Abstract
Introduction
Ovarian clear cell carcinoma (OCCC) is a subtype of ovarian cancer characterized by highly aggressive and poor prognosis. However, it is unclear what factors are associated with OCCC recurrence and death. The study aimed to evaluate whether residual tumor diameter after primary debulking surgery, or other clinicopathological features play roles in predicting survival outcome in stage II–IV OCCC patients.
Material and Methods
We present a retrospective study of OCCC patients with stage II–IV in our department from 2010 to 2015. Kaplan–Meier method was used to draw a survival curve. Survival analysis was performed using Log-rank test for univariate analysis and COX proportional risk regression model for multivariate analysis.
Results
In this cohort of 78 patients who underwent primary debulking surgery, 47 patients had disease recurrence and 32 cases died. On univariate analysis, FIGO stage, residual tumor diameter and ascites were significant predictors of 3-year PFS (P values<0.05) and OS (P values<0.05). On multivariate analysis, the residual tumor diameter was an independent prognostic factor for 3-year PFS and OS (P values<0.05). The outcomes of patients in residual-free group were significantly better than those in the residual tumor diameter 0–1cm and >1cm group (PFS: P=0.000, OS: P=0.001), but there was no significant difference in prognosis between 0–1cm and > 1cm group (P values >0.05). Greater residual tumor diameter predicted progression on cox analysis in patients with stage III, but not for patients with stage IV.
Conclusion
Residual tumor diameter is prognostic after surgery for OCCC. Achieving no residual disease will significantly improve the prognosis in advanced OCCC patients.
Introduction
Epithelial ovarian cancer (EOC) is the fifth most common cause of female cancer death worldwide.Citation1 Ovarian Clear Cell carcinoma (OCCC) is a distinct histological subtype that accounts for 5–10% of all EOC, which has the worst prognosis among all subtypes.Citation2–Citation4 Although the prognosis for OCCC patients with stage I is relatively good, the clinical outcomes of patients with stage II–IV OCCC remain less favorable than patients with serous carcinoma due to its disease aggressiveness and chemotherapy resistance.Citation5,Citation6
The standard treatments for OCCC are similar to that of EOC, which consist of maximal debulking surgery combined with adjuvant platinum-based chemotherapy.Citation7 However, compared with other EOC subtypes, OCCC is not sensitive to platinum-based chemotherapy, so the relapse rate is high and the prognosis is poor.Citation8–Citation10 The JGOG 3016 trial revealed that dose-dense chemotherapy improves both PFS and OS in EOC with stage II–IV, but these benefits were not observed in OCCC subgroup.Citation3 Meanwhile, some prior studies have found residual tumor volume to be an important factor in the progression of EOC,Citation11 but there are no studies on the clinical outcomes between different treatment strategies on OCCC. Therefore, our study retrospectively analyzed the clinicopathological data of 78 patients with stage II–IV OCCC, to further examine the general characteristics, clinical outcomes, and treatment approaches.
Materials and Methods
This is a retrospective study focusing on the clinical characteristics on confirmed cases of OCCC patients. A total of 120 patients diagnosed OCCC in Zhongnan Hospital of Wuhan University from 2010 to 2015. We collected and analyzed 78 cases who were evaluated preoperatively by clinicians and received initial standard treatment (debulking surgery and postoperative platinum-based chemotherapy). According to the medical records, information included age, menopausal status, initial level of CA125, International Federation of Gynecology and Obstetrics (FIGO) stage at initial diagnosis.Citation12 Residual tumor diameter, ascites, histopathologic type and postoperative chemotherapy cycles. This case series was approved by the Institutional Ethics Board of Zhongnan Hospital of Wuhan University (Wuhan, Hubei, China, No. 2,020,041). As discussed by the IRB, the study did not exceed the minimum risk, exemption of written informed consent did not adversely affect any patient rights or welfare, the requirement for written informed consent was hence waived. The clinical study process strictly abide by the principle of patient data confidentiality, and compliance with the Declaration of Helsinki.
We defined the survival outcome through routine appointment out-patient clinic, returning visit or telephones follow-up. In this study, the patient follow-up period started the date of surgery. All patients were followed up every 3 months for the first year, later every six months until December 2018. Data were collected on the clinical and pathologic characteristics of the study women, including age, pre-operative serum cancer antigen 125 (CA-125), ascites, residual tumor size, stage, lymph node (LN) excision and metastasis, cycles of CT after surgical staging. Among them, the evaluation of surgical effect: the ideal reduction was the diameter of postoperative residual tumor ≤1 cm (R1), residual tumor>1 cm (R2) was the non-ideal reduction, and the residual tumor being zero (R0) was the complete reduction. Progression-free survival (PFS) was defined in months from the time of diagnosis to that of tumor progression or patient death or the last follow-up. The criteria for tumor progression should meet at least one of the following conditions: elevation of tumor markers, imaging recurrence, clinical symptoms, pathological evidence after reoperation. Overall survival (OS) was defined in months from the time of diagnosis to patient death or the end of follow-up.
SPSS 17.0 software was used for statistical analysis. Proportions were calculated for categorical data while medians and ranges were used for continuous variables. Kaplan–Meier method was used to draw a survival curve. Survival analysis was conducted using Log-rank test for univariate analysis and COX proportional risk regression model for multivariate analysis. The value of P<0.05 was considered statistically significant.
Results
A total of 78 patients with stage II–IV OCCC were enrolled in our retrospective study. summarizes the data on the patient’s baseline characteristics. The median age was 50 years (26–75 years) and the average age was 51.4 ± 8.6 years. Fifty cases were post-menopausal at diagnosis and the median age at menopause was 49.0 years. According to FIGO stage, 16 cases stage II, 53 cases in stage III and 9 cases in stage IV; the median level of CA-125 was 147.4 IU/mL (range: 8.7–6746 IU/mL). All patients underwent debulking surgery. Regarding the residual tumor diameter, 38 cases were 0 cm, 24 cases were 0–1 cm, and 16 cases were >1 cm. Eighteen patients were diagnosed with lymph node metastasis; histopathological type was mixed in 70 cases; After 3–9 cycles of platinum-based chemotherapy, ≤6 cycles were used in 43 cases and >6 cycles were used in 35 cases. Surgical procedures are listed in Supplementary Table 1.
Table 1 Clinicopathological Characteristics of OCCC Patients with II–IV Stage
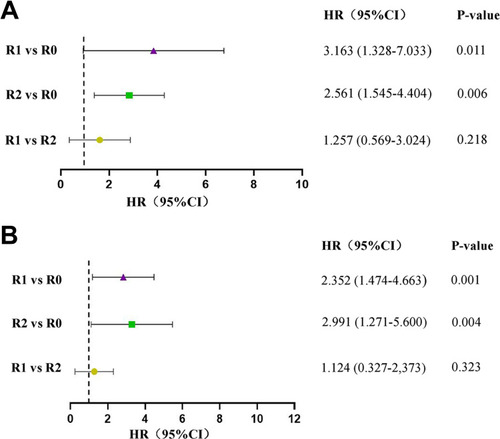
The median follow-up time was 20 months, with 47 recurrence and 32 deaths among all 78 patients. The 3-year PFS and OS were 31.7% and 48.2%, respectively. In the univariate analysis, FIGO stage, residual tumor, and ascites were relevant factors for PFS (P<0.05). Meanwhile, FIGO stage, residual tumor, ascites, and lymph node resection were important factors for OS (P<0.05). In multivariate analysis, we found that residual tumor was an independent prognostic factor affecting both PFS and OS in OCCC patients ().
Table 2 Univariate and Multivariate Analyses of Prognostic Factors for OCCC Patients
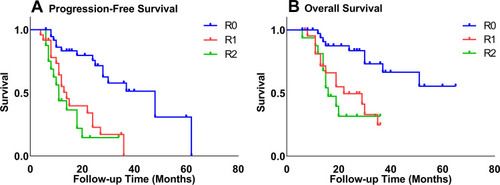
We divided the population into sub-groups according to the residual tumor diameters and conducted the Kaplan–Meier survival curves. The 3-year PFS rates for the residual tumor diameters of 0cm, 0–1cm, and >1cm were 51.3%, 17.0%, and 14.6%, respectively, and the 3-year OS rates were 66.5%, 33.0%, and 31.6%, respectively. As shown in , the outcomes of patients in the residual-free group were significantly higher than those in the residual tumor diameter 0–1cm and >1cm group (PFS: P=0.000, OS: P=0.001). Meanwhile, there was no significant difference in prognosis between 0–1cm and > 1cm group (P>0.05).
Figure 1 Kaplan–Meier curves of progression-free (A) survival and overall survival (B) stratified by extent of residual disease.
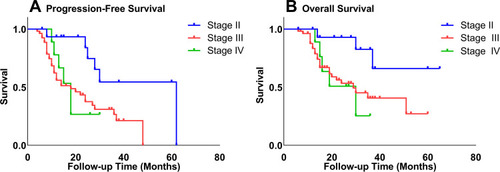
Besides, according to the estimation of the Kaplan–Meier survival curve, the 3-year PFS rates of stage II, III, and IV patients were 54.4%, 26.5%, and 26.7%, respectively, and the 3-year OS rates were 66.0%, 40.6%, and 25.4%, respectively. As shown in , the prognosis of stage II patients was significantly higher than those of stage III (PFS: P=0.004, OS: P=0.018) and stage IV patients (PFS: P=0.029, OS: P=0.013).
Figure 2 Kaplan–Meier curves of progression-free (A) survival and overall survival (B) stratified by FIGO stage.
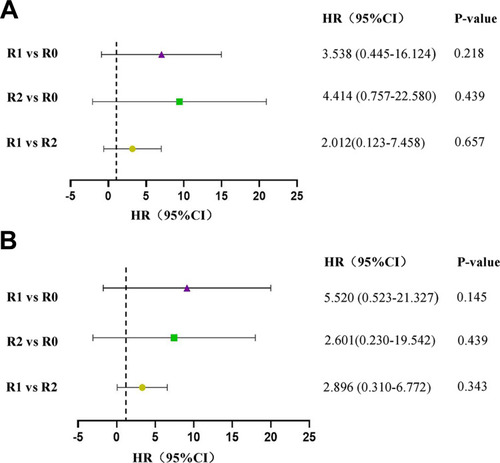
We then conducted the subgroup analysis of residual tumor diameter on different FIGO stage. For patients with stage III, both the 3-year PFS and OS of R1 and R2 groups were significantly lower when compared with R0 group, with P values under 0.05. There was no significant difference in prognosis between R1 and R2 group (P>0.05) (). However, residual tumour diameters did not reveal significant effects on neither 3-year PFS nor 3-year OS in patients with stage IV ().
Discussion
In 1973, OCCC formally became an independent clinical subtype of ovarian tumors and was divided into epithelial ovarian cancer. As was reported,Citation13 the average age of OCCC was younger than that of serous ovarian cancer. The average age in this study was 51.4 years, indicating that the age of OCCC at diagnosis tended to be younger. The premenopausal and postmenopausal incidence rates in this group of patients were similar, suggesting that the relationship between menopausal age and the occurrence of OCCC have not been obvious. In this study, the 3-year PFS and OS were 31.7% and 48.2%, respectively. This result coincided with the conclusion in other studies that the median survival time of advanced OCCC was distinctly lower.Citation14,Citation15
Primary cytoreductive surgery is an important treatment for patients with stage II–IV epithelial ovarian cancer. A meta-analysis pointed that the more complete the debulking, the better the outcomes, so clinicians should make a maximal effort to remove all gross disease.Citation16 Due to the relatively small incidence of OCCC in epithelial ovarian cancer, there is no prospective study on the effect of cytoreductive surgery on the prognosis of OCCC patients. Sioulas et alCitation17 found that patients with stage IIIC ovarian cancer who achieved no residual after initial cytoreductive surgery had the longest median PFS (26.7 months) and the longest median OS (83.4 months). The prognosis of patients with 1–10 mm of residual disease is better than that of patients with >10 mm of residual disease, and the difference is statistically significant. In a retrospective study by Takano et al,Citation18 the median survival time of postoperative residual tumor diameters of 0 cm, 0–1 cm, > 1 cm in patients with stage III–IV OCCC was 39, 7, and 5 months. The prognosis of complete resection of all visible disease was significantly better, but there was no difference in the prognosis of 0–1cm and >1cm groups. In this study, the 3-year PFS of the residual tumor diameters of 0cm, 0–1cm, and >1cm were 51.3%, 17.0%, and 14.6%, respectively, and the 3-year OS were 66.5%, 33.0%, and 31.6%, respectively. In multivariate analysis, residual tumor diameter was an independent prognostic factor affecting recurrence and survival. This result is consistent with Takano M’s study, indicating that cytoreductive surgery in patients with advanced OCCC must achieve no residual to improve the prognosis. In advanced ovarian cancer, the residual tumor diameter is less than 1cm, that is, the ideal reduction can significantly improve the prognosis,Citation19,Citation20 but it does not seem to be applicable to patients with advanced OCCC. In our study, there is no difference in prognosis between patients with 0–1cm and > 1cm of residual disease. We believe that OCCC patients with residual disease after surgery have a significantly poorer prognosis. Considering the existence of the cytotoxicity of the chemotherapy drugs and the resistance to platinum, advanced OCCC patients have a great chance to relapse in a short period of time, and are led to a worse prognosis. Therefore, in ovarian clear cell carcinoma, only when surgery achieves no remnants can recurrence be delayed and over-all survival time be prolonged.
Malignant ascites is one of the complications of ovarian cancer, it is more common in patients with recurrence, and is closely related to poor prognosis.Citation21,Citation22 Marleen et alCitation23 reported that ascites was an independent factor for OS, and patients with ascites volume >1000 mL had poorer treatment outcomes. However, a retrospective study of 140 patients with advanced EOCs found that the relationship between ascites and clinical outcomes was not apparent.Citation24 In our series, 37.2% of patients represented obvious ascites during intraoperative evaluation. Univariate analysis showed that ascites was a related factor affecting prognosis. The median PFS and OS in the patients with ascites were 13 and 19 months, which were significantly lower than those without ascites. Ascites is closely related to the prognosis of OCCC, but whether ascites is an independent factor remains to be further exploration.
Postoperative supplementation with platinum-based chemotherapy is one of the important treatments for ovarian cancer. Compared to serous and non-serous cancer patients, the former with six cycles of postoperative chemotherapy had a potential survival advantage. Among them, the response rate of OCCC to platinum chemotherapy drugs is only 11.1%,Citation9 so how to choose the postoperative chemotherapy regimen of OCCC has always been controversial. For advanced OCCC, the current NCCN guidelines point out that “there is no sufficient evidence to prove that >6 cycles of chemotherapy can benefit patients’ survival, so six cycles of chemotherapy are recommended.” In clinical practice, clinicians use more than 6 cycles of chemotherapy for some patients based on the patient‘s condition and personal experience. Bertelsen et alCitation25 conducted three randomized trials that compared the survival benefits of 5–6 cycles and 8, 10 and 12 cycles of chemotherapy, respectively, and showed that the best duration of first-line chemotherapy for advanced ovarian cancer was not more than 6 cycles. Therefore, it is generally believed that patients with stage II–IV ovarian cancer can be objectively relieved with six cycles of chemotherapy after surgery. It is not clear whether it is applicable to OCCC patients. In this study, patients with ≤6 cycles and >6 cycles group, the median PFS were 24 and 22 months, the 3-year PFS were 25.9% and 29.7%. The median OS was 37 and 30 months, the 3-year OS was 50.0% and 47.8%, respectively. This result shows that for patients with advanced OCCC, there is no significant difference between the prognostic effects of chemotherapy for >6 cycles and ≤6 cycles. Our results were similar to the study by Tozzi et al, which reported the use of neo-adjuvant chemotherapy did not increase the rate of CR and did not reduce the complexity and the morbidity of the surgery.Citation26
The importance of maximal cytoreduction to eliminate residual disease has become widely accepted in the primary treatment of serous epithelial ovarian carcinoma (SOC) patients.Citation27 However, our study was the first to show that residual tumor size is the independent prognostic factor in OCCC patients. Several studies have suggested the differences in prognosis between OCCC and SOC. Oliver suggests that the prognosis of ovarian cancer patients was influenced by histology, in early-stage patients, PFS was better for OCCC than for SOC, but in late-stage patients, OCCC was significantly associated with decreased OS.Citation28 Our study further investigated the prognostic factors related to II–IV stage OCCC patients. In addition, our results demonstrate that greater residual tumor diameter predicted progression on Cox analysis in patients with stage III, but not for patients with stage IV. The possible reasons could be due to the distant metastases and tumour progression, OCCC patients with stage IV who achieved R0 went through more aggressive surgery procedures, which lead to decreased immunological function and worse survival outcomes. Although residual tumor volume is mainly determined by surgery procedures, study of Lee also suggests that tumor biology is another important factor for advanced-stage ovarian cancer patients who achieved R0.Citation29 Though the small sample size limits the representativity of the observation, we believe our finding can serve as an informative starting points for further investigation when larger cohort from a wide range of centers becomes available.
In general, the residual tumor diameter is an independent prognostic factor for PFS and OS in patients with stage II–IV OCCC. During the process of disposing OCCC, clinicians should improve the thoroughness of tumor cytoreductive surgery, follow the principle of no residual as much as possible, and select the appropriate chemotherapy cycles for achieving a better prognosis.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
This study was approved by the Institutional Ethics Board of Zhongnan Hospital of Wuhan University (Wuhan, Hubei, China, No. 2020041). The study is funded by grants National Natural Science Foundation of China 8197103302/H16 (Hong-Bing Cai); and the National Natural Science Foundation of China 82002770/H16 (Mengyuan Dai). Editorial support was provided by the institutional ethics board of Zhongnan Hospital of Wuhan University (No. 2020029).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.2159031912902
- Kim SI, Lee JW, Lee M, et al. Genomic landscape of ovarian clear cell carcinoma via whole exome sequencing. Gynecol Oncol. 2018;148(2):375–382. doi:10.1016/j.ygyno.2017.12.00529233531
- Sugiyama T, Okamoto A, Enomoto T, et al. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial. J Clin Oncol. 2016;34(24):2881–2887. doi:10.1200/JCO.2016.66.901027400948
- Schnack TH, Hogdall E, Nedergaard L, Hogdall C. Demographic clinical and prognostic factors of primary ovarian adenocarcinomas of serous and clear cell histology-a Comparative Study. Int J Gynecol Cancer. 2016;26(1):82–90. doi:10.1097/IGC.000000000000058526569060
- Glasspool RM, McNeish IA. Clear cell carcinoma of ovary and uterus. Curr Oncol Rep. 2013;15(6):566–572. doi:10.1007/s11912-013-0346-024114188
- Del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol. 2012;126(3):481–490. doi:10.1016/j.ygyno.2012.04.02122525820
- Fujiwara K, Shintani D, Nishikawa T. Clear-cell carcinoma of the ovary. Ann Oncol. 2016;27(Suppl 1):i50–i52. doi:10.1093/annonc/mdw08627141072
- Oseledchyk A, Leitao MM Jr, Konner J, et al. Adjuvant chemotherapy in patients with stage I endometrioid or clear cell ovarian cancer in the platinum era: a surveillance, epidemiology, and end results Cohort Study, 2000–2013. Ann Oncol. 2017;28(12):2985–2993. doi:10.1093/annonc/mdx52528950307
- Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88(11):2584–2589. doi:10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-510861437
- Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99(4):653–658. doi:10.1111/j.1349-7006.2008.00747.x18377417
- Fang C, Zhang Y, Zhao L, Chen X, Xia L, Zhang P. The relationship between retroperitoneal lymphadenectomy and survival in advanced ovarian cancer patients. BMC Cancer. 2020;20(1):654. doi:10.1186/s12885-020-07144-132660444
- Oncology FCoG. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125(2):97–98. doi:10.1016/j.ijgo.2014.02.00324630859
- Machida H, Matsuo K, Yamagami W, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO-JSOG joint study. Gynecol Oncol. 2019;153(3):589–596. doi:10.1016/j.ygyno.2019.03.24330905436
- Suh DH, Kim JW, Kang S, Kim HJ, Lee KH. Major clinical research advances in gynecologic cancer in 2013. J Gynecol Oncol. 2014;25(3):236–248. doi:10.3802/jgo.2014.25.3.23625045437
- Takahashi K, Takenaka M, Kawabata A, Yanaihara N, Okamoto A. Rethinking of treatment strategies and clinical management in ovarian clear cell carcinoma. Int J Clin Oncol. 2020;25(3):425–431. doi:10.1007/s10147-020-01625-w31989349
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi:10.1200/JCO.2002.20.5.124811870167
- Sioulas VD, Schiavone MB, Kadouri D, et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol. 2017;145(1):15–20. doi:10.1016/j.ygyno.2017.02.02328238354
- Takano M, Kikuchi Y, Yaegashi N, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94(10):1369–1374. doi:10.1038/sj.bjc.660311616641903
- Suidan RS, Zhou Q, Iasonos A, et al. Prognostic significance of the number of postoperative intraperitoneal chemotherapy cycles for patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2015;25(4):599–606. doi:10.1097/IGC.000000000000038925664437
- Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33(13):1460–1466. doi:10.1200/JCO.2014.55.989825800756
- Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–282. doi:10.1038/nrc343223426401
- Kim S, Kim B, Song YS. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 2016;107(9):1173–1178. doi:10.1111/cas.1298727297561
- van Vliet MM, Schreuder HW, Pasker-de Jong PC, et al. Centralisation of epithelial ovarian cancer surgery: results on survival from a peripheral teaching hospital. Eur J Obstet Gynecol Reprod Biol. 2015;192:72-78. doi:10.1016/j.ejogrb.2015.06.013
- Park JY, Kim DY, Suh DS, et al. Significance of ovarian endometriosis on the prognosis of ovarian clear cell carcinoma. Int J Gynecol Cancer. 2018;28(1):11–18. doi:10.1097/IGC.000000000000113628930811
- Bertelsen K, Grenman S, Rustin GJ. How long should first-line chemotherapy continue? Ann Oncol. 1999;10(Suppl 1):17–20. doi:10.1016/S0923-7534(20)31479-410219448
- Tozzi R, Giannice R, Cianci S, et al. Neo-adjuvant chemotherapy does not increase the rate of complete resection and does not significantly reduce the morbidity of Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer. Gynecol Oncol. 2015;138(2):252–258. doi:10.1016/j.ygyno.2015.05.01026003142
- Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26(4):336–342. doi:10.3802/jgo.2015.26.4.33626197773
- Oliver KE, Brady WE, Birrer M, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG oncology/Gynecologic Oncology Group experience. Gynecol Oncol. 2017;147(2):243–249. doi:10.1016/j.ygyno.2017.08.00428807367
- Lee YJ, Lee JY, Nam EJ, Kim SW, Kim S, Kim YT. Rethinking radical surgery in interval debulking surgery for advanced-stage ovarian cancer patients undergoing neoadjuvant chemotherapy. J Clin Med. 2020;9:4.