Abstract
Background
Although prior studies have shown that marital status affects the prognosis of patients with gastric cancer, its time-varying effects are not well understood. We aimed to investigate the changes in marital status’ impact over a 10-year follow-up time among patients with gastric cancer (GC) in the United States.
Materials and Methods
All patients with gastric cancer diagnosed between 2004 and 2008 in the Surveillance, Epidemiology, and End Results (SEER) database were retrieved. Married patients and unmarried patients (single, separated, divorced or widowed) with complete survival time were selected for comparisons. A total of 14,545 patients who had clinical data and follow-up information available were enrolled. We used Kaplan–Meier analyses and time-dependent flexible parametric models to estimate time-varying hazard ratios (HRs).
Results
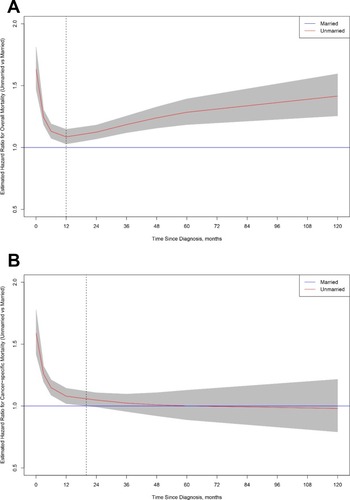
Unmarried GC patients had worse overall and cancer-specific survival compared with married patients (log-rank test: P < 0.001 and P < 0.001, respectively). The time-varying analysis found that unmarried patients had a significantly higher risk of overall mortality during the 10-year follow-up time, with the lowest adjusted hazard ratio (HR) at 12 months after diagnosis (HR at 12 months, 1.08; 95% CI, 1.03–1.15). For cancer-specific mortality, the time-varying adjusted HR of unmarried patients was significantly higher initially (HR at 12 months, 1.08; 95% CI, 1.02–1.14) but decreased to null after 20 months (HR at 24 months = 1.04; 95% CI = 0.99–1.11).
Conclusion
Unmarried patients had a higher risk of cancer-specific mortality during the 20 months after gastric cancer diagnosis, which may be an appropriate time frame for intervention.
Introduction
Gastric cancer (GC) is the sixth most common malignant tumor worldwide and the second most frequent cause of cancer-related death after lung cancer.Citation1,Citation2 In the United States, there were 27,510 new cases of GC and 11,140 GC deaths estimated by the American Cancer Society in 2019.Citation3
Unmarried status was shown to have significant adverse survival effects for different types of cancer.Citation4–Citation8 With regard to GC, prior studies have shown that being unmarried may also increase overall mortality and cancer-specific mortality risk in patients with GC.Citation9–Citation11 However, data on the impact of marital status across a longer follow-up time period are lacking. Studying this association has been complicated by the fact that a differential mortality hazard by marital status over time violates the proportional hazards assumption of the Cox regression model.Citation12
In this study, we aimed to explore changes in the prognostic effect of marital status over a 10-year follow-up time by using the Surveillance, Epidemiology and End Results (SEER) database.
Materials and Methods
Case Selection
Data on all gastric adenocarcinomas for each patient were obtained from the SEER database from 2004 to 2008. Patients with antecedent malignancy before gastric cancer were excluded. The SEER program covers approximately 26% of the United States population. All patients had information on marital status, 5 patients were less than 5 years old, 2222 patients were missing tumor stage, 28 patients were missing information on race, 2740 patients were missing information on tumor grade, and 46 patients were missing information on residence type. We excluded these patients in our analysis. A total of 14,545 patients fulfilled the above criteria and were included in further analyses.
Study Variables
The adjusted marital status hazard ratios were adjusted for age, sex and race, treatment, tumor characteristics and socioeconomic status. According to the SEER database, marital status was described as married (including common law), single (never married), separated, divorced, and widowed. In this study, the unmarried patients included single, separated/divorced, and widowed patients. Race/ethnicity was classified as white, black, and others. Tumor location was classified as cardia or noncardia/NOS according to the International Classification of Diseases for Oncology (third edition) (ICD-O-3). The TNM classification system was defined by the AJCC Cancer Staging Manual (the sixth edition). Residence type was determined at the county level by linkage to the 2003 US Department of Agriculture rural–urban continuum codes. Educational status and median household income were obtained from the county level of 2000 US Census American Community Survey (ACS).
Statistical Analysis
Baseline patient characteristics were compared with the t test or χ2 test, as appropriate. Nonparametric analyses were performed by the Kaplan–Meier method. Median survival time was calculated by Kaplan–Meier method. Median follow-up time was calculated by reverse Kaplan–Meier method.Citation13,Citation14 Statistical significance was assessed using the log-rank test. Time-varying analyses were performed using flexible parametric models, where the logarithm of the baseline hazard function was modeled as a natural cubic spline function of log time.Citation15–Citation17 Marital status was treated as a covariate with a time-varying effect (nonproportional hazards) by including an interaction with time. For model selection, we explored multiple degrees of freedom for the baseline mortality hazard and time-dependent effect and assessed the goodness of fit using the Akaike Information Criterion (AIC). Flexible parametric models were fitted with 6 and 3 degrees of freedom for the baseline mortality hazard and the time-dependent effect, respectively. Statistical tests were 2‐sided with an α value of 0.05. We did not obtain informed consent from the patients because we analyzed deidentified cancer registry data.
Ethics Statement
Our study was carried out in accordance with the principles of the Declaration of Helsinki. The SEER was public-use data: informed consent was waived. And our study was deemed exempt from institutional review board approval by Queen Mary Hospital, The University of Hong Kong.
Results
Patient Characteristics
Among the 14,545 patients enrolled, 12,166 deaths were observed. The median survival time for enrolled patients was 13 months. The median follow-up time for enrolled patients was 122 months. The patients’ baseline characteristics are shown in . The unmarried patients were, on average, 3.4 years older than the married controls (P < 0.001). Unmarried status was more common among female patients than among male patients (P < 0.001). In addition, black patients showed a higher unmarried rate than patients of other races (P < 0.001) ().
Table 1 Baseline Characteristics
Unadjusted Analysis of Long-term Outcome Associated with Marital Status
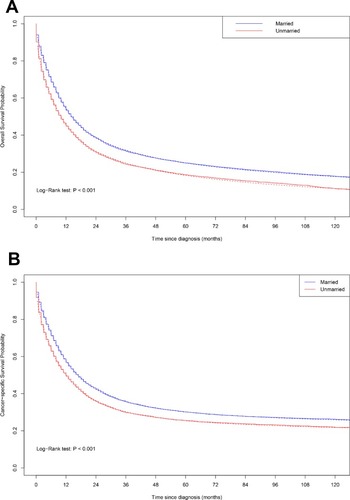
Kaplan–Meier analyses and a flexible parametric model based on marital status were performed for enrolled patients (). A statistically significant difference in both overall survival (log-rank test: P<0.001) and cancer-specific survival (log-rank test: P<0.001) was observed according to marital status. The 10-year overall survival rates for the unmarried group and the married group were 11.1% (95% CI, 10.3% - 12.0%) and 17.8% (95% CI, 17.0% - 18.7%), respectively.
Time-Varying Multivariable Analysis of Long-term Survival Associated with Marital Status
We used flexible parametric modeling to perform a time-varying analysis of the long-term effects of marital status at diagnosis for GC patients, adjusting for classic patient characteristics (age, sex, race), tumor characteristics (tumor location, pathological grade, histological type, tumor stage) and socioeconomic conditions (household incomes, education, residence type). The findings from the time-varying hazard ratio analysis for overall mortality are shown in . Compared with married patients, unmarried patients had a significantly higher mortality risk at the time immediately after their first diagnosis; their risk then declined to the lowest point at 12 months after diagnosis (HR at 12 months, 1.08; 95% CI, 1.03–1.15), and finally increased thereafter in the following 9 years of follow-up time (HR at 120 months = 1.42, 95% CI = 1.26–1.60) (). For cancer-specific mortality analysis, findings from the time-varying HR analysis suggested that unmarried patients were only at significantly higher mortality risk during approximately the first 20 months after GC diagnosis compared with married patients () (HR at 12 months = 1.08; 95% CI, 1.02–1.14; HR at 24 months = 1.04; 95% CI, 0.99–1.11). The subgroup analyses found that the high hazard ratios for both overall and cancer-specific mortality among unmarried patients during early follow-up time were observed regardless of the component of unmarried status or tumor stage (Figures S1 and S2).
Discussion
In this study, we used data from a population-based database in the United States. Our study showed that unmarried GC patients experienced a higher risk of overall and cancer‐specific mortality than married control GC patients throughout a 10-year follow-up period. Moreover, findings from the time-varying analysis suggested that the relative overall and cancer-specific mortality risk changes over follow-up time. Specifically, compared with married patients, the cancer-specific mortality risk for unmarried patients was significantly higher during the first 20 months after GC diagnosis, and then the risk gradually attenuated to null. To the best of our knowledge, this has not been reported previously. This 20-month period may become a “window of opportunity” for social interventions.
Until now, it has generally been accepted that there must be some factors (e.g., psychological, physiological or socioeconomic factors), not marital status per se, that may be an innate reason for the effect of marriage on survival outcome. Based on this assumption, many potential underlying etiologies are proposed to explain the adverse effects of unmarried status on survival outcomes.
Because the cancer-specific mortality risk of unmarried patients mainly happens in the early follow-up period, medical access and adherence may be important factors for the relationship between marital status and survival outcomes. Unmarried patients showed more advanced stage than married patients when first diagnosed,Citation6,Citation10,Citation18 which may reflect worse access to care for unmarried than married patients. Although there is limited evidence in cancer, unmarried patients display a higher risk of medication nonadherence than married patients in numerous other chronic diseases.Citation19–Citation21 Depression strongly affects medical nonadherenceCitation22 and may also be a mediator of the association between marital status and medical adherence.Citation6 Efforts to improve medical access and adherence among unmarried patients may be effective. If we assume that improved social support could improve outcomes in unmarried patients, the first 20 months after diagnosis may be an appropriate time for interventions.
Mental health is also commonly thought to play a role in the relationship between marital status and survival outcomes. Patients with cancers have a higher prevalence of mental disorders in comparison with the normal controls.Citation23,Citation24 These psychological conditions have been associated with worse survival outcomes in multiple studies.Citation25–Citation27 Single patients or patients with family disruption are more likely to experience depression, anxiety and psychological distress than married patients,Citation28,Citation29 which may be a reason for the poor outcome of cancer patients. Screening for mental health disorders and early structural intervention, if needed, among unmarried patients with cancers may be a feasible management approach.Citation30,Citation31 A previous meta-analysis that only included randomized controlled trials indicated that psychosocial interventions have survival benefits during the short-term period after cancer diagnosis,Citation32 which is consistent with the novel concept of the “window of opportunity” in our study. Additionally, other benefits, such as improvement of quality of life, have been demonstrated for mental health interventions and should not be ignored.Citation33
Physiologically, unmarried patients have been demonstrated to be at higher risk of cardiovascular diseasesCitation34 and dementia.Citation35 Comorbidities have included chronic conditions that affect long-term survival in multiple cancers,Citation36 although the cancer type and severity may be determinants of the magnitude and presence of this effect.Citation37–Citation39 Moreover, some stress-related endocrine and immunological changes among unmarried patients may also play a role in their survival.Citation40,Citation41
Financial hardship and increased medical costs have commonly been reported in the cancer survivor’s family in the United States.Citation42,Citation43 These factors are commonly thought to be a mediator in the relationship between marital status and survival outcomes. However, until now, no sufficient evidence has supported financial hardship as a mediator of the association between marital status and survival outcomes.Citation5 Consistent with previous research,Citation4,Citation5 only minimal changes in the HRs for death among unmarried patients between models adjusted and not adjusted for socioeconomic conditions in the multivariate models in our study (data not shown).
The risk of overall mortality, although decreased during the first 12 months, was persistently significantly higher in unmarried patients than in married patients. However, the higher risk of overall mortality among unmarried people is present not only in GC patients but also in the general elderly population.Citation44 Interventions that improve the overall survival of unmarried people in the general population may also be beneficial to cancer patients.
The strengths of this study include its population-based study design, large sample size, and extensive duration of follow-up. However, there are limitations. First, changes in marital status during the survival period were not available in the SEER databases. However, changes in marital status may not be an important reason for declining cancer-specific mortality hazard ratios for unmarried patients because the risk of overall mortality increases gradually during long-term follow-up, which is consistent with the general elderly population.Citation44 Second, the impact of other family caregivers in addition to the spouse was not analyzed in this study. Whether accessing alternative caregivers or getting remarried could improve outcomes for unmarried patients is still unclear.
Despite these potential limitations, our study indicates that the risk of cancer-specific mortality for unmarried GC patients was significantly higher initially but decreased to null after 20 months. Given that efforts on medical and social support may improve the outcomes of cancer patients, this time period is an appropriate opportunity for interventions.
Figure 1 Overall survival and cancer-specific survival by marital status. Log-rank test: (A) Overall survival: married versus unmarried, P < 0.001; (B) Cancer-specific survival: married versus unmarried, P < 0.001. Solid lines show the Kaplan–Meier analysis, and dotted lines show the flexible parametric model.
Disclosure
The authors report no conflicts of interest in this work.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6 ):394–424. doi:10.3322/caac.v68.630207593
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2 ):87–108. doi:10.3322/caac.2126225651787
- Society. AC. Cancer facts & figures 2019 estimates. Available from: www.cancer.gov. Accessed 1210, 2019.
- Martinez ME, Anderson K, Murphy JD, et al. Differences in marital status and mortality by race/ethnicity and nativity among California cancer patients. Cancer. 2016;122(10 ):1570–1578. doi:10.1002/cncr.2988627065455
- Gomez SL, Hurley S, Canchola AJ, et al. Effects of marital status and economic resources on survival after cancer: a population-based study. Cancer. 2016;122(10 ):1618–1625. doi:10.1002/cncr.2988527065317
- Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31 ):3869–3876. doi:10.1200/JCO.2013.49.648924062405
- Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8 ):1273–1278. doi:10.1002/cncr.v121.825524565
- Costa LJ, Brill IK, Brown EE. Impact of marital status, insurance status, income, and race/ethnicity on the survival of younger patients diagnosed with multiple myeloma in the United States. Cancer. 2016;122(20 ):3183–3190. doi:10.1002/cncr.v122.2027548407
- Lagergren J, Andersson G, Talback M, et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer. 2016;122(2 ):207–212. doi:10.1002/cncr.v122.226447737
- Jin JJ, Wang W, Dai FX, et al. Marital status and survival in patients with gastric cancer. Cancer Med. 2016;5(8 ):1821–1829. doi:10.1002/cam4.75827264020
- Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) database. Sci Rep. 2016;6:21098. doi:10.1038/srep2109826876653
- Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pelissier S. Variables with time-varying effects and the cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010;10:20. doi:10.1186/1471-2288-10-2020233435
- Mathoulin-Pelissier S, Gourgou-Bourgade S, Bonnetain F, Kramar A. Survival end point reporting in randomized cancer clinical trials: a review of major journals. J Clin Oncol. 2008;26(22 ):3721–3726. doi:10.1200/JCO.2007.14.119218669458
- Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer. 1995;72(2 ):511–518. doi:10.1038/bjc.1995.3647640241
- Royston P. Flexible parametric alternatives to the cox model, and more. Stata J. 2001;1(1 ):1–28. doi:10.1177/1536867X0100100101
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2 ):265–290. doi:10.1177/1536867X0900900206
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15 ):2175–2197. doi:10.1002/sim.120312210632
- Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21 ):3125–3130. doi:10.1001/jama.1987.034002100670273669259
- Wu JR, Lennie TA, Chung ML, et al. Medication adherence mediates the relationship between marital status and cardiac event-free survival in patients with heart failure. Heart Lung. 2012;41(2 ):107–114. doi:10.1016/j.hrtlng.2011.09.00922054720
- Stephens MA, Fekete EM, Franks MM, Rook KS, Druley JA, Greene K. Spouses’ use of pressure and persuasion to promote osteoarthritis patients’ medical adherence after orthopedic surgery. Health Psychol. 2009;28(1 ):48–55. doi:10.1037/a001238519210017
- Murota H, Takeuchi S, Sugaya M, et al. Characterization of socioeconomic status of Japanese patients with atopic dermatitis showing poor medical adherence and reasons for drug discontinuation. J Dermatol Sci. 2015;79(3 ):279–287. doi:10.1016/j.jdermsci.2015.05.01026255207
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14 ):2101–2107. doi:10.1001/archinte.160.14.210110904452
- Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1(5 ):343–350. doi:10.1016/S2215-0366(14)70313-X26360998
- Kaiser NC, Hartoonian N, Owen JE. Toward a cancer-specific model of psychological distress: population data from the 2003-2005 National Health Interview Surveys. J Cancer Surviv. 2010;4(4 ):291–302. doi:10.1007/s11764-010-0120-320213535
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115(22 ):5349–5361. doi:10.1002/cncr.2456119753617
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11 ):1797–1810. doi:10.1017/S003329170999228520085667
- Lu D, Andrae B, Valdimarsdottir U, et al. Psychological distress is associated with cancer-specific mortality among patients with cervical cancer. Cancer Res. 2019;79:3965–3972. doi:10.1158/0008-5472.CAN-19-0116
- Scott KM, Wells JE, Angermeyer M, et al. Gender and the relationship between marital status and first onset of mood, anxiety and substance use disorders. Psychol Med. 2010;40(9 ):1495–1505. doi:10.1017/S003329170999194219939327
- Hope S, Rodgers B, Power C. Marital status transitions and psychological distress: longitudinal evidence from a national population sample. Psychol Med. 1999;29(2 ):381–389. doi:10.1017/S003329179800814910218928
- Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2(8668 ):888–891. doi:10.1016/S0140-6736(89)91551-12571815
- Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50(9 ):681–689. doi:10.1001/archpsyc.1993.018202100150028357293
- Xia Y, Tong G, Feng R, Chai J, Cheng J, Wang D. Psychosocial and behavioral interventions and cancer patient survival again: hints of an adjusted meta-analysis. Integr Cancer Ther. 2014;13(4 ):301–309. doi:10.1177/153473541452331424613928
- Mulick A, Walker J, Puntis S, et al. Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT oncology-2 and 3 trials. Lancet Psychiatry. 2018;5(4 ):321–326. doi:10.1016/S2215-0366(18)30061-029544711
- Wong CW, Kwok CS, Narain A, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. 2018;104(23 ):1937–1948. doi:10.1136/heartjnl-2018-31300529921571
- Sommerlad A, Ruegger J, Singh-Manoux A, Lewis G, Livingston G. Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry. 2018;89(3 ):231–238. doi:10.1136/jnnp-2017-31627429183957
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4 ):337–350. doi:10.3322/caac.v66.426891458
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5 ):373–383. doi:10.1016/0021-9681(87)90171-83558716
- Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2 ):197–203. doi:10.1016/0895-4356(92)90016-G1573438
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1 ):8–27. doi:10.1097/00005650-199801000-000049431328
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12 ):994–1000. doi:10.1093/jnci/92.12.99410861311
- Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl ):S163–S170. doi:10.1016/j.bbi.2012.07.01922884416
- Yabroff KR, Dowling EC, Guy GP Jr., et al. Financial hardship associated with cancer in the united states: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3 ):259–267. doi:10.1200/JCO.2015.62.046826644532
- O’Neill CB, Atoria CL, O’Reilly EM, LaFemina J, Henman MC, Elkin EB. Costs and trends in pancreatic cancer treatment. Cancer. 2012;118(20 ):5132–5139. doi:10.1002/cncr.2749022415469
- Manzoli L, Villari P, Pirone GM, Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med. 2007;64(1 ):77–94. doi:10.1016/j.socscimed.2006.08.03117011690