showed no significant effect on TP73-AS1 (F) (*p<0.05).
Upregulating of TGF-β1 by TP73-AS1 Regulated CRC Cell Migration and Invasion
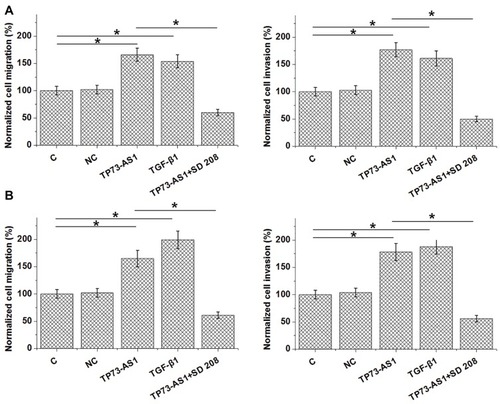
Analysis of Transwell migration and invasion data by one-way ANOVA and Tukey’s test showed that TP73-AS1 and TGF-β1 overexpression caused promoted migration and invasion of both CR4 (, p<0.05) and RKO (, p<0.05) cells. TGF-β inhibitor treatment caused inhibited migration and invasion of CRC cells and attenuated effects of TP73-AS1 and TGF-β1 overexpression.
Figure 4 Upregulating of TGF-β1 by TP73-AS1 regulated CRC cell migration and invasion. Transwell migration and invasion data showed that TP73-AS1 and TGF-β1 overexpression caused promoted migration and invasion of both CR4 (A) and RKO (B) cells. TGF-β inhibitor treatment caused inhibited migration and invasion of CRC cells and attenuated effects of TP73-AS1 and TGF-β1 overexpression (*p<0.05).
Discussion
The present study mainly investigated the role of TP73-AS1 in CRC. We found that TP73-AS1 has prognostic value for CRC and overexpression of TP73-AS1 may promote CRC cell migration and invasion through the upregulation of TGF-β1.
Previous studies showed that TP73-AS1 was a key player in many types of cancer, such as osteosarcomaCitation11 and bladder,Citation12 indicating that TP73-AS1 may also participate in CRC. Interesting, in one study Jia et al showed that TP73-AS1 is downregulated in CRC and inhibited cell proliferation by sponging miR-103 to regulate PTEN.Citation13 In contrast, in another study, Cai et al reported that TP73-AS1 was overexpressed in CRC and promoted cancer cell proliferation, migration and invasion by upregulating TGFαCitation14 In this study we found TP73-AS1 was upregulated in CRC tissues but was not affected by clinical stages. Therefore, the TP73-AS1 was not further upregulated with the development of CRC. It is possible that TP73-AS1 was upregulated at the beginning of the formation of CRC tumors and participates in the whole procedure of cancer development. Interestingly, our follow-up study showed that high TP73-AS1 level was closely correlated with the poor survival of CRC patients. Therefore, detecting the expression of TP73-AS1 in cancer tissues may predict the survival of CRC patients.
TGF-β signaling is a critical player in cancer development.Citation15 Although the role of TGF-β in regulating the proliferation of cancer cells is contradictory in different types of cancer, it is generally believed that the activation of TGF-β has enhancing effects on cancer cell migration and invasion.Citation16 It has been reported TP73-AS1 interacts with epithelial-to-mesenchymal transition (EMT) pathway to promote cancer development,Citation12 while TGF-β signaling is a key player in EMT.Citation17,Citation18 In the present study, we showed that TP73-AS1 was likely an upstream activator of TGF-β signaling, and this interaction participates in the regulation of cancer cell migration and invasion. However, the mechanism of the interaction between these 2 factors is unknown. It has been reported that TGF-β in cancer can be regulated by miR-142,Citation19 which can also interact with TP73-AS1.Citation20 Therefore, miR-142 may be a mediator between TP73-AS1 and TGF-β. We will test this possibility and explore other mediators in our future studies.
Conclusion
In conclusion, TP73-AS1 was upregulated in CRC and overexpression of TP73-AS1 resulted in TGF-β upregulation, which in turn led to the promoted migration and invasion of CRC cells.
Disclosure
The author reports no conflicts of interest in this work.
References
- Jemal A, Parkin DM, Bray F. Patterns of cancer incidence, mortality, and survival. Cancer Epidemiol Prev. 2017;8.
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4 ):271–289. doi:10.3322/caac.v66.427253694
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3 ):177–193. doi:10.3322/caac.2139528248415
- Marijnen CA, Kapiteijn E, van de Velde CJ, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20(3 ):817–825. doi:10.1200/JCO.20.3.81711821466
- Glynne-Jones R, Debus J. Improving chemoradiotherapy in rectal cancer. Oncologist. 2001;6 Suppl 4:29–34. doi:10.1634/theoncologist.6-suppl_4-29
- Bockelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54(1 ):5–16. doi:10.3109/0284186X.2014.97583925430983
- Carethers JM, Jung BH. Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology. 2015;149(5 ):1177–1190 e1173. doi:10.1053/j.gastro.2015.06.04726216840
- Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4 ):452–463. doi:10.1016/j.ccell.2016.03.01027070700
- Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126(8 ):2775–2782. doi:10.1172/JCI8442127479746
- Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2 ):155–165. doi:10.1038/modpathol.2012.16022996375
- Chen X, Zhou Y, Liu S, et al. LncRNA TP73-AS1 predicts poor prognosis and functions as oncogenic lncRNA in osteosarcoma. J Cell Biochem. 2018.
- Tuo Z, Zhang J, Xue W. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem Biophys Res Commun. 2018;499(4 ):875–881. doi:10.1016/j.bbrc.2018.04.01029625110
- Jia Z, Peng J, Yang Z, et al. Long non-coding RNA TP73‑AS1 promotes colorectal cancer proliferation by acting as a ceRNA for miR‑103 to regulate PTEN expression. Gene. 2019;685:222–229. doi:10.1016/j.gene.2018.11.07230472379
- Cai Y, Yan P, Zhang G, Yang W, Wang H, Cheng X. Long non-coding RNA TP73-AS1 sponges miR-194 to promote colorectal cancer cell proliferation, migration and invasion via up-regulating TGFalpha. Cancer Biomark. 2018;23(1 ):145–156. doi:10.3233/CBM-18150330010111
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2 ):117–129.11586292
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer – a double-edged sword. Trends Cell Biol. 2001;11(11 ):S44–S51. doi:10.1016/s0962-8924(01)02130-411684442
- Song J. EMT or apoptosis: a decision for TGF-beta. Cell Res. 2007;17(4 ):289–290. doi:10.1038/cr.2007.2517426696
- Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1 ):76–84. doi:10.1097/CCO.0b013e32835b637123197193
- Lei Z, Xu G, Wang L, et al. MiR-142-3p represses TGF-beta-induced growth inhibition through repression of TGFbetaR1 in non-small cell lung cancer. FASEB J. 2014;28(6 ):2696–2704. doi:10.1096/fj.13-24728824558198
- Yang G, Song R, Wang L, Wu X. Knockdown of long non-coding RNA TP73-AS1 inhibits osteosarcoma cell proliferation and invasion through sponging miR-142. Biomed Pharmacother. 2018;103:1238–1245. doi:10.1016/j.biopha.2018.04.14629864904
