Abstract
Purpose
PRDX (Peroxiredoxin) family has involved in breast cancer tumorigenesis from the evidence obtained from cell lines, human tissues and mouse models. Nonetheless, the diversified expression patterns, coupled with the prognostic values of PRDX family, still require explanation. This study aimed at investigating the clinical importance and biological of PRDXs in breast cancer.
Patients and methods
Specimens of paraffin sections used for immunohistochemistry were collected from the hospital and the remaining patient information was retrieved from online databases. The expression and survival data of PRDXs in patients with breast cancer were from ONCOMINE, GEPIA, Kaplan–Meier Plotter. cBioPortal, Metascape, String, Cytoscape and DAVID were used to predict functions and pathways of the changes in PRDXs and their frequently altered neighbor genes. Immunohistochemistry was used to detect the expression of PRDXs in breast cancer.
Results
We discovered the expression levels of PRDX1-5 were higher in breast cancer tissues than in normal tissues, whereas the expression level of PRDX6 was observed as lower in the former one in comparison with that of the latter one. There existed a correlation between the expression levels of PRDX4, 5 and the advanced tumor stage. Survival analysis revealed that the expression of PRDXs were all associated with relapse-free survival (RFS) in all of the patients with breast cancer. Eventually, we discovered significant regulation of the cellular oxidant detoxification and detoxification of ROS by the PRDX changes, together with obtaining the core modules of genes (TXN, TXN2, TXNRD1, TXNRD2, GPX1 and GPX2) linked to the PRDX family of genes in breast cancer.
Conclusion
The PRDX family is widely involved in the development of breast cancer and affects the prognosis of patients. The functions and pathways of the changes in PRDXs and their frequently altered neighbor genes can be further verified by wet experiments.
Introduction
Peroxiredoxin (PRDX) is an antioxidant enzyme, commonly expressed in living organisms. Its physiological action primarily involves scavenging the free radicals and avoiding the impairment of cellular free radicals. As currently known, there are six PRDX subtypes (PRDX1-6) in mammals, which are usually segregated into 1-Cys as well as 2-Cys that depend on the number of cysteine remains, of which PRDX1-5 is associated with the 2-Cys subtype, whereas PRDX6 is the 1-Cys subtype.Citation1,Citation2 As indicated by the sequence homology analysis, the amino acid sequence of PRDX1-4 shows an elevated level of similarity, while PRDX5 possesses a low similarity with the other protein amino acid sequences belonging to the same family, the similarity between them is approximately 10%. On the other hand, the similarity of PRDX6 with the other protein sequences belonging to the same family is extremely high at 40%. There are different distributions of each subtype in cells. PRDX1, PRDX2 and PRDX6 are highly expressed in cytoplasm, and PRDX3 is mostly expressed in mitochondria. PRDX5 does not have any specificity in the subcellular localization expression, besides being expressed in a number of organelles.
The PRDX family of proteins scavenges not just oxygen species, but also reactive nitrogen and sulfur-free radicals, together with safeguarding the cells from the impairment by the reactive oxygen species by means of this phenomenon. PRDX family proteins have a close association with the level of malignancy, recurrence, prognosis, or considered good therapeutic targets of a variety of tumors, for instance, colorectal cancer,Citation3–Citation5 liver cancer,Citation6–Citation8 ovarian cancer,Citation9,Citation10 lung cancer,Citation11–Citation15 glioblastoma,Citation16,Citation17 prostate cancer,Citation18,Citation19 esophageal squamous cell carcinoma,Citation20 bladder cancer,Citation21 pancreatic cancerCitation22 and so on.
Breast cancer refers to a common malignant tumor and a leading cause of cancer-related deaths in females across the globe. At present, the treatment methods of breast cancer include surgical treatment, in addition to chemotherapy, endocrine therapy, targeted therapy and combination therapy. The death from breast cancer in both the regions of North America and the European Union (EU) has recently declined, which can be majorly ascribed to the timely detection, coupled with the productive systemic treatments. Nonetheless, breast cancer continues to constitute the most frequent reason leading to death from cancer in the less developed nations, together with being the second to lung cancer in more advanced nations.Citation23 As known to all of us, early detection and early treatment are the key to cancer treatment. That is why it is deemed as extremely pivotal to detect breast cancer through the detection of early indicators.
Till today, the role of the PRDX family in developing and treating breast cancer has been extensively mentioned. Fiskus WCitation24 is of the view that breast cancer cells are more sensitive to the ROS-induced DNA damage and cytotoxicity following the limitation of the antioxidant activity of PRDX1. In accordance with Desmetz C,Citation25 PRDX2 manifested substantially augmented reactivity in the primary breast cancer as well as CIS (carcinoma in situ) in comparison with healthy controls. Luthra SCitation26 suggested that PRDX2 gene expression has the potential to constitute a predictor for the early or less aggressive Lump breast cancers; in addition, higher expressed oxidative stress as antioxidant genes GSTK1, together with PRDX2 and PRDX3, is associated with the ER-positivity breast cancer as well as the prediction of longer survival. Also, the elevated expression of PRDX4 in the primary breast tumor had a consistent relationship with metastasis at 5 years.Citation27 Chang XZ et alCitation28 are of the belief that upregulating the peroxiredoxin 6 improved not just the proliferation but also the invasion of breast cancer cells in vitro.
To our optimal understanding, bioinformatics analysis has yet been put to the application for the investigation of the function of PRDXs in breast cancer. Hence, based on the analyses of thousands of gene expression or changes in the copy numbers that are published on the internet, we carried out the analysis of the expression of PRDXs in those patients, who had breast cancer, comprehensively for the purpose of determining not just the expression patterns but also the underlying roles, and distinguished prognostic values.
Materials And Methods
Ethics Statement
The current research work received approval from the Academic Committee of The Fourth Affiliated Hospital of Anhui Medical University and was carried out in accordance with the rules put forward in the Declaration of Helsinki. The retrieval of each and every dataset was carried out from the publishing literature, accordingly confirming that all of the written informed consents were attained.
ONCOMINE Analysis
ONCOMINE gene expression array datasets,Citation29 which is an internet-based cancer microarray database, was put to use for the analysis of the transcription levels of PRDXs in varying cancers. The comparison of the mRNA expressions of PRDXs in the medical cancer samples was carried out with that in the normal controls, with the help of the Students’ t-test, aimed at generating a p value. Both the cut-off of p value and fold change were stated as 0.01 and 2, correspondingly.
GEPIA (Gene Expression Profiling Interactive Analysis) Dataset
GEPIA refers to a recently created interactive webserver to analyze the RNA sequencing expression data of 9736 tumors as well as 8587 normal specimens from the TCGA and the GTEx projects, with the use of a standard processing pipeline. GEPIA offers customizable roles, for instance, tumor/normal differential expression analysis, profiling in accordance with the types of cancer or pathological stage, the patient survival analysis, similar gene detection, correlation analysis and dimensionality reduction analysis.Citation30
The Kaplan–Meier Plotter
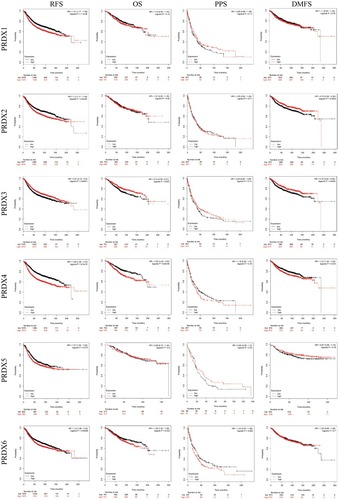
The evaluation of the prognostic value of PRDXs mRNA expression was carried out with the use of an online database, Kaplan–Meier Plotter,Citation31 containing the gene expression data, in addition to the survival information associated with 3955 medical breast cancer patients.Citation32 For the purpose of analyzing the relapse-free survival (RFS), the overall survival (OS), distant metastasis-free survival (DMFS) and post-progression survival (PPS) of the patients having breast cancer, the patient specimens were segregated into two cohorts in accordance with the median expression (high vs low expression), followed by being evaluated with the help of a Kaplan–Meier survival plot, having the hazard ratio (HR) with 95% confidence intervals (CI), coupled with the log-rank p value. The JetSet best probe set of PRDXs were just selected for the achievement of the Kaplan–Meier plots, wherein the Number-at-risk is highlighted below the key plot.
TCGA Data And cBioPortal
The Cancer Genome Atlas possesses not just the sequencing but also the pathological data dealing with 30 different cancers.Citation33 The breast cancer (TCGA, Provisional) dataset that includes the data from 1108 cases with pathology reports was chosen for additionally analyzing PRDXs with the use of cBioPortal.Citation34 The genomic profiles counted on mutations, in addition to putative copy-number alterations (CNA) from GISTIC, mRNA expression z-scores (RNA Seq V2 RSEM) and protein expression Z-scores (RPPA). The calculation of the co-expression and network was carried out in accordance with the cBioPortal’s online instruction.
Metascape, String, Cytoscape And DAVID
PRDX1-6, together with the other 22 commonly changed neighbor genes from cBioPortal’s network online instruction, was input into MetascapeCitation35,Citation36 and StringCitation37 for the purpose of gene annotation and analysis. Aimed at constructing the core PPI network (protein interaction network), we input the differential genes into the String database for the analysis, together with visualizing the results with Cytoscape.Citation38,Citation39 Each node in the network is a representation of a single gene, and the lines existing between the points indicate the functional connections that exist between the two molecules in the network, and the thickness of lines is differentiated in accordance with the combined score. MCODE is a plugin in cytoscape, which performs the analysis of the interrelationships between different gene-encoded proteins and key modules. The plugin sets the following parameters with regard to the tightly connected module regions in the PPI network: MCODE score > 5, degree cut-off = 2, node score-off = 0.2, Max depth = 100, and k-score = 2. In addition, the functions of PRDXs and the genes significantly associated with PRDX alterations were predicted in accordance with the Kyoto Encyclopedia of Genes and Genomes (KEGG) in the Database for Annotation, Visualization, and Integrated Discovery (DAVID).Citation40,Citation41
Immunohistochemistry
Immunohistochemistry for PRDXs were carried out on all of the tissue specimens. The tissue specimens were cut into 4 µm parts on the silanised glass slides. Protein expression was figured out using 2-step immunohistochemistry. Briefly, both the dewaxing and hydrated parts were treated using 0.3% hydrogen peroxide in methanol for a period of 15 mins at the RT, aimed at blocking the endogenous peroxidase activity, followed by washing in PBS (thrice for 3 mins each), whereby antigen retrieval was carried out in citrate buffer (cat. no. P0081; Beyotime Institute of Biotechnology, Haimen, China; pH 6.0) for a period of 10 mins at a temperature of 100°C. Subsequent to three more PBS washes (3 min each), staining of the parts was done using PRDX antibodies () for 2 hrs at a temperature of 37°C, followed by washing again using PBS (thrice for 3 mins each). Thereafter, the incubation of the parts was carried out using horseradish peroxidase (HRP) IgG antibody polymer () for 1 hr at a temperature of 37°C, which proceeded to three PBS washes (3 mins each). All of the parts underwent the treatment using 50–100 μl diaminobenzadine working solution (DAB Horseradish Peroxidase Color Development kit; cat. no. ZLI 9019; Zsbio, Beijing, China) at the RT for a period between 3 and 5 mins, which proceeded to a wash in PBS. Each and every part underwent counterstaining using haematoxylin for 1 min at the RT to make the morphology of the tissue possible to be observed with the use of a light microscope.
Table 1 Antibody Name, Company, Catalog And Concentration For Immunohistochemistry
Results
Transcriptional Levels Of PRDXs In Patients With Breast Cancer
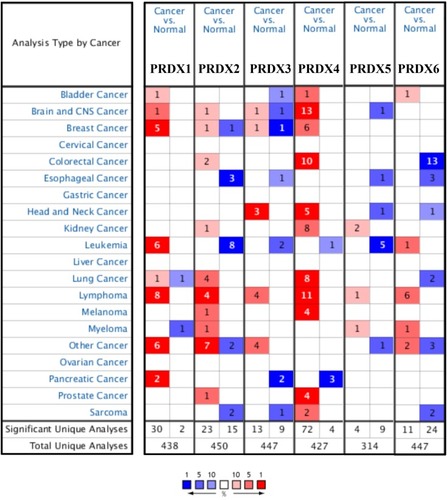
We carried out the comparison of the transcriptional levels of PRDXs in cancers with those in the normal specimens through the use of ONCOMINE databases (). The significant upregulation of the mRNA expression levels of PRDX1 and PRDX4 was carried out in the patients having breast cancer in five and six datasets.
In Zhao’s dataset,Citation42 PRDX1 is overexpressed in lobular breast carcinoma as well as invasive ductal breast carcinoma with a fold change amounting to 3.224 and 3.531. PRDX4 is also overexpressed in comparison with that in the normal specimens, which have a fold change amounting to 2.035 and 2.468. Together with that, PRDX2 is also overexpressed in invasive ductal breast carcinoma having a fold change amounting to 2.322 in the same dataset. PRDX1 overexpression is found in ductal breast carcinoma in situ epithelia having a fold change amounting to 2.546 in Ma’s dataset.Citation43 CurtisCitation44 showed another mRNA expression factor with augmented expression, suggesting that PRDX1 has a fold change amounting to 2.373 in those patients, who have medullary breast carcinoma, a fold change amounting to 2.001 in those patients, who have invasive ductal breast carcinoma as compared with that in the patients having normal breast tissues. In addition, PRDX4 has a fold change amounting to 2.008 in those patients, who have medullary breast carcinoma in this dataset. In addition, PRDX3 is highly expressed in invasive breast carcinoma stroma having a fold change amounting to 2.298 in Finak’s dataset.Citation45 There are significantly different transcriptional levels of PRDX4 in ductal breast carcinoma (fold change = 2.103 and 2.189) from the ones in the normal specimens in Sorlie’s datasetCitation46 and Richardson’s datasetCitation47 ().
Table 2 The Significant Changes Of PRDXs Expression In Transcription Level Between Different Types Of Breast Cancer And Normal Tissues (ONCOMINE Database)
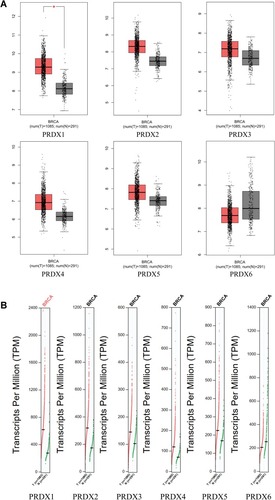
With the use of GEPIA (Gene Expression Profiling Interactive Analysis) dataset,Citation48 we carried out the comparison of the mRNA expression of PRDX elements between breast cancer and normal tissues. As suggested by the findings, the expression levels of PRDX1-5 were observed as higher in breast cancer tissues as compared with that in the normal ones; on the other hand, the expression level of PRDX6 was observed as lower in the former one as compared with that in the latter one ( and ).
Association Existing Between The mRNA And Protein Levels Of PRDXs And The Clinicopathological Parameters Of The Patients Having Breast Cancer
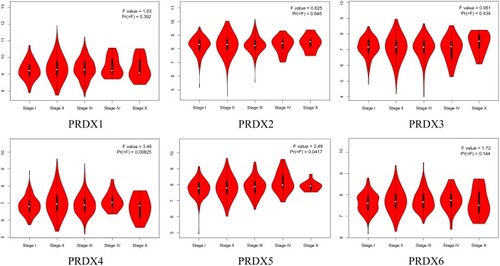
We carried out the analysis of the expression of PRDXs with the tumor stage for breast cancer. Moreover, the expression of PRDX4 and PRDX5 were substantially statistically significant with the clinical-pathological stage of breast cancer, having a p-value amounting to 0.00825 and 0.0417. Nonetheless, other cohorts did not have a significant difference ().
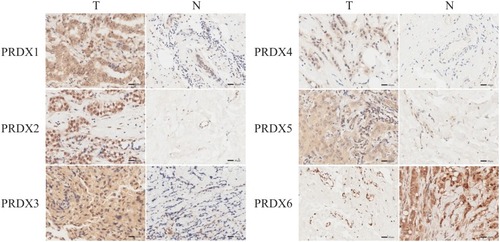
We carried out IHC for the purpose of testing the PRDX proteins expression in breast cancer tissues as well as their counterparts, together with examining the expression of PRDXs. We found that PRDX1-5 proteins were more highly expressed in the cancer tissues than in the normal, whereas the expression level of PRDX6 was observed as lower in the former one in comparison with that the latter one. ().
Relationship Of The Augmented mRNA Expression Of PRDXs With The Enhanced Prognosis Of The Patients Having Breast Cancer
We additionally investigated the clinical productivity of PRDXs in the survival of those patients, who have breast cancer. The use of Kaplan–Meier Plotter tools was made for analyzing the relationship existing between the mRNA levels of PRDXs and the survival of 3955 clinical breast cancer patients through the use of the publicly accessible datasets.Citation31 As indicated by both the Kaplan–Meier curve and log-rank test analyses, the augmented PRDX1/2/4/5/6 mRNA levels, coupled with the lowered PRDX3 mRNA levels, had a significant association with the RFS (p < 0.05) of each and every patient having breast cancer (). In addition, the patients, who had breast cancer with the elevated mRNA levels of the PRDX4/6 or low mRNA levels of PRDX3, were likely to be predicted as having weak OS, PPS, and DMFS.
Predicted Functions And Pathways Of The Changes In PRDXs And Their Frequently Altered Neighbor Genes In Patients With Breast Cancer
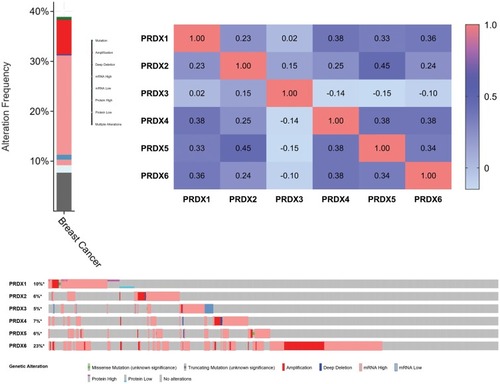
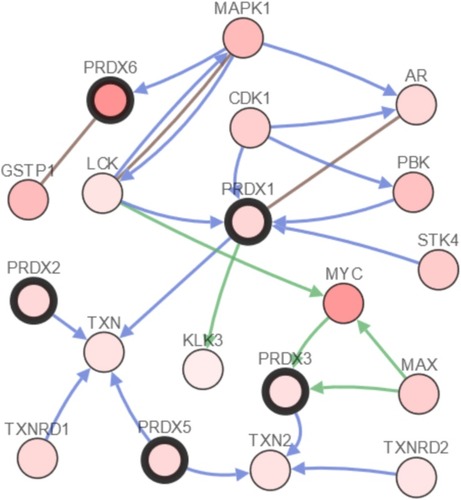
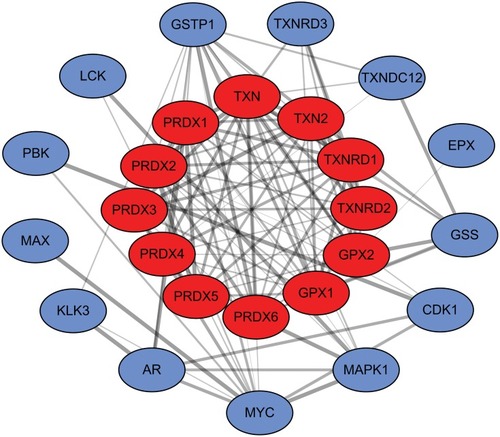
We performed the analysis of the PRDX changes, relationships, and networks through the use of the cBioPortal online instrument for breast cancer (TCGA, Provisional). PRDXs were altered in 425 samples out of those 1093 patients, who had breast cancer (38.88%). The mRNA levels detected in 217 cases were observed as augmented. Among the 425 cases, 217 cases (51.06%) were detected with an increase in the mRNA levels, whereas 84 (19.76%) were detected having multiple alterations (). Besides that, we performed the calculation of the relationships of PRDXs with one another through the analysis of their mRNA expression (RNA Seq V2 RSEM) by means of the cBioPortal online instrument for breast cancer (TCGA, Provisional), together with including the Pearson’s correction. Also, the PRDXs correlations are presented in . Subsequent to that, we developed the network for PRDXs as well as for the 22 most frequently altered neighbor genes. As the findings suggested, MYC, MAPK1 and GSTP1 had a close association with PRDX changes ().
The roles of PRDXs and the genes, which had a significant association with PRDX changes, were predicted by Metascape and String. We figured it out that GO:0098869 (cellular oxidant detoxification) and R-HAS-3299685 (Detoxification of Reactive Oxygen Species) had undergone significant regulation by the PRDX changes. Hsa00480 (Glutathione metabolism), hsa05200 (Pathways in cancer), GO:0009617 (response to bacterium) and hsa00450 (Selenocompound metabolism) also showed involvement in the regulation caused by PRDX alterations ().
Figure 8 Heatmap of enriched terms across PRDXs and other 22 most frequently altered neighbor gene lists, colored by p-values (Metascape).
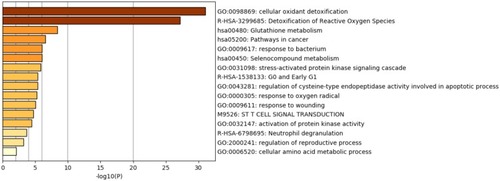
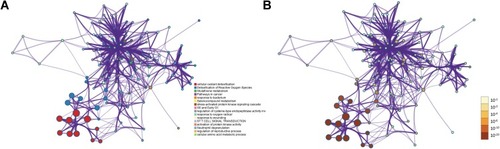
Aimed at further capturing the associations existing between the terms, a subset of enriched terms was chosen and rendered as a network plot, in which the terms having a similarity >0.3 are linked by edges. Subsequent to that, we chose the terms that had the best p-values. The network is visualized with the use of Cytoscape, wherein every node is a representation of an enriched term, together with being colored first in accordance with its cluster ID () and with its p-value afterward (). The top three terms included: cellular oxidant detoxification, Detoxification of Reactive Oxygen Species and Glutathione metabolism. In addition, we also calculated the core modules of genes (TXN, TXN2, TXNRD1, TXNRD2, GPX1 and GPX2) linked to the PRDX family of genes in breast cancer () that was likely to significantly contribute to the development of breast cancer associated with PRDXs.
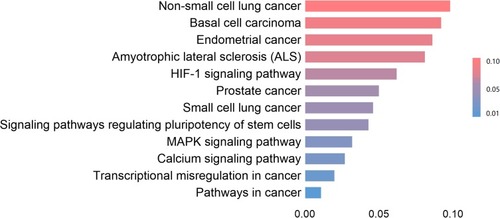
KEGG analysis is capable of defining the pathways associated with the roles of PRDX changes and the commonly changed neighbor genes. In addition, 12 pathways associated with the roles of PRDX changes in breast cancer were observed by means of KEGG analysis ().
Discussion
As indicated earlier, the PRDX family is extensively expressed in a variety of tumors. Even though the function of PRDXs in the tumorigenesis and the prognosis of a number of cancers has been verified partly, the further bioinformatics analysis of breast cancer is still required to be carried out. The current research work refers to the first time exploring the mRNA expression as well as the prognostic (RFS, OS, PPS and DMFS) values of varying PRDX factors in breast cancer. As we expect, our research is going to put forward fresh ideas for the research of clinical diagnosis, therapeutic targets and tumor development mechanisms of breast cancer.
An extensive study of the PRDX1 in breast cancer has been carried out, besides its high expression in breast cancer tissues and cell lines.Citation49,Citation50 In addition to that, other research works have revealed the fact that the high mRNA expression of PRDX1 in human breast carcinoma is pertinent to the higher tumor grade,Citation51 whereas the elevated expression of cytoplasmic PRDX1 linked to a significant risk of local recurrence following the radiotherapy.Citation52
In recent years, it has been discovered that PRDX1 has dual roles of tumor suppressor and cancer promotion in breast cancer, besides being associated with the expression and function of estrogen receptor, having a potential value in not just diagnosing but treating breast cancer as well.Citation53,Citation54 Moreover, the function of PRDX1 in tumors is extremely intricate. It is capable of promoting the proliferation and differentiation of tumor cells through the regulation of H2O2 levels,Citation55 and the repression of PRDX1 expression causes the elevated levels of ROS-stimulated phosphorylation of p38 MAPKα, together with promoting the H2O2-stimulated senescence in breast cancer cells.Citation56 Other studies have figured out that PRDX1 has the potential to suppresses tumor cell deathCitation50 and promote tumor growth.Citation57 In addition, it was explored that the drug resistance formation accompanies a substantial rise in the expression of PRDX1 gene in the breast cancer cell strains.Citation58
However, there are a number of reports suggesting that PRDX1 is likely to play the role of a tumor suppressor in breast cancer. Cao J et alCitation59 have figured out that PRDX1 safeguards the tumor-suppressive role of PTEN phosphatase from ROS-stimulated, besides inhibiting the Ras-stimulated mammary tumors. Furthermore, the overexpression of miR-510 results into the lower PRDX1 that, as a response, augments the function of PI3K/Akt pathway, in addition to promoting the cell and tumor growth in breast cancer.Citation60 With regard to our research work, ONCOMINE datasets and TCGA datasets shed light on the fact that the expression of PRDX1 was substantially higher in human breast cancer as compared with that in normal tissues. The expression of PRDX1 and TNM stage did not have a significant statistical difference. With the help of the Kaplan–Meier Plotter, we figured out the prognostic value of PRDX1 in those patients, who had breast cancer. An elevated PRDX1 expression had a significant association with the weak RFS in each and every patient having breast cancer, who underwent follow-up for a period of more than 250 months.
PRDX2 manifested substantially augmented reactivity in primary breast cancer and carcinoma in situ in comparison with the healthy controls.Citation25 KuronoCitation61 puts forward that PRDX2 levels had significant differences between nipple discharge specimens from the patients having and not having breast cancer. Together, LacombeCitation62 also confirmed the same point between node-negative early-stage breast cancer and ductal carcinoma in situ and the healthy individuals. StresingCitation63 indicates that PRDX2 constitutes a targetable “metabolic adaptor” driver protein that is involved in the selective development of breast metastatic cells in the lungs through their protection against oxidative stress. In accordance with our report, the expression of PRDX2 in breast cancer tissues was observed as higher in comparison with that in normal ones. Nonetheless, PRDX2 expression did not have a correlation with the TNM stage of those patients, who had breast cancer. An elevated PRDX2 expression had a significant correlation with the weak RFS and DMFS in each and every patient having LC.
Pendharkar N et alCitation64 demonstrated the fact that PRDX3 and PRDX1 constitute a panel of markers, capable of discriminating the luminal B HER2 positive and HER2 enriched subtypes of breast cancer, respectively. Cytoplasmic PRDX 3 immunostaining had an approximately substantial correlation with the poorer breast cancer-specific survival. However, PRDX3 was not independent of the conventional prognostic factor in multivariate analysis.Citation65 Moreover, the silencing of the PRDX3 gene inhibits cell proliferation in breast cancer.Citation66 LiuCitation67 shed light on the fact that the knockdown of ECHS1, as well as PRDX3 by RNAi, augmented the apoptosis stimulated by PP2 in breast cancer cell line MCF-7; in addition, the overexpression of PRDX3 hampered the PP2-stimulated apoptosis in MCF-7. As indicated in , and , the expression of PRDX3 in breast cancer tissues was observed as higher in comparison with that in the normal ones; nonetheless, this expression did not have a correlation with the tumor phase in the patients having breast cancer. Nevertheless, surprisingly, a low PRDX3 expression had a significant correlation with the weak RFS, OS and DMFS in all of the patients, who had LC, appearing as inconsistent with the function of PRDX3 as an oncogene. It seems to be inconsistent with the content reported in the above literature.Citation64
Castellana’s qPCR dataCitation68 confirmed the fact that the expression of PRDX4 had a significant difference in invasive lobular breast carcinomas vs normal. TiedemannCitation69 has demonstrated that secreted L-plastin, as well as PRDX4, mediates osteoclast activation by human breast cancer cells. In the same manner as before, the breast carcinoma cells MCF7, together with MDA-MB-231, secreted PRDX4; also, the elevated expression of PRDX4 in the primary breast tumor had a consistent association with the metastasis at 5 years.Citation27 As our report suggests, we illustrated that the expression of PRDX was observed as higher in breast cancer tissues as compared with that in the normal ones. Besides that, this expression had an evident correlation with the tumor phase in the patients having breast cancer. Interestingly, an elevated PRDX4 expression had a significant correlation with the weak RFS, OS and DMFS in each and every patient having breast cancer.
PRDX5 is extensively reported in a number of tumors, for instance, endometrial cancer,Citation70 colon cancer,Citation71 gastric cancerCitation72 and so on. Nonetheless, there have not been an expression and prognosis role in PRDX5 reported in breast cancer. Kim’s research workCitation72 shed light on the fact that the expression of PRDX5 had a significant correlation with not just the size of the tumor but also the depth of tumor, and lymphatic invasion in the patients having gastric cancer. Also, the overexpression of PRDX5 improved carcinogenicity through the increase in the proliferation as well as invasiveness of gastric cancer cells by means of the upregulation of Snail. In addition, PRDX5 is likely to constitute a latent determinant, which may make a contribution to the weak prognosis of gastric cancer by means of the enhancement of the mesenchymal phenotype. In the current report, we illustrated the fact that the expression of PRDX5 in breast cancer tissues was higher as compared with that in the normal ones; together with that, this expression had an apparent correlation with the phase of the tumor in those patients, who had breast cancer. Furthermore, a higher PRDX5 expression had a significant correlation with the weak RFS in each and every patient having breast cancer.
Goncalves’s dataCitation73 put forward that MCF-7 cells express the same kinds of levels of PRDX6 in comparison with MCF-10A cells. Besides that, they also verify the cytoprotective function of peroxiredoxins in normal cells, in addition to revealing a nonredundant synergistic impact of both Prdx1 and Prdx6 on MCF-10A cells. The overexpression of PRDX6 results into a more invasive phenotype as well as the metastatic capability of human breast cancer.Citation28 Seibold’s findingsCitation74 provide evidence that the genetic variant in PRDX6 is likely to alter the prognosis in breast cancer patients. In accordance with our report, we illustrated the fact that the expression of PRDX6 was observed as lower in breast cancer tissues as compared with that in the normal ones. Moreover, there exists no correlation with the TNM stage. A higher PRDX6 expression had a significant correlation with the weak RFS, and OS in all of the patients, who had breast cancer.
Conclusions
Peroxiredoxins (PRDXs) refer to a pervasive family of antioxidant enzymes, which is termed as catalyzing the peroxide reduction, aimed at balancing the levels of cellular hydrogen peroxide (H2O2) that is considered as necessary for the purpose of cell signaling and metabolism, besides acting as a regulator of redox signaling.Citation75 Redox signaling is termed as a crucial part of cell signaling pathways that have involvement in regulating not just the cell development but also metabolism, hormone signaling, immune regulation and numerous physiological roles.Citation76,Citation77 In the current research work, we carried out the systemic analysis of the expression as well as the prognostic value of PRDXs in breast cancer. In addition, we offer a comprehensive understanding of not only the heterogeneity but also the intricacy of the molecular biology of breast cancer. By means of the analysis of various online databases, we finally discovered the fact that the cellular oxidant detoxification and detoxification of ROS underwent significant regulation by the PRDX changes, attained the core modules of genes (TXN, TXN2, TXNRD1, TXNRD2, GPX1 and GPX2) correlated with the PRDX family of genes in breast cancer. Subsequent to that, we are going to further clarify the molecular mechanisms of PRDXs in breast cancer with the use of wet experiments.
Author Contributions
G. W. has provided constructing the idea for research. G.W., W. C. Z. and A. M. X. have provided planning methodology to reach the conclusion. G. W., W. C. Z., Y. H. B. and S. Y. T. are mainly responsible for data analysis by online databases. Taking responsibility for the execution of the experiments and edition of pictures has been provided by H. Z. and H. X. Z. Reviewing the article before submission not only for spelling and grammar but also for its intellectual content has been provided by G.W. and W. C. Z. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No. 81572350 to A. M. X.).
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
References
- Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid Redox Signal. 2012;16(6):506–523. doi:10.1089/ars.2011.426022114845
- Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7(5–6):768–777. doi:10.1089/ars.2005.7.76815890023
- Lu W, Fu Z, Wang H, Feng J, Wei J, Guo J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells’ survival by protecting cells from oxidative stress. Mol Cell Biochem. 2014;387(1–2):261–270. doi:10.1007/s11010-013-1891-424234423
- Lu W, Fu Z, Wang H, Feng J, Wei J, Guo J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/beta-catenin signaling. Cancer Lett. 2014;343(2):190–199. doi:10.1016/j.canlet.2013.10.00224125860
- Song IS, Jeong YJ, Jeong SH, et al. FOXM1-induced PRX3 regulates stemness and survival of colon cancer cells via maintenance of mitochondrial function. Gastroenterology. 2015;149(4):1006–1016 e1009. doi:10.1053/j.gastro.2015.06.00726091938
- Guo X, Noguchi H, Ishii N, et al. The association of peroxiredoxin 4 with the initiation and progression of hepatocellular carcinoma. Antioxid Redox Signal. 2019;30(10):1271–1284. doi:10.1089/ars.2017.742629687726
- Fang Y, He J, Janssen HLA, Wu J, Dong L, Shen XZ. Peroxiredoxin 1, restraining cell migration and invasion, is involved in hepatocellular carcinoma recurrence. J Dig Dis. 2018;19(3):155–169. doi:10.1111/1751-2980.1258029377617
- Sun QK, Zhu JY, Wang W, et al. Diagnostic and prognostic significance of peroxiredoxin 1 expression in human hepatocellular carcinoma. Med Oncol. 2014;31(1):786. doi:10.1007/s12032-013-0786-224297309
- Chung KH, Lee DH, Kim Y, et al. Proteomic identification of overexpressed PRDX 1 and its clinical implications in ovarian carcinoma. J Proteome Res. 2010;9(1):451–457. doi:10.1021/pr900811x19902980
- Wang XY, Wang HJ, Li XQ. Peroxiredoxin III protein expression is associated with platinum resistance in epithelial ovarian cancer. Tumour Biol. 2013;34(4):2275–2281. doi:10.1007/s13277-013-0769-023564483
- Jo M, Yun HM, Park KR, et al. Lung tumor growth-promoting function of peroxiredoxin 6. Free Radic Biol Med. 2013;61:453–463. doi:10.1016/j.freeradbiomed.2013.04.03223643677
- Soini Y, Kinnula VL. High association of peroxiredoxins with lung cancer. Lung Cancer. 2012;78(2):167. doi:10.1016/j.lungcan.2012.08.01322981900
- Wei Q, Jiang H, Xiao Z, et al. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci U S A. 2011;108(17):7004–7009. doi:10.1073/pnas.101301210821487000
- Kim JH, Bogner PN, Ramnath N, Park Y, Yu J, Park YM. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13(13):3875–3882. doi:10.1158/1078-0432.CCR-06-289317606720
- Jiang H, Wu L, Mishra M, Chawsheen HA, Wei Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am J Cancer Res. 2014;4(5):445–460.25232487
- Deighton RF, Le Bihan T, Martin SF, et al. Interactions among mitochondrial proteins altered in glioblastoma. J Neurooncol. 2014;118(2):247–256. doi:10.1007/s11060-014-1430-524728830
- Khalil AA. Biomarker discovery: a proteomic approach for brain cancer profiling. Cancer Sci. 2007;98(2):201–213. doi:10.1111/j.1349-7006.2007.00374.x17233837
- Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011;71(5):1637–1646. doi:10.1158/0008-5472.CAN-10-367421343392
- Ummanni R, Barreto F, Venz S, et al. Peroxiredoxins 3 and 4 are overexpressed in prostate cancer tissue and affect the proliferation of prostate cancer cells in vitro. J Proteome Res. 2012;11(4):2452–2466. doi:10.1021/pr201172n22424448
- Gong F, Hou G, Liu H, Zhang M. Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med Oncol. 2015;32(2):455. doi:10.1007/s12032-014-0455-025579166
- Jiang L, Ren J, Xiao X, et al. Proteomic analysis of bladder cancer by iTRAQ after Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene treatment. Biol Chem. 2013;394(10):1333–1342. doi:10.1515/hsz-2013-020123893687
- Taniuchi K, Furihata M, Hanazaki K, et al. Peroxiredoxin 1 promotes pancreatic cancer cell invasion by modulating p38 MAPK activity. Pancreas. 2015;44(2):331–340. doi:10.1097/MPA.000000000000027025426613
- Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-827865536
- Fiskus W, Coothankandaswamy V, Chen J, et al. SIRT2 deacetylates and inhibits the peroxidase activity of peroxiredoxin-1 to sensitize breast cancer cells to oxidant stress-inducing agents. Cancer Res. 2016;76(18):5467–5478. doi:10.1158/0008-5472.CAN-16-012627503926
- Desmetz C, Bascoul-Mollevi C, Rochaix P, et al. Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin Cancer Res. 2009;15(14):4733–4741. doi:10.1158/1078-0432.CCR-08-330719584157
- Luthra S, Chandran U, Diergaarde B, Becich M, Lee AV, Neumann CA. Expression of reactive species related genes is associated with patient survival in luminal B breast cancer. Free Radic Biol Med. 2018;120:170–180. doi:10.1016/j.freeradbiomed.2018.03.01129545070
- Rafiei S, Tiedemann K, Tabaries S, Siegel PM, Komarova SV. Peroxiredoxin 4: a novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett. 2015;361(2):262–270. doi:10.1016/j.canlet.2015.03.01225779674
- Chang XZ, Li DQ, Hou YF, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007;9(6):R76. doi:10.1186/bcr178917980029
- Oncomine. Availalble from: https://www.oncomine.org/. Accessed 117, 2019.
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx24728407145
- Kaplan Meier-plotter. Availalble from: https://kmplot.com/analysis/. Accessed 117, 2019.
- Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi:10.1007/s10549-009-0674-920020197
- Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi:10.1038/nature1141223000897
- cBioPortal for cancer genomics. Availalble from: https://www.cbioportal.org/. Accessed 117, 2019.
- Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi:10.1038/s41467-019-09234-630944313
- Metascape. Availalble from: http://www.metascape.org/.
- STRING: functional protein association networks. Availalble from: https://string-db.org/. Accessed 117, 2019.
- Cytoscape: an open source platform for complex network analysis and visualization. Availalble from: https://cytoscape.org/. Accessed 117, 2019.
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.123930314597658
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.21119131956
- DAVID functional annotation bioinformatics microarray analysis. Availalble from: https://david.ncifcrf.gov/. Accessed 117, 2019.
- Zhao H, Langerod A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15(6):2523–2536. doi:10.1091/mbc.e03-11-078615034139
- Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11(1):R7. doi:10.1186/bcr222219187537
- Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi:10.1038/nature1098322522925
- Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. doi:10.1038/nm176418438415
- Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi:10.1073/pnas.19136709811553815
- Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi:10.1016/j.ccr.2006.01.01316473279
- GEPIA (Gene Expression Profiling Interactive Analysis). Availalble from: http://gepia.cancer-pku.cn/. Accessed 117, 2019.
- van de Moosdijk AA, van Amerongen R. Identification of reliable reference genes for qRT-PCR studies of the developing mouse mammary gland. Sci Rep. 2016;6:35595. doi:10.1038/srep3559527752147
- Wang X, He S, Sun JM, Delcuve GP, Davie JR. Selective association of peroxiredoxin 1 with genomic DNA and COX-2 upstream promoter elements in estrogen receptor negative breast cancer cells. Mol Biol Cell. 2010;21(17):2987–2995. doi:10.1091/mbc.e10-02-016020631257
- Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi:10.1186/1756-9966-28-9319566940
- Woolston CM, Storr SJ, Ellis IO, Morgan DAL, Martin SG. Expression of thioredoxin system and related peroxiredoxin proteins is associated with clinical outcome in radiotherapy treated early stage breast cancer. Radiother Oncol. 2011;100(2):308–313. doi:10.1016/j.radonc.2011.05.02921641069
- O’Leary PC, Terrile M, Bajor M, et al. Peroxiredoxin-1 protects estrogen receptor alpha from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014;16(4):R79. doi:10.1186/bcr369125011585
- Bajor M, Zych AO, Graczyk-Jarzynka A, et al. Targeting peroxiredoxin 1 impairs growth of breast cancer cells and potently sensitises these cells to prooxidant agents. Br J Cancer. 2018;119(7):873–884. doi:10.1038/s41416-018-0263-y30287919
- Coumans JV, Gau D, Poljak A, Wasinger V, Roy P, Moens PD. Profilin-1 overexpression in MDA-MB-231 breast cancer cells is associated with alterations in proteomics biomarkers of cell proliferation, survival, and motility as revealed by global proteomics analyses. OMICS. 2014;18(12):778–791. doi:10.1089/omi.2014.007525454514
- Turner-Ivey B, Manevich Y, Schulte J, et al. Role for Prdx1 as a specific sensor in redox-regulated senescence in breast cancer. Oncogene. 2013;32(45):5302–5314. doi:10.1038/onc.2012.62423334324
- Wang R, Liu Y, Liu L, et al. Tumor cells induce LAMP2a expression in tumor-associated macrophage for cancer progression. EBioMedicine. 2019;40:118–134. doi:10.1016/j.ebiom.2019.01.04530711520
- Kalinina EV, Berezov TT, Shtil AA, et al. Expression of peroxiredoxin 1, 2, 3, and 6 genes in cancer cells during drug resistance formation. Bull Exp Biol Med. 2012;153(6):878–881. doi:10.1007/s10517-012-1849-723113308
- Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28(10):1505–1517. doi:10.1038/emboj.2009.10119369943
- Guo QJ, Mills JN, Bandurraga SG, et al. MicroRNA-510 promotes cell and tumor growth by targeting peroxiredoxin1 in breast cancer. Breast Cancer Res. 2013;15(4):R70. doi:10.1186/bcr346423971998
- Kurono S, Kaneko Y, Matsuura N, et al. Identification of potential breast cancer markers in nipple discharge by protein profile analysis using two-dimensional nano-liquid chromatography/nanoelectrospray ionization-mass spectrometry. Proteomics Clin Appl. 2016;10(5):605–613. doi:10.1002/prca.v10.526970563
- Lacombe J, Mange A, Bougnoux AC, Prassas I, Solassol J. A multiparametric serum marker panel as a complementary test to mammography for the diagnosis of node-negative early-stage breast cancer and DCIS in young women. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1834–1842. doi:10.1158/1055-9965.EPI-14-026724957886
- Stresing V, Baltziskueta E, Rubio N, et al. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene. 2013;32(6):724–735. doi:10.1038/onc.2012.9322430214
- Pendharkar N, Gajbhiye A, Taunk K, et al. Quantitative tissue proteomic investigation of invasive ductal carcinoma of breast with luminal B HER2 positive and HER2 enriched subtypes towards potential diagnostic and therapeutic biomarkers. J Proteomics. 2016;132:112–130. doi:10.1016/j.jprot.2015.11.02426642762
- Karihtala P, Kauppila S, Soini Y, Arja Jukkola V. Oxidative stress and counteracting mechanisms in hormone receptor positive, triple-negative and basal-like breast carcinomas. BMC Cancer. 2011;11:262. doi:10.1186/1471-2407-11-26221693047
- Chua PJ, Lee EH, Yu Y, Yip GW, Tan PH, Bay BH. Silencing the Peroxiredoxin III gene inhibits cell proliferation in breast cancer. Int J Oncol. 2010;36(2):359–364.20043069
- Liu X, Feng R, Du L. The role of enoyl-CoA hydratase short chain 1 and peroxiredoxin 3 in PP2-induced apoptosis in human breast cancer MCF-7 cells. FEBS Lett. 2010;584(14):3185–3192. doi:10.1016/j.febslet.2010.06.00220541551
- Castellana B, Escuin D, Perez-Olabarria M, et al. Genetic up-regulation and overexpression of PLEKHA7 differentiates invasive lobular carcinomas from invasive ductal carcinomas. Hum Pathol. 2012;43(11):1902–1909. doi:10.1016/j.humpath.2012.01.01722542108
- Tiedemann K, Sadvakassova G, Mikolajewicz N, et al. Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl Oncol. 2019;12(3):462–474. doi:10.1016/j.tranon.2018.11.01430583289
- Byun JM, Kim SS, Kim KT, et al. Overexpression of peroxiredoxin-3 and −5 is a potential biomarker for prognosis in endometrial cancer. Oncol Lett. 2018;15(4):5111–5118. doi:10.3892/ol.2018.790929541251
- Ahn HM, Yoo JW, Lee S, Lee HJ, Lee HS, Lee DS. Peroxiredoxin 5 promotes the epithelial-mesenchymal transition in colon cancer. Biochem Biophys Res Commun. 2017;487(3):580–586. doi:10.1016/j.bbrc.2017.04.09428431931
- Kim B, Kim YS, Ahn HM, et al. Peroxiredoxin 5 overexpression enhances tumorigenicity and correlates with poor prognosis in gastric cancer. Int J Oncol. 2017;51(1):298–306. doi:10.3892/ijo.2017.401328535004
- Goncalves K, Sullivan K, Phelan S. Differential expression and function of peroxiredoxin 1 and peroxiredoxin 6 in cancerous MCF-7 and noncancerous MCF-10A breast epithelial cells. Cancer Invest. 2012;30(1):38–47. doi:10.3109/07357907.2011.62938222236188
- Seibold P, Hall P, Schoof N, et al. Polymorphisms in oxidative stress-related genes and mortality in breast cancer patients–potential differential effects by radiotherapy? Breast. 2013;22(5):817–823. doi:10.1016/j.breast.2013.02.00823489758
- Chen L, Na R, Ran Q. Enhanced defense against mitochondrial hydrogen peroxide attenuates age-associated cognition decline. Neurobiol Aging. 2014;35(11):2552–2561. doi:10.1016/j.neurobiolaging.2014.05.00724906890
- Mishra M, Jiang H, Wu L, Chawsheen HA, Wei Q. The sulfiredoxin-peroxiredoxin (Srx-Prx) axis in cell signal transduction and cancer development. Cancer Lett. 2015;366(2):150–159. doi:10.1016/j.canlet.2015.07.00226170166
- Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–569. doi:10.1016/j.cell.2015.10.00126496603