Abstract
Malignant bowel obstruction (MBO) is a frequent complication in advanced cancer patients, especially in those with abdominal tumors. Clinical management of MBO requires a specific and individualized approach that is based on disease prognosis and the objectives of care. The global prevalence of MBO is estimated to be 3% to 15% of cancer patients. Surgery should always be considered for patients in the initial stages of the disease with a preserved general status and a single level of occlusion. Less invasive approaches such as duodenal or colonic stenting should be considered when surgery is contraindicated in obstructions at the single level. The priority of care for inoperable and consolidated MBO is to control symptoms and promote the maximum level of comfort possible. The spontaneous resolution of an inoperable obstructive process is observed in more than one third of patients. The mean survival is of no longer than 4–5 weeks in patients with consolidated MBO. Polymodal medical treatment based on a combination of glucocorticoids, strong opioids, antiemetics, and antisecretory drugs achieves very high symptomatic control. This review focuses on the epidemiological aspects, diagnosis, surgical criteria, medical management, and factors influencing the spontaneous resolution of MBO in advanced cancer patients.
Introduction
Malignant bowel obstruction (MBO) is a frequent complication in patients with advanced cancer, especially of digestive or gynecological origin. Bowel obstruction is any mechanical or functional obstruction of the intestine that prevents physiological transit and digestion. This is a generic definition that is widely accepted by most authors and includes very different benign or malignant clinical situations. An international consensus group recently proposed an operative, specific definition of MBO with the aim of unifying the diagnostic criteria of this complication. According to this definition, the diagnostic criteria of MBO are: (a) clinical evidence of bowel obstruction, (b) obstruction distal to the Treitz ligament, (c) the presence of primary intra-abdominal or extra-abdominal cancer with peritoneal involvement, and (d) the absence of reasonable possibilities for a cure.Citation1
The clinical management of MBO requires a specific and individualized approach based on disease prognosis and the objectives of care. Palliative surgery – the only treatment able to restore digestive transit in consolidated MBO – must always be considered, but it should not be routinely performed. The decision making process is difficult, especially in advanced phases of cancer and depends on the level of obstruction, the presence of single or multiple occlusive levels, the extent of the cancer, associated comorbidities, and the performance status of the patient. When surgical or minimally invasive surgical approaches are not possible, a devastating clinical picture develops, which leads to intense symptoms, rapid deterioration of the patient’s general status, and a short life expectancy. At this time, palliative medical treatment aimed at reducing symptoms and providing the highest level of comfort possible becomes the priority of care.
This review focuses on the epidemiological aspects, diagnosis, surgical criteria, medical management, and factors influencing the spontaneous resolution of MBO in advanced cancer patients.
Epidemiological aspects
The global prevalence of MBO is estimated to range from 3% to 15% of cancer patients, reaching 20%–50% in patients with ovarian cancer and 10%–29% in patients with colon cancer.Citation2–Citation5 Primary cancers of abdominal origin that most frequently produce MBO are those of the colon (25%–40%), ovary (16%–29%), stomach (6%–19%), the pancreas (6%–13%), the bladder (3%–10%), and the endometrium (3%–11%).Citation2,Citation5–Citation9 The primary cancers of extra-abdominal origin most frequently leading to MBO due to peritoneal infiltration are those of the breast (2%–3%) and melanoma (3%).Citation2,Citation5 The mean age of the patients presenting MBO is 61 years (from 58–65 years) and 64% (59%–69%) are women (). The mean time from the initial diagnosis of cancer to MBO is 14 months (13–15 months). The diagnosis of cancer coincides with the episode of MBO in 22% (13%–32%) of the cases in surgical series and in 2% in studies of patients with advanced or terminal diseases. One quarter of advanced and terminal cancer patients with this complication have presented previous episodes of intestinal obstruction (mean 1.37 subocclusive episodes per patient, SD ± 0.7).Citation5 The spontaneous resolution of the occlusive picture occurs in 36% (31%–42%) of patients with inoperable MBO. In these cases, the rate of recurrence of obstruction is greater than 60%. In a series of surgical cases, the average survival ranges from 3 to 8 months, including patients treated with palliative surgery. In advanced cancer patients with inoperable MBO, the mean survival rate is no longer than 4–5 weeks. Likewise, six-month life expectancy is approximately 50% in surgical patients and 8% in patients with inoperable MBO.Citation5–Citation9
Table 1 Characteristics and outcome of malignant bowel disease
The higher overall frequency of MBO in women can be explained by the high incidence of this complication in ovarian cancers. The reason for the better survival observed in the surgical series is obvious; the MBO is diagnosed at an earlier stage of the disease when palliative surgery is still an option in most of the cases (80%–40%).
Although these data are of great interest, the current research is far from revealing the overall incidence or prevalence of this complication in cancer patients. The global context of these studies is limited since most of these data are based on selected subpopulations or retrospective case series with different outcomes and heterogeneous diagnostic criteria.
Physiopathology
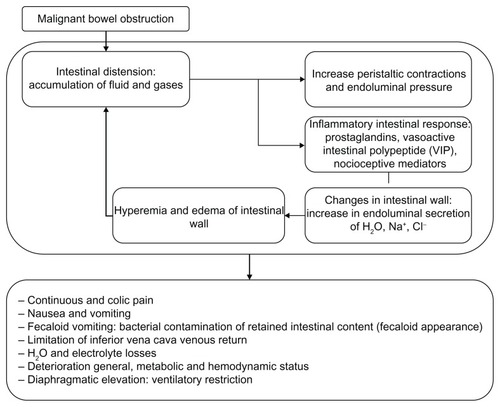
MBO may appear at any time during the evolution of the disease, but is more frequent in cases of advanced cancer (). Obstruction may originate in the small (61%) or large bowel (33%) or in both simultaneously (20%).Citation10 Obstruction may be complete or partial and may appear as a subocclusive crisis or may involve one or multiple intestinal levels. In advanced and inoperable patients, multiple occlusive levels are presented in 80% of cases and peritoneal carcinomatosis is previously diagnosed in more than 65% of cases.Citation5 Abdominal tumor growth may lead to MBO by extrinsic intestinal compression, endoluminal obstruction, intramural infiltration, or extensive mesenteric infiltration (). Intraluminal tumors may occlude the bowel lumen or provoke intussusception. Intramural infiltration through the mucosa may obstruct the lumen or impair peristaltic movements. Mesenteric and omental tumor involvement may angulate the bowel and provoke extramural bowel occlusion. Infiltration of the enteric or celiac plexus may cause severe impairment in peristalsis and consequent obstruction due to dysmotiliy. Factors that may favor the appearance of MBO, but are not directly dependent on abdominal tumor growth include paraneoplastic neuropathies, chronic constipation, intestinal dysfunction induced by opioids, inflammatory bowel disease, renal insufficiency, dehydration, mesenteric thrombosis, surgical adherences, and radiogenic fibrosis.
Table 2 Physiopathology of MBO (I)
Fluid retention and intestinal gases proximal to the occlusive level produce a marked increase in endoluminal intestinal pressure. This abdominal distension favors the release of 5-HT3 by the intestinal enterochromaffin cells which, in turn, activates the enteric interneuronal system through its different mediators (P substance, nitric oxide, acetylcholin, somatostatin, and vasoactive intestinal peptide). This stimulates the secretomotor neurons that are especially mediated by the vasoactive intestinal peptide, which leads to splanchnic vasodilatation and hypersecretion of the cells of the intestinal crypts. The consequences of these phenomena are the appearance of intense intestinal edema, an increase in the secretions retained, and endoluminal pressure, all of which are mechanisms that perpetuate the physiopathological process of MBO.Citation2,Citation7,Citation11–Citation13
Clinical manifestations
The onset of MBO may be subacute with the presence of colic pain, abdominal distension, nausea, and vomiting, which spontaneously cease (subocclusive crisis). The prevalence of symptoms in consolidated MBO are nausea 100%, vomiting 87%–100%, colic pain 72%–80%, pain due to distension 56%–90%, and the absence of stools or emission of gases in the previous 72 hours in 85%–93%.Citation2,Citation5,Citation7,Citation8 In upper MBO, the nausea is intense and presents early, the vomiting is numerous and with an aqueous, mucous or biliary appearance and has little odor. Vomiting in lower obstruction usually occurs later, is dark, and has a strong odor. Bacterial liquefaction of the retained intestinal content in the zone proximal to the obstruction confers the characteristic appearance and smell of fecaloid vomit. Cases with partial obstruction may present liquid stools due to bacterial liquefaction of the digestive content and intestinal hypersecretion. The colic pain is due to giant peristaltic waves and spasms in the bowel with increased endoluminal pressure and no possibility of effective transit. Intestinal distension and tumoral infiltration of the abdominal structures are responsible for the continuous pain.Citation2,Citation10,Citation14 During physical examination, abdominal distension is noted and is more marked in lower obstructions and in the changes in peristaltism. At the onset, borborygmus, fighting peristalsis, may be heard on auscultation. On consolidation of MBO, the peristalsis may decrease or even cease to present isolated metallic sounds thereafter due to hydroaerial tension on auscultation. In patients with advanced cancer, MBO is also associated with anemia (70%), hypoalbuminemia (68%), alterations in hepatic enzymes (62%), dehydration and prerenal renal dysfunction (44%), cachexia (22%), ascites (41%), palpable abdominal tumor masses (21%), and marked cognitive deterioration (23%).Citation5
Radiological diagnosis
Plain radiography of the abdomen in a biped position is the imaging method of choice for the detection of suspected MBO and is also used to assess the patient’s evolution after treatment. The radiological signs of MBO are distension of the intestinal loops, fluid retention, and gases with the presence of air-fluid levels in the zone proximal to the occlusion as well as a reduction in gas and stools in the segments distal to the obstruction. In upper occlusions, distension of the loops and air-fluid levels may be absent. Radiological techniques using contrast may be necessary to evaluate the surgical approach. Barium contrast provides excellent radiological definition, but is not absorbed and may become impacted, thereby compromising other tests or endoscopic maneuvers. In many cases, these imaging tests are limited by the presence of nausea and vomiting, which may prevent the ingestion of radiographic contrast or increase the risk of aspiration pneumonia.
Gastrografin provides similar radiologic definition and its hyperosmotic character may, in some cases, favor the resolution of obstructions in the small bowel. In fact, a recent meta-analysis confirms a reduction in the need for surgical intervention and hospital stay in patients with occlusion after the administration of Gastrografin.Citation15 Computerized tomography (CT) provides a high possibility for the diagnosis of the extension of the neoplasm and, on many occasions, the level of obstruction. The diagnostic sensitivity of CT in determining the obstruction level is of 93%, with a specificity of 100% and a predictive value of 83%–94%, which is significantly higher than that provided by abdominal echography and simple radiology.Citation16,Citation17 The precision of the diagnosis of peritoneal carcinomatosis by CT is scarce, with a predictive value of less than 20% if the peritoneal lesions are less than 0.5 cm or if they are located in the pelvis, mesenterium, or small bowel.Citation18,Citation19 The sensitivity of magnetic resonance (MR) in diagnosing of the extension of the neoplasm and the level of the obstruction is 93%–95%, with a specificity of 63%–100% and a predictive value of 81%–96%. One study on the diagnostic possibilities of MR compared with CT in MBO, showed the significant superiority of MR in terms of sensitivity, specificity, and predictive value.Citation21
In summary:
– Plain abdominal radiography is sufficient in most cases to confirm the diagnosis of MBO.
– One should consider the use of contrast radiography, CT, or MR, when the patient’s general condition was good prior to the complication, the extent of the cancer is unknown, when a unique occlusive level is suspected, and when the cancer is potentially operable.
– Contrast radiography determines, with a reasonable degree of accuracy, the site or sites of obstruction and the degree of obstruction. It may rule out a bowel occlusion due to motility disorder (opioid-induced intestinal dysfunction, pseudo-obstruction).
– CT or MR should be reserved for cases where precise radiological information is needed to facilitate adequate decision making regarding surgery (ie, tumor characteristics in the site of obstruction, the presence of lymph nodes, and the intra- and extra-abdominal metastatic spread).
Treatment
The decision making process in advanced oncologic patients requires individualized evaluation based on the extension of the neoplasm, the global prognosis, the possibility of specific cancer treatments, associated comorbidities, the general status, and the particular options available to the duly informed patient. Possible treatments include surgery, endoscopic palliation, digestive aspiration, and symptomatic palliative pharmacologic therapy.
Surgery in MBO
The aim of surgery is to reestablish digestive permeability, and should always be considered in patients in the initial stages of the disease, with a preserved general status and a single level of occlusion. Studies involving a series of surgical cases of MBO have shown a 30-day mortality of 25% (9%–40%), postsurgical morbidity of 50% (9%–90%), a rate of reobstruction of 48% (39%–57%), and a median survival of 7 months (2–12 months).Citation6,Citation9,Citation10,Citation23–Citation28
Age, advanced disease, malnutrition, and deterioration in the general status are considered factors of poor prognosis even in cases where surgery may technically be possible.Citation10,Citation14 A study on patients with colon cancer undergoing surgery for MBO reported an increase in surgical mortality associated with age, with an OR of 1.85 for each 10-year interval of age above 65 years. Using the American Society of Anesthesia scale to measure deterioration of general status, surgical mortality (Odds Ratio 3.3) increased in patients with a score ≥ 2, compared to those with a score < 2.Citation29 Furthermore, surgical mortality is three-fold greater in patients with deficient nutritional status and hypoalbuminemia.Citation9,Citation30 The presence of ascites greater than 3000 mL and palpable tumor masses is statistically associated with a poor surgical prognosis.Citation9,Citation30
The mean survival for patients with ovarian cancer undergoing surgery for MBO is significantly higher in those without ascites and previous chemotherapy.Citation31–Citation33 Pelvic and abdominal radiotherapy prior to MBO is associated with a high rate of surgical complications and an increase in operative mortality in patients with gynecological cancer, a fact that has not been confirmed in cancer of other etiologies.Citation9,Citation32 Medical treatment prior to surgery for MBO is based on absolute diet, parenteral hydration, nasogastric aspiration, and antiemetic and analgesic drugs. The aims of these measures are to control the symptoms, reestablish the hydroelectrolytic balance, favor spontaneous resolution, and gain the time necessary to establish a diagnostic process to facilitate individualized surgical decisions. With these measures, adequate control of the symptoms is achieved in 80% of cases if absolute diet and nasogastric aspiration are maintained. It is reasonable to assume that nasogastric aspiration at the onset of the obstruction may favor spontaneous resolution since it drastically reduces endoluminal pressure. However, longterm nasogastric aspiration is uncomfortable for the patient and has intense secondary effects (eg, esophagitis, gastroesophageal reflux, nasal erosions, and bronchoaspiration). In a series of surgical cases, spontaneous resolution is presented in 30% of patients within a mean time of less than 8 days after diagnosis. For this reason, and considering the hypothesis that nasogastric aspiration may improve the rate of spontaneous resolution, there is no reason to maintain this measure over a prolonged period of time greater than the possible estimations of resolution in patients in whom surgery has been ruled out and in whom adequate control of the symptomatology may be obtained with intensification of palliative treatment.
Taking these data into account, most researchers consider the following factors to limit the indication of surgery in MBO: elderly age, malnutrition or cachexia, peritoneal carcinomatosis, multiple occlusive levels, palpable abdominal masses, previous malnutrition, refractory ascites, symptomatic extra-abdominal metastatic disease, deteriorated general status, renal or hepatic insufficiency, previous abdominal pelvic radiotherapy, and the absence of the possibility for specific oncologic treatments.Citation2,Citation10
Endoscopic palliation and stents
The use of stents has spread in recent years as an endoscopic alternative for the treatment of proximal small bowel obstructions and the colon. The rate of symptomatic control and tolerance of ingestion after the placement of a duodenal stent for duodenal obstruction is 90%. Studies comparing surgical gastrojejuostomy with the placement of a duodenal stent showed no significant differences in technical success (100% versus 90%), in early (6% versus 7%) or late complications (17% versus 18%), and in persistent occlusive symptomatology (9% versus 8%), respectively. The mean survival is 105 days for the group treated with a duodenal stent compared to 165 days in the surgical group.Citation34 The most frequent late complications after duodenal stent placement are hemorrhage or perforation (1.2%), migration of the device (5%), and stent obstruction (18%),Citation35 which is sometimes susceptible to repermeabilization using a ND Yang laser.Citation36 Successful insertion of a stent in colon cancer ranges from 80%–100% of the cases and improves the symptomatology in more than 75% of the patients. The mean duration of colonic stent permeability is 106 days (66–88 days). The most frequent complications of this technique are immediate or differed perforation (4.5%), migration (11%), and obstruction (12%).Citation37,Citation38 Recurrence of obstruction due to tumor growth through the mesh or endoluminal at the ends of the stent may require the placement of a second additional stent after repermeabilizing the lumen with a laser or photodynamic therapy.
In summary, self-expanding metal stents can be considered a good option in patients with a single point of obstruction in whom palliative surgery has been ruled out or in those who do not want to undergo surgery.
Percutaneous endoscopic gastrostomy by aspiration
As mentioned previously, longterm aspiration using a nasogastric tube is uncomfortable and may produce severe secondary effects. Percutaneous endoscopic gastrostomy may be a highly effective and safe alternative for patients in whom surgery is ruled out and in those who cannot control their symptoms without maintaining digestive aspiration. In MBO secondary to advanced ovarian cancer, the success of percutaneous endoscopic placement is 94% (94%–98%), which achieves adequate control of symptomatology in 84% of the patients during a mean time of 70 days, even in cases presenting peritoneal carcinomatosis, ascites, or gastric infiltration.Citation39,Citation40
Parenteral nutrition in MBO
The aim of total parenteral nutrition (TPN) is the recovery of nutritional status in patients who are candidates for surgery. The indication for TPN in advanced oncological patients with inoperable MBO is more controversial. TPN is an invasive technique that requires specific training for use and frequent monitoring of electrolytes and hydration. It also predisposes patients to infection (central venous access), thrombosis, diarrhea, liver dysfunction, and hyperglycemia. The scarce studies that have evaluated the efficacy of longterm TPN for inoperable MBO have reported a mean survival rate of 4–6 months, a rate of complications associated with the procedure greater than 13%, and maintained stability of nutritional parameters of only 2–3 months prior to death. These studies concluded that only 30% of the patients surviving for more than 3 months, benefit from the application of TPN.Citation41,Citation42 Routine use of TPN in MBO is not recommended in inoperable patients. The indication for long term TPN should be made with caution and should be reserved for patients with a preserved general status prior to MBO, slow growing tumors, the possibility of response to chemotherapy, reasonable expectations of survival longer than 3 months, and without severe extra-abdominal complications by the extension of the neoplasm.
Palliative treatment
In 1985, Baines et al demonstrated that pharmacologic treatment, specifically palliative treatment for inoperable MBO, may provide adequate symptomatic control with measures aimed at maintaining the maximum comfort possible.Citation4 Palliative treatment for MBO has the following objectives: control of nausea, vomiting, and pain, allowing minimum food intake, avoiding or withdrawing nasogastric aspiration, and favoring hospital discharge under control at home or in healthcare centers. This treatment is based on the use of antiemetic, potent analgesic, glucocorticoid, and antisecretor drugs in combination with the most comfortable route of administration to allow its application within the homecare setting.Citation2,Citation4
More than 80% of patients with MBO present continuous pain and high-intensity colic.Citation4,Citation5,Citation7,Citation8 The administration of analgesics for the treatment of MBO should be adjusted to the analgesic scale of the World Health Organization (WHO), which has demonstrated an efficacy rate greater than 80% in cancer patients.Citation43–Citation46 According to the European Society of Palliative Care and the WHO, morphine is the opioid of choice in the absence of controlled clinical trials comparing different opioids in this indication.Citation47 Some authors have reported that oxycodone may be more effective than other opioids for visceral pain treatment given its action on the kappa opioid receptors, although this has yet to be confirmed in controlled clinical studies.Citation48 A meta-analysis of five controlled clinical trials by Tassinari et al in 2009 confirmed that fentanyl is a potent opioid that produces less constipation as a secondary effect.Citation49 A recent descriptive analysis of MBO in advanced cancer shows that more than 60% of the patients were treated with potent opioids prior to the occlusive episode and more than 80% required these drugs for analgesia during the episode. In this study, no statistically significant differences were observed in the rate of spontaneous resolution under symptomatic treatment among patients treated with a potent opioid prior to or during the episode of MBO versus those who did not receive this type of drug.Citation5 The opioid dose should be titrated individually for adequate pain relief. The subcutaneous, intravenous, sublingual, or transdermal route for opioid administration should be used frequently because nausea and vomiting do not allow for oral administration.
Antiemetic treatment uses drugs from three pharmacological groups: anti-cholinergic, dopamine antagonists, and serotonin antagonists (5-HT3). The dopamine antagonists are divided into benzamides (metoclopramide), butyrophenones (haloperidol), and phenothiazines (chlorpromazine). Metoclopramide blocks the dopamine receptors (D2) at the central and peripheral level. Its action facilitates the release of acetylcholine and at high doses (>120 mg/day) antagonizes the 5-HT3 receptors. The mixed, central, and peripheral actions confer an antiemetic and prokinetic digestive effect on metoclopramide. The usual metoclopramide doses range from 40–120 mg/day. Haloperidol and phenothiazines (chlorpromazine and levomepromazine) are neuroleptic drugs that block the dopamine receptors at the central level only. They have a potent antiemetic, but not prokinetic, action. Among these drugs, haloperidol is considered the most ideal because it produces less somnolence and anticholinergic effects. Haloperidol doses range from 5–15 mg/day, which can be administered in divided doses subcutaneously or intravenously, or by continuous subcutaneous or intravenous infusion. Scopolamine and hyoscine butylbromide are anticholinergic drugs that have an antiemetic action that acts by blocking acetylcholine at the central and peripheral levels, and the peripheral level, respectively, in association with a clear antisecretor effect. Hyoscine butylbromide doses range from 40–120 mg/day. The serotonin (5-HT3) receptor antagonists, as ondansetron or granisetron, can be useful for emesis control in the treatment of MBO. A recent noncontrolled Phase II study demonstrated an index greater than 80% for the antiemetic control of MBO using granisetron (5-HT3 receptor antagonist), even in cases that have not responded to typical antiemetic treatment.Citation7 The ondansetron dose ranges from 12–24 mg/day, and the granisetron dose ranges from 1–3 mg/day. These drugs are usually well tolerated. Headache, dizziness, and constipation are the most commonly reported side effects associated with their use.
Glucocorticoids possess an antiemetic action, the mechanism of which is not well known, and an anti-inflammatory action that reduces peritumoral edema. Therefore, most researchers recommend glucocorticoids in the palliative treatment of MBO. A meta-analysis of three controlled clinical trials published in 1999 demonstrate that the use of glucocorticoids, particularly dexamethasone at a dose ranging from 6–16 mg, collaborates with the antiemetic action and favors the spontaneous resolution of MBO in advanced gynecological and digestive cancer. In this meta-analysis, the rate of spontaneous resolution was 62%–68% in patients treated with glucocorticoids compared to 33%–57% in those receiving placebo.Citation32,Citation50,Citation51
The objective of antisecretor drugs is to reduce intestinal hypersecretion, and secondarily, to improve nausea, vomiting, and pain. Anticholinergic drugs (scopolamine, hyoscine, and butylbromide) have traditionally been the antisecretors of choice. Octreotide, a somatostatin analog, provides a more specific and prolonged antisecretor effect. The pharmacologic activity of octreotide is mediated by the inhibition of the secretion of vasoactive intestinal peptides. This pharmacologic activity reduces hydroelectrolytic retention in the intestinal lumen, as well as gastric secretions, intestinal motility, biliary flow, splanchnic hypervascularization, and intestinal parietal edema. Furthermore, it increases the absorption of water and the production of intestinal mucous.Citation52,Citation53 Different studies on the effectiveness of octreotide at doses from 200–600 μg/day have shown a clear reduction in intestinal secretions, the eventual possibility of withdrawing the nasogastric tube, and a high grade of antiemetic and analgesic response with no relevant adverse effects.Citation2,Citation8,Citation54–Citation58 Two controlled clinical studies have compared the antiemetic, analgesic, and antisecretory efficacy of octreotide (300 μg/day) versus hyoscine butylbromide (60 mg/day) in the treatment of MBO. In both studies, the efficacy of octreotide was statistically greater in all the parameters of response (reduction in digestive hypersecretion and control of nausea and vomiting). Citation54–Citation56 A Phase II study demonstrated that a long-acting formulation of octreotide (LAR Depot) in combination with corticosteroids is useful and safe for the treatment of MBO due to peritoneal carcinomatosis.Citation59 A recent review of the literature concludes that despite the limited number of controlled clinical trials, octreotide is the antisecretory agent of choice for the treatment of MBO based on the results from 15 consistent studies and the experience acquired from 20 years of its use.Citation60 Histamine-2 antagonists and proton pump inhibitors are useful for reducing gastric secretions. A recent meta-analysis confirmed that ranitidine is more effective than proton pump inhibitors as an antisecretory agent. Based on these data, the authors hypothesized that ranitidine would be useful as an adjuvant drug in antisecretory therapy for the treatment of MBO and suggested the development of specific research to confirm these findings.Citation61,Citation62
The palliative treatment of MBO is polymodal and based on the combined use of different drugs active in controlling symptoms. According to most researchers and the recently published guidelines of clinical practice from the National Comprehensive Cancer Network,Citation63 the initial treatment for inoperable MBO is the combined use of analgesia with opioids, antiemetics, antisecretors, glucocorticoids, and hydratation with endovenous sera. It is reasonable to consider that the continuous infusion of fentanyl using an intravenous, subcutaneous route, or transdermic device may be the method of choice for its lesser influence on the intestinal motility and better tolerance in dehydrated patients. In complete MBO, the antiemetic of choice is haloperidol since the prokinetic effect of metoclopramide may paradoxically increase pain and nausea.Citation2 Antagonists of the 5-HT3 receptors (ondansetron or Granisetron) may be an alternative for patients who have had an inadequate response to previous antiemetic treatments.Citation7 The initial use of glucocorticoids is recommended due to their antiemetic effect and reduction of intestinal edema, which may facilitate the spontaneous resolution of the occlusive picture.Citation32,Citation50,Citation51 Most researchers recommend the early use of octreotide or an antisecretor drug due to its clear superiority over other anticholinergic drugs.Citation2,Citation54–Citation59 Nasogastric aspiration should only be considered for the treatment of inoperable MBO in the absence of a symptomatic response to polymodal palliative treatment. The rate of control for nausea, vomiting, and pain using different variations of this polymodal palliative treatment strategy in inoperable MBO is greater than 80%, with spontaneous resolution in more than 30% of cases.Citation2,Citation4,Citation5,Citation7,Citation8,Citation10,Citation56 The estimated median survival in cases of inoperable MBO is 1 month with a 6-month life expectancy rate of less than 8%.Citation5
Factors affecting the spontaneous resolution of inoperable MBO
The spontaneous resolution of the occlusive picture occurs in 30%–40% of patients with inoperable MBO.Citation5–Citation9,Citation64 Little is known about the factors that may influence the spontaneous resolution of this complication. Surgical studies describe patients undergoing surgery, but do not report the evolution of inoperable patients.
A prospective cohort study was conducted by the Catalan Institute of Cancer in 2007, which included 100 patients diagnosed with inoperable MBO who were selected out of 885 patients visited by the Palliative Care Hospital Support Team (MBO prevalence = 11.3%). Twenty-five percent of these patients had previous episodes of MBO with spontaneous resolution (overall mean of 1.37 episodes per patient; SD ± 0.7; range 1–4). An extensive record of the clinical characteristics of these patients was documented. Over 80% of patients had multiple obstructive levels and more than 60% showed a peritoneal carcinomatosis with radiological or cytological confirmation. The spontaneous resolution of inoperable MBO with symptomatic treatment was observed in 42% of patients. Resolution occurred within the first 7 days after diagnosis in 92% of patients. In the follow-up procedure, the index of intestinal reobstruction was 74%. The mean overall survival rate was 23 days (95% CI = 16.8–29.4). Clinical data for all patients were stratified according to their specific evolution (spontaneous resolution versus no resolution) in order to indentify the factors influencing the spontaneous resolution of MBO. The mean survival was 12 days (95% CI = 9.0–14.1) for patients with no spontaneous resolution of MBO, and 57 days for patients with complete resolution (P < 0.001). In the group of patients who did not present MBO resolution, some showed tolerance to minimal food intake, mainly liquids, without recovery of normal digestive transit and with the need to maintain antiemetic and antisecretory treatment. The mean survival rate of these patients (persistent subobstruction) was 23 days (95% CI = 3.9–36), which is lower than the full resolution cases and higher than those patients who did not tolerate the intake of liquids at any time (P < 0.001). A multivariate analysis of the clinical characteristics of the patients assessed at the time of inclusion in the study reveals the most relevant factors influencing the consolidation and nonresolution of MBO. These are: cognitive failure, cachexia, dyspnea at rest, palpable abdominal tumors, hepatic failure, upper intestinal obstruction, and dehydration.Citation5,Citation64
It is important to know the risk of nonresolution of MBO in order to carefully establish therapeutic measures, adjust real expectations, and accurately report them to the patient and family.
It is relevant to determine if there are pharmacological and nonpharmacological measures for preventing reobstruction in patients who demonstrate the spontaneous resolution of MBO. Some researchers suggest that a low residue diet, avoidance of osmotic laxatives, or use of longterm antisecretory drugs (eg, long-acting octreotide) may improve the likelihood of further obstructive episodes. However, this question remains unanswered. A pilot study conducted in 2005 including 15 ovarian cancer patients diagnosed with inoperable MBO, documented peritoneal carcinomatosis. These patients were treated with the immediate release octreotide and thereafter with long-acting octreotide administered monthly. Sixty percent of patients received at least one dose of long-acting octreotide. Twenty percent of the patients presented full recovery of digestive transit. These patients continued the antisecretory therapy with long-acting octreotide over a mean time of 9 months (3–15 months).Citation65 This long period of treatment with long acting octreotide, even higher to survival of many studies without this drug, may suggest that maintenance of antisecretory therapy may prevent new episodes of MBO. However, from data of this study it is not definitively possible to conclude that the long-acting octreotide is useful in preventing new episodes of the MBO, because patients were also treated with chemotherapy, which obviously influences the evolution of the MBO, and that new obstructive episodes were not reported clearly. At present the measures for preventing intestinal reobstruction remain under debate and may be the focus of future research.
Summary of key points
– MBO is a frequent complication in advanced oncological patients, especially in those with abdominal tumors. The prevalence and incidence of global MBO in cancer and different primary organs requires elucidation since most studies are retrospective and based on a series of cases or contain heterogeneous criteria for outcomes and diagnoses.
– The initial diagnosis of MBO is fundamentally based on anamnesis, physical examination, and simple radiology of the abdomen. Radiological techniques with contrast, CT, and MR may increase the diagnostic precision related to tumor extension and the level of obstruction, which is often necessary for decision making and evaluation related to the indication for surgery or endoscopic palliation.
– Surgery is the only therapeutic measure that may reestablish digestive transit and allow – according to the evolution of the patient – treatment with the intention of eradicating or palliating the obstruction by intestinal bypass. However, its indication should be assessed carefully on an individualized manner, especially in patients with advanced cancer due to the high rate of surgical mortality and morbidity. Most experts claim the factors limiting surgery in MBO are advanced age, previous malnutrition, and the presence of multiple occlusive levels, extra-abdominal metastatic disease, refractory ascites, deteriorated general status, previous abdominal radiotherapy, and the absence of active oncological treatments.
– Self-expanding duodenal and colonic stents are highly effective and safe alternatives in patients with a single level of occlusion and who are considered inoperable.
– Percutaneous gastrostomy allows for more comfortable and safe longterm digestive decompression than nasogastric aspiration in patients with inoperable MBO and symptomatology that is inadequately controlled by symptomatic treatment.
– Palliative medical treatment of inoperable MBO is polymodal and based on the combined use of glucocorticoids, antiemetics, antisecretors, and potent analgesic opioids. Due to their antiemetic action and reduction of perilesional edema, glucocorticoids are indicated in the initial phases of this complication and may increase the rate of spontaneous resolution. Most experts concur that the antiemetics of choice are neuroleptics (haloperidol) and rule out the use of prokinetic drugs because of the risk of increasing pain if the obstructive process is not functional. Antagonists of 5-HT3 receptors are effective for controlling emesis in the treatment MBO, even in cases where the patient’s response to antiemetics is insufficient. The limitation of these considerations is they are based on the opinion of experts or noncontrolled clinical studies (Phase II).
– Abdominal pain in MBO is highly prevalent, of great intensity, and often requires the use of potent opioid drugs. No controlled clinical trials have compared the different potent opioids in this indication. Fentanyl is the opioid that least affects intestinal motility, which has been demonstrated by controlled clinical studies of different indications of MBO.
– Antisecretor drugs improve nausea, vomiting, and pain with an important reduction in intestinal hypersecretion proximal to the occlusive process. According to the results of several controlled clinical studies, octreotide, an analog of somatostatin and a potent antisecretor drug, has shown a clearly superior efficacy with anticholinergic drugs. As mentioned previously, the implementation or maintenance of digestive aspiration with a nasogastric tube or percutaneous gastrostomy is only useful if the polymodal palliative pharmacological treatment cannot achieve adequate symptomatic control.
– Symptomatic control is very high with the medical treatment strategy and spontaneous resolution is observed in more than one third of patients.
– The life expectancy of advanced oncological patients from the diagnosis of inoperable MBO is short, with an estimated mean survival rate no longer than 4 weeks.
– The most relevant factors influencing the consolidation and nonresolution of MBO include cognitive failure, cachexia, dyspnea at rest, palpable abdominal tumors, hepatic failure, upper intestinal obstruction, and dehydration.
Disclosure
The authors declare no conflict of interest in this work.
References
- AnthonyTBaronTMercadanteSReport of the clinical protocol committee: development of randomized trials for malignant bowel obstructionJ Pain Symptom Manage200734Suppl 1S49S5917544243
- RipamontiCEassonAMGerdesHManagement of malignant bowel obstructionEur J Cancer20084481105111518359221
- DvoretskyPMRichardsKAAngelCRabinowitzLBeechamJBBonfiglioTASurvival time, causes of death, and tumor. Treatment related morbidity in 100 women with ovarian cancerHum Pathol19881911127312793181948
- BainesMOliverDJCarterRLMedical management of intestinal obstruction in patients with advanced malignant disease – a clinical and pathological studyLancet1985284629909932414614
- TucaACodorniuNGarzónSerranoGMalignant bowel obstruction due to advanced cancer in palliative care: Observational and descriptive study5th Research Forum of European Association for Palliative Care Poster: 462May 2008Trodheim, Norway
- MillerGBomanJShrierIGordonPHSmall bowel obstruction secondary to malignant disease: an 11-year auditCan J Surg200043535335811045093
- TucaARocaRSalaCEfficacy of granisetron in the antiemetic control of nonsurgical intestinal obstruction in advanced cancer: a phase II clinical trialJ Pain Symptom Manage200937225927018789638
- ArvieuxCLavalGStefaniLVillardMLMestralletJPCardinNProtocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital CenterJ Pain Symptom Manage200631650251216793490
- BlairSLChuDZSchwarzEOutcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancerAnn Surg Oncol20018863263711569777
- RipamontiCTwycrossRBainesMfor Working Group of the European Association for Palliative CareClinical-practice recommendations for management of bowel obstruction in patients with end-stage cancerSupport Care Cancer20019422323311430417
- BennettMILivingstoneHJCostelloPAllenKRDeggTJSymptom scores, serotonin and 5-hydroxyindole acetic acid levels in cancer patients with and without bowel obstructionPalliat Med2007321215715917344265
- Jimenez-GarcıaABalongo-GarcıaRAlconeroFFIntestinal wall damage in simple ileus in rabbits: immune modulator role of somatostatineHepatogastroenterology200451581030103615239240
- GoyalRKHiranoIEnteric nervous systemN Engl J Med199633417110611158598871
- HelyeLEassonAMSurgical Approaches to Malignant Bowel ObstructionJ Support Oncol20086310511318402300
- BrancoBCBarmparasGSchnürigerBInabaKChanLSDemetriadesDSystematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstructionBr J Surg201097447047820205228
- SuriSGuptaSSudhakarPJVenkataramuNKSoodBWigJDComparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstructionActa Radiol199940442242810394872
- ThompsonWMKilaniRKSmithBBAccuracy of abdominal radiography in acute small bowel obstruction: does reviewer experience matter?AJR Am J Roentgenol20071883W233W23817312028
- de BreeEKoopsWKrögerRvan RuthSWitkampAJZoetmulderMAPeritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreementJ Surg Oncol2004862647315112247
- JacquestPJelinekJSStevesMASugarbakerPHEvaluation of computed tomography in patients with peritoneal carcinomatosisCancer1993725163116368348494
- LowRChenSBaroneRDistinguishing benign from malignant bowel obstruction in patients with malignancy: findings at MR imagingRadiology2003228115716512832579
- BeallDFortmanBLawlerBReganFImaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomographyClin Radiol200257871972412169282
- HaHKShinBSLeeSIUsefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancyAJR Am J Roentgenol19981716158715939843293
- ChanAWoodrufRKIntestinal obstruction in patients with wide-spread intra- abdominal malignancyJ Pain Symptom Manage1992763393421517649
- LauPVLorenzPGResults of surgery for malignant bowel obstruction in advanced, unresectable, recurrent colorectal cancerDis Colon Rectum199336161647677982
- JongPSturgeonJJamiesonCGBenefit of palliative surgery for bowel obstruction in advanced ovarian cancerCan J Surg19953854544577553472
- YazdiGPMiedemaBWHumphreyLJHigh mortality after abdominal operation in patients with large volume malignant ascitesJ Surg Oncol199662293968649047
- WoolfsonRGJenningsKWhalenGFManagement of bowel obstruction in patients with abdominal cancerArch Surg199713210109310979336507
- SunXLiXManagement of bowel obstruction in advanced ovarian cancer: an analysis of 57 casesZhonghua Zhong Liu Za Zhi199517139427656786
- TekkisPKinsmanRThompsomMStamatakisJThe Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancerAnn Surg20042401768115213621
- BryanDRadbodRBerekJAn analysis of surgical versus a chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancerInt J Gynecol Cancer200616112513416445622
- ParkerMCBainesMJIntestinal obstruction in patients with advanced malignant diseaseBr J Surg1996831128653326
- FeuerDJBroadleyKEShepherdJHBartonDPSystematic review of surgery in malignant bowel obstruction in advanced gynecological and gastrointestinal cancerGynecol Oncol199975331332210600282
- ZoetmulderFAHelmerhorstTJvan CoevordenFWolfsPELeyerJPHartAAManagement of bowel obstruction in patients with advanced ovarıan cancerEur J Cancer199430A11162516287833134
- JeurninkSMvan EijckCHSteyerbergEWKuipersEJSiersemaPDStent versus a gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic reviewBMC Gastroenterol200771817559659
- DormannAMeisnerSVerinNWenk LangASelf-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectivenessEndoscopy2004366054315202052
- HoltAPPatelMAhmedMMPalliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice?Gastrointest Endosc20046061010101715605026
- WattAMFaragherIGGriffinTTRiegerNAMaddernGJSelf-expanding Metallic Stents for Relieving Malignant Colorectal Obstruction: A Systematic ReviewAnn Surg20072461243017592286
- KhotUPLangAWMuraliKParkerMCSystematic review of the efficacy and safety of colorectal stentsBr J Surg2002899109612190673
- CampagnuttaECannizzaroRGalloAPalliative treatment of upper intestinal obstruction by gynecological malignancy: the usefulness of percutaneous endoscopic gastrostomyGynecol Oncol19966211031058690280
- PothuriBMontemranoMGerardiMPercutaneous endoscopic gastrostomy tube placement in malignat bowel obstruction due to ovarıan carcinomaGynecol Oncol20059633033415661217
- FanBGParentera l nutrition prolongs the survival of patients associated with malignant gastrointestinal obstructionJPEN J Parenter Enteral Nutr200731650851017947608
- BozzettiFCozzaglioLBiganzoliEQuality of life and length of survival in advanced cancer patients on home parenteral nutritionClin Nutr200221428128812135587
- World Health OrganizationCancer Pain Relief – with a guide to opioid availabilitysecond editionWHOGeneva1996 Available in internet: http://whqlibdoc.who.int/publications/9241544821.pdfAccessed May 2012
- VentafriddaVTamburiniMCaraceniADe ConnoFNaldiFA validation study of the WHO method for cancer pain reliefCancer19875948508563802043
- ZechDFGrondSLynchJHertelDLehmannKAValidation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective studyPain199563165768577492
- MercadanteSPain treatment and outcomes for patients with advanced cancer who receive follow-up care at homeCancer19998581849185810223581
- HanksGWConnoFChernyNfor Expert Working Group of the Research Network of the European Association for Palliative CareMorphine and alternative opioids in cancer pain: the EAPC recommendationsBr J Cancer200184558759311237376
- RileyJEisenbergEMüller-SchwefeGDrewesAMArednt-NielsenLOxycodone: a review of its use in the management of painCurr Med Res Opin200824117519218039433
- TassinariDSartoriSTamburiniETransdermal fentanyl as a front-line approach to moderate-severe pain: a meta-analysis of randomized clinical trialsJ Palliat Care200925317218019824278
- LavalGGirardierJLassauumiereJMLeducBHaondCSchaererRThe use of steroids in the management of inoperable intestinal obstruction in terminal cancer patients: do they remove the obstruction?Palliat Med200014131010717717
- HardyJRLingPJMansiJPitfalls in placebo controlled trials in palliative care: dexamethasone for the palliation of malignant bowel obstructionPalliat Med1998121643744310621863
- NevilleRFieldingPCambriaRPModlinIVascular responsiveness in obstructed gutDis Colon Rectum19913432292351999129
- NellgardPBojoLCassutoJImportance of vasoactive intestinal peptide and somatostatin for fluid losses in small bowel obstructionScand J Gastroenterol19953054644697638573
- RipamontiCMercadanteSGroffLZeccaEDe ConnoFCassucioARole of octreotide, scopolamine butylbromide and hydration in symptom control of patients with inoperable bowel obstruction having a nasogastric tube – a prospective, randomized clinical trialJ Pain Symptom Manage2000191233410687323
- MercadanteSRipamontiCCasuccioAZeccaEGroffLComparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstructionSupport Care Cancer20008318819110789958
- MercadanteSCasuccioAMangioneSMedical Treatment for inoperable malignant bowel obstruction: A qualitative systematic reviewJ Pain Symptom Manage200733221722317280927
- WatariHHosakaMWakuiYA Prospective Study on the Efficacy of Octreotide in the Management of Malignant Bowel Obstruction in Gynecologic CancerInt J Gynecol Cancer Epub February 14, 2012
- HisanagaTShinjoTMoritaTMulticenter prospective study on efficacy and safety of octreotide for inoperable malignant bowel obstructionJpn J Clin Oncol8201040873974520410056
- LavalGRousselotHToussaint-MartelSSALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstructionBull Cancer212012992E1E922265994
- MercadanteSPorzioGOctreotide for malignant bowel obstruction: twenty years afterCrit Rev Oncol Hematol Epub January 23, 2012
- ClarkKLamLCurrowDReducing gastric secretions-a role for histamine 2 antagonists or proton pump inhibitors in malignant bowel obstruction?Support Care Cancer12200917121463146819290549
- ClarkKLamLTGibsonSCurrowDThe effect of ranitidine versus proton pump inhibitors on gastric secretions: a meta-analysis of randomised control trialsAnaesthesia200964665265719453319
- http://www.nccn.org [homepage on the Internet]Pallaitive CarePractice Guidelines in Oncology of National Comprehensive Cancer NetworkAccessed February 19, 2011 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#palliativeAccessed May 2012
- TucaAMartínezEGüellEGómez BatisteXMalignant bowel obstructionMed Clin (Barc)2010135837538120452630
- MatulonisUASeidenMVRocheMLong-acting octreotide for the treatment and symptomatic relief of bowel obstruction in advanced ovarian cancerJ Pain Symptom Manage200530656356916376743