Abstract
Purpose
Systemic inflammatory cell ratio, neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR) are used as prognostic indicators for several types of tumors. The purpose of this study was to evaluate the predictive value of inflammatory markers for pathological response and prognosis in breast cancer patients receiving neoadjuvant chemotherapy (NAC).
Methods
In this study, we collected data of 203 breast cancer patients who underwent surgery after receiving standard neoadjuvant therapy. The effects of NLR, PLR, and LMR on the disease-free survival (DFS) of patients with breast cancer were analyzed by χ2 test and Cox regression analyses.
Results
We found that 27 of the 203 patients (13.3%) had local or distant metastases. The peripheral blood NLR, PLR, and LMR areas under the curve (AUC) were 0.674 (0.555–0.793), 0.630 (0.508–0.753), and 0.773 (0.673–0.874), respectively. The optimal cutoff values were 3.0, 135, and 6.2, respectively. Univariate and multivariate analyses revealed that LMR was related to the pathological complete response (pCR) rates and breast cancer DFS (P < 0.05). Among all patients, those with low LMR, HER-2 positive, and lymph node status (N2–3) demonstrated poor DFS.
Conclusion
Our study thus demonstrated that LMR can act as a potential marker for predicting the efficacy and prognosis of patients with breast cancer.
Introduction
Breast cancer is presently the most-threatening disease affecting women’s healthCitation1 with unclear comprehension on postoperative recurrence and distant metastasis, thereby warranting urgent research focus. Neoadjuvant chemotherapy is a commonly used treatment approach for the treatment of breast cancer patients. The pathological complete response (pCR) of neoadjuvant chemotherapy is associated with longer disease-free survival (DFS) and overall survival of patients.Citation2 Although there are a variety of treatment methods available for breast cancer treatment with the continuous development of new drugs, some breast cancer patients continue to experience recurrence and metastasis,Citation3 which seriously affects their quality of life. Therefore, there is an urgent need for biomarkers that can predict the treatment outcomes and patient survival so as to facilitate the identification of patients who are most likely to benefit from the treatment.
Past researches have demonstrated that inflammation affects all stages of tumorigenesis.Citation4 Inflammation promotes the occurrence and development of a tumor, and the tumor provides conditions encouraging the persistence of inflammation, which complements each other.Citation5,Citation6 Altered inflammation hence not only promotes the occurrence and development of tumor but also promotes tumor metastasis.Citation7,Citation8 The neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR) are important indicators of the inflammatory response exerted during the prognosis of colorectal cancer, gastric cancer, lung cancer, and other malignant tumors.Citation9–Citation11
PLR is an independent predictor of breast cancer. The clinical prognostic effect of elevated PLR is better than that of NLR and LMR.Citation12 Some past studies also assert that NLR has a better predictive efficiency.Citation13 In a study on breast cancer patients conducted in Spain, high LMR and low NLR were found to be associated with a lower risk of recurrence.Citation14 In a study conducted in South Korea, LMR indicated the potential to strongly predict DFS and OS in breast cancer.Citation15 Considering the ease of measurement and reproducibility of NLR, LMR, and PLR, they have been increasingly studied as an independent factor in the survival of breast cancer patients. However, the importance of these indicators to determine the effect of breast cancer treatment and the associated prognostic value remains controversial. Therefore, we aimed to verify the relationship among PLR, NLR, and LMR as well as the effect of neoadjuvant chemotherapy and DFS on breast cancer so as to screen more valuable predictors.
In this study, the clinical and follow-up data of 203 breast cancer patients were analyzed to investigate the value of peripheral blood NLR, PLR, and LMR in predicting postoperative recurrence of breast cancer and to provide appropriate references for clinical applicability.
Materials and Methods
Study Subjects
Patients with breast cancer (confirmed by pathology) at the Henan Cancer Hospital during March 2017-December 2018 were included in this analysis. The basic patient demographics, pathological information, and prognosis information were collected for processing.
A total of 203 female patients (aged: 27–71 years) with complete clinicopathological data and those who had completed neoadjuvant therapy at our hospital were included in the analyses.
All patients were followed-up regularly after the surgery. We calculated the DFS of the patients from the time of surgery to the time of disease relapse or the last follow-up. All patients were recorded to be alive at the last follow-up.
Inclusion and Exclusion Criteria
Entry Criteria
(1) all patients were confirmed diagnosed with invasive breast cancer via hollow needle biopsy. The levels of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) were all determined by immunohistochemistry. (2) The clinical stage of the disease was stage II–III breast cancer. The size of the tumor was confirmed by color Doppler ultrasound or magnetic resonance imaging (MRI), and the status of axillary lymph nodes was found to be positive or suspiciously positive by hollow needle biopsy. The clinical tumor-node-metastasis (cTNM) cancer staging system was performed with reference to the 7th edition of the American Joint Committee on Cancer (AJCC). (3) The complete follow-up data were recorded for all enrolled patients. (4) No serious cardiopulmonary diseases, hematological diseases, and contraindications to chemotherapy were recorded for any patients.
Exclusion Criteria
Patients with advanced breast cancer, other systemic diseases that could not tolerate chemotherapy, inflammatory breast cancer, and other malignant tumors were excluded.
After completing the standard neoadjuvant therapy, all patients underwent surgery, postoperative sequential radiotherapy, or endocrine therapy according to their specific condition.
Information Collection and Follow-Up
The cell counts of platelets, neutrophils, lymphocytes, and monocytes in the blood samples of all study patients were enumerated and judged by a professional laboratorian in the laboratory department of our center.
The clinical data of the patients were collected, including age, surgery date, menstrual status, treatment solutions, family history, tumor size, lymph node metastasis, molecular classification, radiotherapy or not, recurrence time, recurrence site, as well as preoperative blood routine results (such as the counts of platelet, neutrophils, and lymphocytes). Finally, the values of NLR, PLR, and LNR were calculated.
The receiver operating characteristic (ROC) curve was plotted for the raw NLR, PLR, and LMR data, and the area under the curve (AUC) was calculated. The Jordan index was calculated according to the sensitivity and specificity, and the point with the largest AUC was selected as the demarcation point. The expression level of NLR, PLR, and LMR was defined as per the optimal cut-off point value.
Statistical Analysis
The Statistical Product and Service Solutions (SPSS) 22.0 was used for statistical analysis. The correlation between the NLR, PLR, and LMR values and the clinical data was analyzed by χ2 test. The different levels of NLR, PLR, and LMR in the two groups were determined by T-test (). Univariate analysis of factors associated with recurrence and metastasis was evaluated by Chi-square test. Independent risk factors affecting prognosis were analyzed by multivariate Cox regression. The Kaplan–Meier survival curve was used to reflect the effects of different levels of NLR, PLR, and LMR on survival.
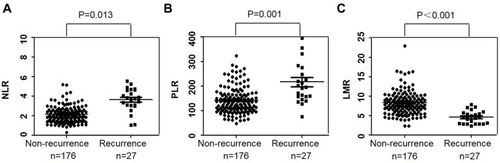
Figure 1 The levels of NLR, PLR, and LMR in the non-recurrent and recurrent groups. (A, B) The levels of NLR and PLR in the recurrent group were significantly higher than those in the non-recurrent group (P < 0.05). (C) The level of LMP in the recurrent group was significantly lower than that in the non-recurrent group (P < 0.05).
Results
Patient Baseline Characteristics
After strict adherence to the inclusion and exclusion criteria, a total of 203 breast cancer patients were included in the analysis, and 71.9% (n =146) of them were diagnosed while being premenopausal. All patients were female of an average age of 46.59 ± 9.39 years. Postoperative local or distant metastasis occurred in 27 patients (13.3%). The median follow-up time of the disease was 31 months (range: 1–39 months).
Based on the expression of ER, PR, and HER2, 98 cases (48.3%) were identified to be luminal, 63 (31.0%) as HER2 positive, and 42 (20.7%) as triple-negative breast cancer (TNBC). The average values of the NLR, PLR, and LMR were 2.26 ± 0.08, 144.79 ± 4.14, and 8.11 ± 0.30, respectively.
Correlation Between NLR, PLR, and LMR Values and the Corresponding Clinicopathological Data
We categorized the patients into 2 groups. The first group included patients who had local or distant organ metastasis after the operation (the recurrence group). The second group included patients who had no episodes of relapse as of the last follow-up (the non-recurrence group).
The levels of NLR and PLR in the non-recurrent group were found to be significantly lower than those in the recurrent group (P < 0.05), and the level of LMR in the non-recurrent group was significantly higher than that in the recurrent group (P < 0.001) ().
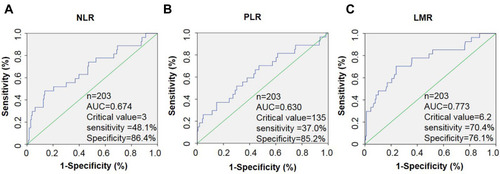
Considering the lack of an exact boundary value of NLR, PLR, and LMR in clinics, we used the ROC curve to determine the boundary value of the largest area. The AUC of NLR, PLR, and LMR were 0.674 (0.555–0.793), 0.630 (0.508–0.753) and 0.773 (0.673–0.874), respectively (). The optimal cutoff value was found to be 3.0 for the NLR (sensitivity = 48.1%, specificity = 86.4%), and 135.0 for the PLR (sensitivity =37.0%, specificity = 85.2%), and 6.2 for the LMR (sensitivity = 70.4%, specificity = 76.1%) (). Based on their respective cutoff values, 33 (16.3%) patients showed high NLR, 103 (50.7%) showed high PLR, and 134 (66.0%) showed high LMR.
Table 1 The Optimal Cut-Off Values Based on Disease-Free Survival (DFS)
Figure 2 The ROC curves of NLR, PLR, and LMR. (A) The optimal cutoff value was 3.0 for the NLR (sensitivity 48.1%, specificity 86.4%, AUC 0.674). (B) The optimal cutoff value was 135.0 for the PLR (sensitivity 37.0%, specificity 85.2%, AUC 0.630). (C) The optimal cutoff value was 6.2 for the LMR (sensitivity 70.4%, specificity 76.1%, AUC 0.773).
The analysis of the clinicopathological data revealed that high PLR and low LMR were significantly correlated with the lymph node metastasis, low LMR level was also significantly correlated with the clinical T stage ().
Table 2 Baseline Characteristics of the Patients According to the NLR, PLR and LMR
Correlations Among NLR, PLR, and LMR Values and pCR
In order to explore the relationship between the inflammatory factors and the sensitivity of chemotherapy, we further analyzed the correlation between inflammatory factors and pCR after neoadjuvant chemotherapy. Overall, pCR was defined as the absence of tumor cells in the breast (breast pCR) and the axillary lymph nodes (axillary pCR) after neoadjuvant chemotherapy. The results of the study revealed that the levels of LMR were significantly related to the axillary pCR and overall pCR in the neoadjuvant chemotherapy patients. Neoadjuvant chemotherapy patients with low LMR showed higher pCR rates and better chemotherapy outcomes ().
Table 3 Analysis of the Correlation Between Inflammatory Factors and pCR
Low LMR Indicated Poor Prognosis in NAC Breast Cancer Patients
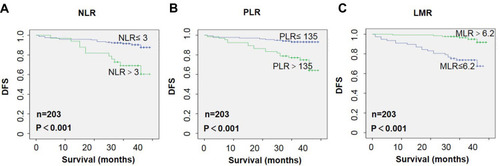
During a median follow-up of 31 months (range: 1–39 months), 27 patients (13.3%) showed local or distant metastasis. First, we performed a single-factor analysis to filter the statistically significant variables. In univariate analysis, we tested the clinicopathological parameters related to DFS as variables, including the age, menstrual status, family history, T-stage, lymph node metastasis, breast cancer subtypes, radiotherapy, and inflammation markers NLR, PLR, and LMR. Univariate analysis revealed that the age, NLR, PLR, LMR, clinical T-stage, HER-2, overall pCR and lymph node metastasis were the factors affecting the prognosis of breast cancer (). The Kaplan–Meier survival curve indicated that the prognosis of breast cancer patients with different levels of NLR, PLR, and LMR is significantly different ().
Table 4 Prognostic Factors for Postoperative Disease-Free Survival in Breast Cancer
Figure 3 Kaplan–Meier analyses for disease-free survival (DFS) of all 203 patients. (A, B) An elevated neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) indicating poor DFS following surgical resection. (C) A low lymphocyte–monocyte ratio (LMR) indicating poor DFS.
The inclusion of the variables of univariate analysis (P<0.05) into the Cox regression model revealed that LMR, Her-2 and lymph node metastasis acted as independent prognostic factors for the DFS.
Our cumulative results indicated that the patients with low LMR (mean DFS: 31.2 vs 38.1 months; HR: 5.660, P = 0.001), Her-2 positive (mean DFS: 33.8 vs 36.7 months; HR: 2.594, P = 0.025), and lymph node metastasis N2–3 (mean DFS: 32.5 vs 38.1 months; HR: 4.930, P = 0.004) have poor DFS ().
Discussion
There has been a total of approximately 2.1 million newly diagnosed cases of breast cancer in 2018 across the world.Citation16 Breast cancer has become a common tumor that seriously endangers women’s physical and mental health as well as their life.Citation17 Postoperative recurrence and metastasis of breast cancer is one of the important causes of death. Screening early scientific and effective predictors of breast cancer patients play an extremely important role in reducing the postoperative recurrence and metastasis and thereby in improving the survival rate.
In-depth analysis of tumor indicated that inflammatory cells play an important role in tumorigenesis and prognosis; especially, specific responses of the body to inflammation affect the prognosis of the cancer patients.Citation18 The greater the malignant degree of the tumor, the more serious is the necrosis and disintegration of the surrounding tissues and the more obvious is the non-specific inflammatory reaction. Notably, the inflammatory mediators can destroy the stability of the normal intracellular environment, which leads to abnormal cell proliferation and further cell deterioration. The relationship among the ratio of the number of neutrophils, platelets, and monocytes in the peripheral blood cells and the prognosis of tumor patients has been studied in several cancers.Citation19 For instance, NLR, PLR, and LMR are all markers related to inflammation that have been reported in relation to tumor and infection. Most of these markers have been widely used as clinical predictors of different diseases.Citation20–Citation22
In this study, we demonstrated the clinical values of NLR, PLR, and LMR in predicting the prognosis of breast cancer. Univariate analysis revealed that the levels of these 3 markers were significantly correlated with the DFS. However, after multivariate adjustment, low LMR was found to be independent inflammatory markers associated with poor DFS, albeit NLR and PLR showed no significant correlation with disease prognosis.
NLR is a common index that reflects the level of inflammation and immunity, and it has been studied in several types of tumors. The increase of the NLR level indicates a decrease in the lymphocyte level or an increase in the neutrophil count relative to that in normal patients. NLR has been proven to be closely associated with the prognosis of tumor patients, with higher PLR suggesting poor prognosis in lung cancer,Citation11 ovarian cancer,Citation23 colorectal cancer,Citation9,Citation24 osteosarcomaCitation25 and gastric cancer.Citation10 Some studies have been conducted on the clinical significance of PLR and NLR in the prognosis of breast cancer.Citation12,Citation26–Citation30
A past study reported that high PLR levels are suggestive of a poor prognosis only in people with normal lymphocyte count, while NLR is an independent predictor of 5-year survival in all populations. The prediction effect of NLR was found to be better than that of PLRCitation29 The prognostic value of NLR reported by another study suggested that NLR is a prognostic indicator for patients with TNBC.Citation28 However, the reports of different studies on this aspect are inconsistent. PLR is an independent predictor of breast cancer. The clinical prognostic effect of PLR is better than that of NLR and LMR.Citation12 In this study, the level of NLR in patients with postoperative recurrence and metastasis was higher than that in patients with no recurrence and metastasis, and the average survival time of patients with a high level of NLR was shorter (mean survival duration: 30.9 vs. 36.8 months). However, after further multivariate correction, NLR was not found to be an independent prognostic factor for DFS of breast cancer. Some of the differences in these reports may be attributed to the differences in the applied inclusion criteria, the races of the study population, and the study design.
An in-depth analysis of the peripheral blood cells revealed that platelets not only play an important role in the process of hemostasis but that they also play a certain role in the prognosis of malignant tumors and inflammatory diseases. Tumor cells can promote platelet proliferation, while platelets can provide raw materials for angiogenic factors, which are beneficial to the migration of intravascular tumor cells.Citation31,Citation32 Past studies have shown that PLR is closely related to the survival outcome of patients with colorectal cancer, gastric cancer, and liver cancer.Citation10,Citation24,Citation33 High levels of PLR were associated with a relative increase in the count of platelets and a relative decrease in the count of lymphocytes. Therefore, the non-specific inflammatory response induced by malignant tumor is expected to increase the platelet count and lymphocytopenia in patients, promote tumor development, and, eventually, contribute to the poor prognosis of tumor patients. The abovementioned clinical studies reported that PLR is a significant prognostic index with a good prospect of clinical application.Citation34 Our results also verify the prognostic value of PLR. Despite no significant correlation found between PLR and lymph node metastasis or tumor size in our study, PLR showed a statistical difference in the survival analysis. Multivariate analysis revealed that PLR was not a prognostic indicator of DFS in neoadjuvant chemotherapy patients. This result is somewhat different from those of some previous studies, although the significance of PLR cannot be denied. The result obtained may be related to the nature of our enrolled population and the sample size.Citation27,Citation35
Low LMR is often used as an indicator of poor prognosis in several types of tumors.Citation22,Citation36,Citation37 Lymphocytes are the basic immune factors for the host to resist all types of malignant tumors. Lymphocytes can infiltrate into the tumor microenvironment and express various factors, such as tumor-infiltrating lymphocytes, thereby affecting and destroying the proliferation and metastasis of tumor cells. They also play a role in immune protection.Citation38 In addition, monocytes can differentiate into tumor-associated macrophages and become the main component of the tumor microenvironment,Citation39 while tumor-associated macrophages possess immunosuppressive effect and can promote angiogenesis around the tumor, tumor spread, and metastasis.Citation40 Therefore, the level of LMR, which is a combined indicator of lymphocytes and monocytes, can be considered as a potential prognostic marker. In this study, the level of LMR in the non-recurrence group was found to be significantly higher than that in the recurrence group. The results of multivariate Cox regression analysis revealed that the LMR value affected the DFS of breast cancer, indicating that patients with low LMR have a poor prognosis (mean survival duration: 31.2 vs 38.1 months; HR = 5.660, P= 0.001).
There are some limitations of our study. For instance, data were collected from a single center, overall survival data were lacking, the follow-up time was short, and the relative sample size was small, among others. Furthermore, the count of inflammatory cells in the peripheral blood may be affected by the patient’s physical status, drug regime, diet, and other inflammatory factors. Thus, further analyses of relevant retrospective studies and large-scale prospective studies are warranted to verify the preliminary results of our study.
We believe that the examination of the relationship among peripheral blood inflammatory markers and tumor recurrence and metastasis would be helpful to further understand the mechanisms involved in breast cancer metastasis so as to provide a reliable theoretical basis in our search for valuable biomarkers.
In summary, the preoperative levels of LMR in the peripheral blood are significantly correlated with the DFS of patients with breast cancer. Low levels of LMR may indicate a higher probability of recurrence and metastasis in breast cancer patients. Thus, it is recommended that postoperative adjuvant therapy be actively improved and relevant indicating factors be monitored and reexamined regularly. LMR is an indicator of the peripheral blood, which is convenient, economical, simple, and reliable to apply in a clinical setting. They are expected to provide a new method for predicting the prognosis of patients with breast cancer and thereby yielding certain guiding value for clinical treatment and prognosis.
Conclusion
The recurrence and metastasis of breast cancer remain a clinical issue that needs to be resolved urgently, which necessitates the establishment of meaningful and valuable predictors. In our research, we systematically analyzed the prognostic value of inflammatory cell markers in breast cancer patients. Our results revealed that low LMR indicated poor prognosis. Although NLR and PLR are not prognostic indicators of DFS in multivariate analysis, the values of NLR and PLR were higher in the recurrence and metastasis groups and related to the tumor size, suggesting that NLR and PLR have certain clinical significance. In general, LMR is a better prognostic marker than NLR and PLR in predicting the prognosis of breast cancer. Thus, we believe that the use of LMR as a prognostic marker would be both economical and convenient for breast cancer patients.
Abbreviations
AUC, area under curve; 95% CI, 95% confidence interval; DFS, disease-free survival; OS, overall survival; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; LMR, lymphocyte–monocyte ratio; TNBC, triple negative breast cancer; HR, hazard ratio; pCR, pathological complete response; cTNM, clinical tumor-node-metastasis; SPSS, Statistical Product and Service Solutions.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This research was conducted in accordance with the standards set out in the Declaration of Helsinki. This study was approved by the Medical Ethics Committee of Henan Cancer Hospital (Research Approval Number: 2,020,051,313). The Medical Ethics Committee of Henan Cancer Hospital did not require patients to agree to review their medical records (On the premise of not disclosing the privacy of patients, doctors can use it for clinical research).
Disclosure
The authors report no conflicts of interest for this work.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi:10.1016/j.ejca.2018.07.00530100160
- Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017;15(10):1216–1223. doi:10.6004/jnccn.2017.015828982747
- Fahad Ullah M. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64. doi:10.1007/978-3-030-20301-6_431456179
- Karki R, Man SM, Kanneganti TD. Inflammasomes and cancer. Cancer Immunol Res. 2017;5(2):94–99. doi:10.1158/2326-6066.CIR-16-026928093447
- Yang L, Lin PC. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 2017;47:185–195. doi:10.1016/j.semcancer.2017.08.00128782608
- Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–184. doi:10.1016/j.semcancer.2017.08.00228823497
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. doi:10.1038/nrc.2016.5227282249
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi:10.1182/blood-2014-08-53158226109205
- Peng H-X, Yang L, He B-S, et al. Combination of preoperative NLR, PLR and CEA could increase the diagnostic efficacy for I-III stage CRC. J Clin Lab Anal. 2017;31(5):e22075. doi:10.1002/jcla.22075
- Liu C, Li X. Stage-dependent changes in albumin, NLR, PLR, and AFR are correlated with shorter survival in patients with gastric cancer. Clin Lab. 2019;65(9). doi:10.7754/Clin.Lab.2019.190132
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.02428838390
- Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936. doi:10.1371/journal.pone.020093630048474
- Azab B, Mohammad F, Shah N, et al. The value of the pretreatment neutrophil lymphocyte ratio vs. platelet lymphocyte ratio in predicting the long-term survival in colorectal cancer. Cancer Biomark. 2014;14(5):303–312. doi:10.3233/CBM-14041625171472
- Marin Hernandez C, Pinero Madrona A, Gil Vazquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476–483. doi:10.1007/s12094-017-1732-028785911
- Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. doi:10.1186/s12885-018-4832-530285668
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- Loke SY, Lee ASG. The future of blood-based biomarkers for the early detection of breast cancer. Eur J Cancer. 2018;92:54–68. doi:10.1016/j.ejca.2017.12.02529413690
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.01321376230
- Shi M, Zhao W, Zhou F, et al. Neutrophil or platelet-to-lymphocyte ratios in blood are associated with poor prognosis of pulmonary large cell neuroendocrine carcinoma. Transl Lung Cancer. 2020;9(1):45–54. doi:10.21037/tlcr.2020.01.17
- Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi:10.1007/s12032-014-0305-025355641
- Meng X, Chang Q, Liu Y, et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: a posteriori and big-data-based. J Clin Lab Anal. 2018;32(2):e22228. doi:10.1002/jcla.22228
- Rajwa P, Zyczkowski M, Paradysz A, Bujak K, Bryniarski P. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci. 2018;22(10):3027–3037. doi:10.26355/eurrev_201805_1506029863247
- Yilmaz E, Coskun EI, Sahin N, Ciplak B, Ekici K. MPV, NLR, and platelet count: new hematologic markers in diagnosis of malignant ovarian tumor. Eur J Gynaecol Oncol. 2017;38(3):346–349.29693870
- Solak Mekic M, Pedisic I, Sobat H, et al. The role of complete blood count parameters in patients with colorectal cancer. Acta Clin Croat. 2018;57(4):624–629. doi:10.20471/acc.2018.57.04.0331168198
- Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016;14:127. doi:10.1186/s12957-016-0889-227125872
- Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. doi:10.1038/bjc.2015.18326022929
- Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One. 2015;10(11):e0143061. doi:10.1371/journal.pone.014306126580962
- Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014;7:1743–1752. doi:10.2147/OTT.S6965725328407
- Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. doi:10.1007/s12032-012-0432-423283648
- Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine. 2018;97(49):e13340. doi:10.1097/MD.000000000001334030544398
- Toiyama Y, Inoue Y, Kawamura M, et al. Elevated platelet count as predictor of recurrence in rectal cancer patients undergoing preoperative chemoradiotherapy followed by surgery. Int Surg. 2015;100(2):199–207. doi:10.9738/INTSURG-D-13-00178.125692418
- Ishii Y, Hamashima T, Yamamoto S, Sasahara M. Pathogenetic significance and possibility as a therapeutic target of platelet derived growth factor. Pathol Int. 2017;67(5):235–246. doi:10.1111/pin.1253028393435
- Suner A, Carr BI, Akkiz H, et al. C-reactive protein and platelet-lymphocyte ratio as potential tumor markers in low-alpha-fetoprotein hepatocellular carcinoma. Oncology. 2019;96(1):25–32. doi:10.1159/00049247330336489
- Zhu Y, Si W, Sun Q, Qin B, Zhao W, Yang J. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget. 2017;8(1):1023–1030. doi:10.18632/oncotarget.1371427906679
- Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi:10.1038/bjc.2014.16324675383
- Kumarasamy C, Sabarimurugan S, Madurantakam RM, et al. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine. 2019;98(24):e14834. doi:10.1097/MD.000000000001483431192906
- Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. Onco Targets Ther. 2017;10:871–877. doi:10.2147/OTT.S12491528243122
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi:10.1093/annonc/mdu45025214542
- Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Sci World J. 2014;2014:521754. doi:10.1155/2014/521754
- Shand FH, Ueha S, Otsuji M, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci U S A. 2014;111(21):7771–7776. doi:10.1073/pnas.140291411124825888
