Abstract
Transvaginal ultrasound (TVUS) represents an accurate and noninvasive technique to investigate endometrial thickness (ET) in the early diagnosis of endometrial cancer (EC). In the literature, for maximum ET there is no consensus on the cutoff value for normal ET in postmenopause for either symptomatic or asymptomatic women. Most patients with EC present with postmenopausal bleeding (PMB) and in these patients is necessary to perform TVUS to evaluate ET as an indicator for endometrial biopsy. On the contrary, if ET is incidentally detected in postmenopausal patients without bleeding, endometrial sampling for a postmenopausal woman without bleeding should not be routinely performed, although it is estimated that up to 15% of EC occurs in women without vaginal bleeding. The aim of our review was to give clinicians necessary and useful knowledge on the role of TVUS and ET for early detection of EC in their daily routine practice. Based on the most important studies in the literature, we summarized that in premenopausal woman with abnormal uterine bleeding, an optimal cutoff for ET has not yet been established. For postmenopausal women with PMB, at low risk, and ET <4 mm, a follow-up scan could be offered, and for women with ET ≥4 mm, office hysteroscopy–guided endometrial sampling is recommended independently of ET results. On the other hand, in postmenopausal women with PMB and at high risk of EC, office hysteroscopy–guided endometrial sampling is necessary. In postmenopausal women without PMB and ET ≥4 mm, arbitrary endometrial sampling is not recommended, but evaluated case by case based on risk factors. In conclusion, there is broad consensus on the importance of TVUS and the need for further investigation based on risk factors of EC.
Introduction
Endometrial cancer (EC) is the most common gynaecological malignancy in developed countries, and its incidence is increasing in relation to certain patient characteristics that have risen sharply in recent decades, such as ageing and obesity.Citation1 The number of new EC cases in Europe in 2018 was 121,578, with 29,638 deaths. Individual risk factors related to the development of EC are nulliparity, obesity, polycystic ovary syndrome, early menarche, and late menopause.Citation2 Women with hereditary nonpolyposis colorectal cancer syndrome have an estimated cumulative incidence of EC of 20%–60% by the age of 70 years. Although most EC is diagnosed in menopause, 5%–30% of cases are diagnosed in premenopause, with a risk of 1.33%.Citation3,Citation4 As for all malignancy, early and accurate diagnosis is the first fundamental step to ensure adequate treatment and maximize overall survival. Transvaginal ultrasound (TVUS) technology in the last 20 years has significantly increased the ability of physicians to investigate the endometrium, and it has been found to be an accurate and noninvasive tool for early diagnosis and also for staging the disease.Citation5
An international consensus (International Endometrial Tumor Analysis group) describing the US characteristics of EC and studies on diagnostic accuracy focusing specifically on endometrial thickness (ET) have been published. In the literature, the diagnostic accuracy of TVUS varies greatly in specific subpopulations, such as pre- and postmenopausal patients. Indeed, during women’s reproductive years, the cyclic changing of the endometrium makes the determination of specific ET cutoff in cases of suspected EC difficult. The situation for postmenopausal women is different. In these patients, the endometrium is not influenced by hormones. Many studies have tried to determine the optimal ET cutoff for assessment of patients that need more invasive investigations like hysteroscopy and endometrial sampling.Citation6,Citation7 Considering these data, we performed a review of the most important literature on this topic in order to give physicians necessary knowledge on the role of TVUS and ET for early detection of EC in their daily routine practice.
Endometrial Thickness in Pre- and Postmenopausal Women
Evaluation of Endometrial Thickness in Premenopausal Women
In premenopausal women, the utility of TVUS for excluding malignant endometrial abnormalities has not been established. Few data are available on the efficacy of ET measurement in premenopausal women with abnormal uterine bleeding (AUB). This cohort of patients is subject to cyclic modification of the endometrium, due to the hormonal cycle. The best time to perform TVUS is on days 4–6 of the menstrual cycle, when ET should be as its thinnest.Citation8,Citation9 At the same time, in clinical practice information on the last menstruation or menstrual status is sometimes missing, so for practical reasons premenopausal patients are examined independently of their cycle day.Citation9–Citation11
Ozdemir et al evaluated ET in 144 premenopausal women with AUB, reporting that ET ≥8 mm showed the best sensitivity and specificity for endometrial abnormality screening, considering both benign and malignant conditions. Endometrial hyperplasia and cancer were diagnosed respectively in 11.8% and 5.5% of patients, and the endometrium was thicker in patients with endometrial hyperplasia (12.71±4.24mm) and EC (15.25±8.23mm).Citation10 Also, Getpook et al found that ET ≤8 mm in premenopausal women with AUB was less likely associated with a pathological endometrium but the mean ET of patients with a final diagnosis of EC was 18.55 mm, so a cutoff of 8 mm was associated with a high false-positive rate.Citation8 Minagawa et al analysed a cohort of 367 premenopausal women with and without AUB. EC was diagnosed in 1.4%: four cases had ET ≥20 mm and in one case ET was 18 mm.Citation12 Dueholm et al tried to describe a cutoff for the exclusion of polyps and hyperplasia in premenopausal patients with AUB, but the authors did not find a specific cutoff to exclude polyps and hyperplasia.Citation13 Dreisler et al investigated premenopausal women without AUB and concluded that measuring ET was not a reliable tool for a diagnosis of focal intrauterine pathology.Citation14
One of the biggest studies published, performed by Kim et al in South Korea, studied a group of 9888 women to determine clinical factors and sonographic findings associated with endometrial hyperplasia or cancer in premenopausal and perimenopausal women. Only endometrial echo abnormality had a significant association with endometrial hyperplasia. There was no significant association between ET and endometrial malignancy or premalignancy lesions.Citation15 For premenopausal women, an optimal cutoff for ET that would help clinicians in daily practice has not yet been established because of the cyclic nature of a normal menstrual cycle and the amount of variation that exists due to anatomical factors like increased parity and uterine size.Citation8,Citation9,Citation16,Citation17
Evaluation of Endometrial Thickness in Postmenopausal Women with Bleeding
TVUS is considered appropriate for an initial evaluation of a patient with postmenopausal bleeding (PMB) to assess ET. ET is the maximum anterior–posterior thickness of the endometrial echo on a long-axis transvaginal view of the uterus.Citation18,Citation19 In the literature, for maximum ET, there is no consensus on a cutoff value for a normal endometrial echo in postmenopause for either symptomatic or asymptomatic women. In fact, through the years, different ET-cutoff values have been proposed as a reliable, noninvasive approach to rule out EC in patients with PMB.
The earliest reports comparing TVS with endometrial sampling consistently found that ET of 4–5 mm or less in women with PMB reliably excluded EC.Citation20–Citation24 In 1998, the first meta-analysis was published, which included data on 5892 women with PMB. This had an important effect on clinical practice, and nowadays is the most quoted paper in guidelines. The authors estimated a 1% risk of EC in women with PMB and ET <5 mm, excluding EC with a sensitivity of 96%.Citation21 Confirming these data, a comprehensive systematic review by Gupta et al was published in 2002. From 57 studies with 9031 patients, the commonest ET cutoffs were 4 mm and 5 mm, but only four studies were identified as being of best quality. Pooling of the results of these four studies for ET 5 mm or less resulted in a likelihood ratio of a negative test of 0.16. Such a likelihood ratio would imply that in a patient with a negative test result, a pretest probability of 10% would change to a posttest probability of 2.5%. As such, the authors concluded that ET ≤5 mm detected by ultrasonography can be used to rule out endometrial hyperplasia or carcinoma with good accuracy.Citation22
On the contrary, in a meta-analysis by Tabor et al, median ET in women with EC was 3.7 times that in unaffected women at the same center. They reported sensitivity of 96% and specificity of 50% with a 4% false-negative rate if the median ET in each study (range 2–6.4 mm) was used as a cutoff. They concluded that this rate was unacceptably high for PMB, so proposed offering endometrial sampling by dilation and curettage (D&C) to all postmenopausal women with vaginal bleeding.Citation23 A new meta-analysis was performed by Timmermans et al aiming to obtain an ET cutoff for the presence of EC with virtually 100% sensitivity. According to the authors, previous data on ET measurement had probably overestimated diagnostic accuracy in the detection of EC. Based on data obtained from 13 studies on 2896 patients, of which 259 had EC, the commonly used cutoffs of 4 mm and 5 mm were found to have sensitivity of 95% and 90%, respectively. A cutoff of 3 mm presented a sensitivity of 98%. The authors thus recommended using 3 mm as a cutoff for exclusion of EC in women with PMB.Citation24
In light of these differing results, guidelines published by the American College of Obstetricians and Gynecologists (ACOG) adjudged that TVUS was appropriate for an initial evaluation of PMB in cases of thin endometrial echo (≤4 mm), given that ET ≤4 mm has an extremely high negative predictive value, >99%, for EC, while specificity varies from 42.1% to 51.5%. However, the college recommended that TVUS be used only for patients with an initial episode of bleeding when the probability of EC and hyperplasia was low enough that no additional testing would be required after a normal TVUS. Instead, endometrial sampling should be the first-line test for women with PMB considered at high risk, eg, in cases of advanced age, obesity, specific medical comorbidities (eg, polycystic ovary syndrome, type 2 diabetes mellitus, atypical glandular cells on screening cervical cytology), and family history of gynaecologic malignancy.Citation25 Another important issue is persistent or recurrent UB and ET <4 mm. This situation requires histological evaluation, because it does not reliably exclude type II EC (serous and clear-cell).Citation26–Citation28
In order to test the ACOG guideline, Saccardi et al conducted a prospective observational study investigating the clinical relevance of ET and AUB with regard to EC. They found a high prevalence (8.5%) of EC in postmenopausal women with bleeding and ET <4 mm, underlining that the value of ET in patients with PMB probably needs to be combined with other risk factors, as recommended by the ACOG guidelines.Citation29 In a large meta-analysis by Long et al on 44 studies and 17,339 women, the authors proposed an ET cutoff of 5 mm as optimal for detection of EC in patients with PMB. Sensitivity was almost identical for cutoffs of ≥3, ≥4, and ≥5 mm (96.2%, 95.7%, and 96.2%, respectively), and for NPV (99.7%, 99.4%, and 99.2%, respectively). Furthermore, by using a cutoff ≥5 mm, it was possible to reduce the number of invasive diagnostic procedures by up to 17%. An intermediate threshold (3–5 mm) requiring repeat imaging further increased the sensitivity of noninvasive testing.Citation30
Evaluation of Endometrial Thickness in Postmenopausal Women Without Bleeding
In current practice, many postmenopausal women without symptoms undergo TVUS as part of a routine gynaecological checkup, eg, to investigate abdominal pain or masses, delineate the adnexa when pelvic examination is inadequate, or evaluate a uterus prolapse, but usually TVUS without indication is not recommended. As such, an incidental finding of endometrial thickening in postmenopausal women without vaginal bleeding represents a clinical management dilemma, because there is a lack of consensus among gynaecologists. The most important international societies have concluded that no screening for EC is recommended in asymptomatic postmenopausal women, so the use of ET measurement should be avoided in the evaluation of EC risk.Citation5,Citation6,Citation18,Citation31,Citation32
The 2001 consensus statement on women with PMB published by the Society of Radiologists in Ultrasound concluded that the indications for tissue sampling of the endometrium in PMB with ET >4–5 mm does not apply to asymptomatic women observed to have a thickened endometrium.Citation33 In 2010, Goldstein et al emphasized that it is inappropriate to investigate every asymptomatic patient with ET >5 mm, emphasising the importance of evaluating risk factors of EC, such as obesity, polycystic ovary syndrome, and diabetes mellitus, in decision-making.Citation34 Others have argued against the use of ET cutoffs in workup for EC. Gambacciani et al performed a retrospective review of 850 postmenopausal women who had been investigated with outpatient hysteroscopy for various causes of endometrial thickening. The authors focused on the 148 asymptomatic postmenopausal women with ET of >4–5 mm and found one case of adenocarcinoma (0.7%), with ET of 16 mm. They found that the use of TVS as a screening tool for endometrial pathology in asymptomatic postmenopausal women generated 93.2% false-positive results. They concluded that in asymptomatic postmenopausal women, endometrial US evaluation is not worthwhile as a screening tool in common clinical practice.Citation35 Confirming these data, the American Cancer Society does not recommend routine screening of asymptomatic patients for EC.Citation36
Smith-Bindman et al in 2004 reported on a theoretical cohort of postmenopausal women and ET cutoff in asymptomatic women. The risk of EC with ET ≥11 mm and calculated EC risk of 6.7% was similar in women with PMB and ET ≥5 mm, with a calculated EC risk of 7.3%, suggesting that endometrial sampling should be performed in asymptomatic postmenopausal women with ET ≥11 mm.Citation37 This recommendation has been included in some clinical guidelines, such as the Society of Obstetricians and Gynaecologists of Canada (SOGC) guidelines.Citation38 According to these, on asymptomatic endometrial thickening, tissue sampling in woman with ET >4–5 mm should not be performed in asymptomatic patients. However, if a woman presents with ET >11 mm or other positive ultrasonographic findings (such as increased vascularity, inhomogeneous endometrium, or particulate fluid), she should be further investigated after accounting for various risk factors, such as age, obesity, diabetes, hypertension, hormone-replacement therapy, tamoxifen, and late menopausal age.Citation38,Citation39
The ACOG has stated that if an ET measurement >4 mm that is incidentally discovered in a postmenopausal patient without bleeding, there is no evidence to recommend other investigation, although an individualized assessment based on patient characteristics and risk factors is appropriate.Citation25 The first meta-analysis that compared the risk of EC in asymptomatic postmenopausal women with ET ≥11 mm versus <11 mm was published in 2018 by Alcázar et al. The authors observed that the risk of EC in women with ET ≥11 mm was almost threefold that of those with ET 5–10 mm.Citation40 Saccardi et al confirmed that a threshold of 4 mm, commonly used in cases of patients with PMB, should not be extrapolated to asymptomatic patients, due to poor positive predictive value. Confirming Smith-Bindman et al and Alcazar et al, they found a significant risk of cancer only for values of ET starting from 11 mm, with 100% sensitivity and 80% specificity for EC detection.Citation29
On the other hand, in a retrospective UK review using an ET cutoff of 10 mm in asymptomatic postmenopausal women. the yield rate of EC and atypical hyperplasia was 1.2% and 2.4%, respectively, among all patients. In patients with ET <10 mm, no malignancy was found on histopathological analysis. Instead, the yield rate of EC and atypical hyperplasia was 1.81% and 3.63%, respectively, among patients referred with ET ≥10 mm, and all these cases had at least one risk factor of endometrial pathology. The authors recommended using ET ≥10 mm as a cutoff for offering endometrial biopsy or hysteroscopy for asymptomatic postmenopausal women. For asymptomatic women with ET 4–10 mm, decisions about further investigations should be made on a case-by-case basis, taking into account such risk factors as diabetes, hypertension, obesity, and additional findings on US.Citation41
Limits of Evaluation of Endometrial Thickness in Postmenopausal Women
Many studies have underlined the inability to complete a meaningful TVUS examination with a reliable measurement of ET in all postmenopausal patients.Citation25,Citation42–Citation44 The ACOG guidelines underlined that the presence of uterine pathology, such as fibroids, adenomyosis, or previous uterine surgery, can increase the difficulty of obtaining a measurement of the endometrial echo. In these cases, failure to adequately evaluate the endometrium by US investigation should always be followed by an alternative method of evaluation like saline or gel-infused sonohysterography and office hysteroscopy.Citation25,Citation26 The use of sonohysterography has an important role in cases where ET exceeds an acceptable cutoff for proper triage for focal versus global pathology.Citation45,Citation46 A recent meta-analysis using “polyps seen on hysteroscopy” as a reference standard reported sensitivity of 85.1% and specificity of 84.5% for sonohysterography. The authors concluded that this exam can be considered a method to stratify women with PMB for further diagnostic workup and treatment with hysteroscopy.Citation47
Endometrial Sampling
For many years, D&C was the preferred method for the evaluation of patients with PMB to obtain an endometrial sample. The most important limitation of this type of sampling is blind endometrial biopsy. Since 1975, researchers have acknowledged that when using this blind diagnostic technique, >50% of the endometrial cavity went unsampled. In fact, if the pathology occupies >50% of the cavity (focal pathology), blind biopsy can potentially miss a diagnosis.Citation25,Citation48 Subsequently, in-office endometrial sampling devices became available. In 1995, Guido et al concluded that the Pipelle endometrial suction curette was an effective office device for evaluating patients at risk of EC; however, tumours localized in a polyp or small area of the endometrium may go undetected.Citation49 These data were confirmed in subsequent studies on women with PMB evaluated by TVUS and Pipelle biopsy. A Pipelle biopsy was able to be performed in only 82% of the women and a sample adequate for diagnosis was obtained in only 27%.Citation50,Citation51
Considering endometrial tissue sampling resulting in findings insufficient for diagnosis is common, if blind sampling does not reveal endometrial hyperplasia or malignancy, the ACOG guidelines underlined the importance to perform further testing, such as office hysteroscopy. Nowadays it is accepted that hysteroscopy is superior to blind endometrial biopsy and D&C and should be considered a complementary diagnostic technique in patients presenting with PMB.Citation52,Citation53 That it is time for hysteroscopy to become the standard of care for ruling out endometrial malignancies was an important conclusion of a recent editorial.Citation54 Office hysteroscopy–guided endometrial sampling is recommended when US investigation is not adequate to evaluate the endometrium in patients with persistent PMB or in cases of blind sampling being nondiagnostic for endometrial hyperplasia or malignancy.Citation25,Citation55
Special Situations
Evaluation of Endometrial Thickness in Postmenopausal Women on Hormone-Replacement Therapy (HRT)
The risk of EC in PMB in postmenopausal women on HRT is significantly less than in women not receiving HRT.Citation55 Causes of vaginal bleeding in these patients are poor compliance with therapy, liver disease, drug interactions, benign gynaecological conditions, such as endometrial or cervical polyps, cervicitis, and nongynaecologic pathology (urinary tract, gastrointestinal tract).Citation56 Hänggi et al evaluated ET in postmenopausal women on HRT, comparing the safety of three HRT regimens (oral micronized 17β-estradiol–oral sequential dydrogesterone versus transdermal 17β-estradiol–oral sequential dydrogesterone versus oral tibolone) and patients not on therapy. With 5 mm ET as cutoff, >75% of biopsies were able to be avoided. In particular ET <5 mm was indicative of an atrophic endometrium, and in these cases endometrial biopsy had no significant additional diagnostic value. In women using sequential estrogen–progestogen HRT, a progestogen-induced secretory state was related to increased ET. However, neither hyperplastic cancer nor EC was reported, and all three HRT were safe with respect to the endometrium.Citation57
Langer et al reported a 99% negative predictive value of TVUS and with an ET threshold of 5 mm for detecting endometrial pathology in asymptomatic postmenopausal women using estrogen-only or combined HRT preparations. No cases of EC were found in women with ET >5 mm, including those using estrogen-only preparations. Instead, a majority of malignant disease cases were found in women with ET >10 mm. Eight cases of complex hyperplasia, three of atypical hyperplasia, and one of adenocarcinoma were found in the study population. Based on this threshold, more than half the women underwent a biopsy, while only 4% had serious disease.Citation58 Mossa et al investigated ET in 587 women on HRT. In postmenopausal women, increased ET (≥5 mm) and increased incidence of bleeding were found. However, these results did not coincide with a higher incidence of malignant pathology in the HRT and control groups. The authors concluded that women with bleeding on HRT should undergo hysteroscopy and biopsy only with ET >8 mm.Citation59
Tamoxifen-Treated Patients
Tamoxifen is a nonsteroidal antiestrogen agent and part of the class of selective estrogen (ER)-receptor modulators. It is used in women with ER-positive breast cancer as adjuvant treatment, for treatment of metastatic breast cancer, and for reduction in breast cancer incidence in high-risk women.Citation60 A Cochrane review affirmed that 5 years of adjuvant tamoxifen treatment substantially improved the 10-year survival rate of women with ER-positive tumours or tumours of unknown ER status by reducing breast cancer recurrence and mortality.Citation61 Studies have demonstrated that therapy with tamoxifen increases the relative risk of EC two- to threefold compared to the general population, due to estrogenic activity on the endometrium.Citation62,Citation63 The increased risk of developing EC is outweighed by the important survival benefit.Citation62–Citation65
Premenopausal Women Treated with Tamoxifen
In premenopause, there is not a statistically significant difference in the incidence of EC in patients treated or untreated with tamoxifen. The only increased risk is the formation of endometrial polyps secondary to the use of tamoxifen.Citation66,Citation67 As such, in patients being treated with tamoxifen, no additional monitoring is necessary, apart from the routine controls.Citation60
Postmenopausal Women Treated with Tamoxifen
Asymptomatic Women
For asymptomatic women treated with tamoxifen, there is low correspondence between TVUS measurement of ET and abnormal pathology, because tamoxifen induces subepithelial stromal hypertrophy and/or glandular cystic atrophy that is often subendometrial or contained within polyps.Citation68,Citation69 Bertelli et al demonstrated that in asymptomatic women who had undergone hysteroscopy with endometrial sampling, malignant pathology was diagnosed in <1% of cases and there was no association between ET and endometrial malignancy.Citation70 Love et al showed that a cutoff of >5 mm had a high false-positive rate of about 46%; however, higher cutoffs, even if they decreases the false-positive rate, at the same time the loss of patients that need further investigation is increased.Citation71 Fung et al demonstrated that in asymptomatic women treated with tamoxifen, periodic screening with TVUS or endometrial sampling was not useful: 32% of 304 treated patients had increased ET, only six had endometrial atypia, and all reported a history of AUB.Citation72 Berliere et al and Vosse et al investigated patients before starting tamoxifen therapy and identified those with polyps before therapy with tamoxifen as high risk. After 5 years of treatment, incidence of atypical hyperplasia was higher than in those without lesions. Also, patients identified as high risk did not benefit from routine endometrial surveillance, but were followed closely.Citation73,Citation74 The ACOG in 2020 and other articles strongly affirmed there are was no benefit from routine surveillance in patients treated with tamoxifen, with the authors underlining the importance of individuating high risk patients (based on the presence of benign endometrial polyps before therapy) and pretreatment screening in postmenopausal women with TVUS and sonohysterography when needed or office hysteroscopy before initiation of tamoxifen therapy.Citation60,Citation72,Citation75–Citation77
Symptomatic Women
Postmenopausal patients with AUB should be investigated because this symptom can be a signal of benign or malignant endometrial pathology.Citation15 Women that present with vaginal bleeding need further investigation. In fact, many papers have reported that >80% of histological endometrial atypia was preceded by AUB.Citation60,Citation76–Citation79ACOG guidelines recommend that any AUB in tamoxifen-treated patients be investigated.Citation60 In women referred with AUB, the literature agrees that hysteroscopic examination with eventual histopathological examination of the endometrium is a preferable tool for the investigation of endometrial status independently of ET.Citation78–Citation82
Conclusion
Based on the most important studies and published guidelines in the literature, TVUS is considered an accurate, appropriate, and noninvasive technique for an initial evaluation of ET for an early diagnosis of EC, in particular in postmenopausal women (). In premenopausal women with or without AUB, an optimal cutoff for ET has not yet been established. Therefore, the routine use of ET alone is not recommended to confirm suspicions of EC or precancerous lesions. Instead, in postmenopausal women, TVUS is appropriate for an initial evaluation of PMB only in those with an initial episode of bleeding and low risk factors of EC or precancerous lesions. In this cohort of patients, in women with ET <4 mm, a follow-up scan can be offered and in women with ET ≥4 mm, office hysteroscopy–guided endometrial sampling is recommended. Instead, in postmenopausal women with PMB and high risk factors of EC, office hysteroscopy–guided endometrial sampling should be the first-line test independently of ET results.
Figure 1 Flowchart of EC risk factors and procedures for postmenopausal woman.
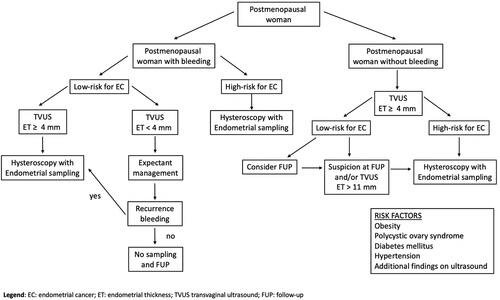
Another important category of patients is women with recurrent PMB and ET <4 mm, who require office hysteroscopy–guided endometrial sampling to exclude type II EC. On the other hand, in postmenopausal women without PMB and ET >4 mm, arbitrary endometrial sampling is not recommended, and the indication for further investigations (ie, office hysteroscopy–guided endometrial sampling) versus a follow-up scan should be evaluated case by case based on EC risk factors. The SOGC clinical practice guidelines proposed ET >11 mm and/or other positive findings on US as a cutoff for performing further investigations, always based on EC risk factors. In women with PMB on HRT, based on available data, office hysteroscopy–guided endometrial sampling should be performed if ET is ≥8 mm. Finally, in women treated with tamoxifen without AUB, TVUS for ET evaluation should not be routinely performed. Instead in women treated with tamoxifen with AUB, hysteroscopic examination with endometrial sampling is a preferable tool for the investigation of endometrial status independently of ET. There is broad consensus on the importance of TVUS and the need for further investigation based on risk factors of EC, but none on the ideal sequence of investigations, in particular in postmenopausal women with or without PMB. Future studies are needed to determine an ideal sequence of investigations to achieve greater accuracy.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39.
- Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105(3):575–580. doi:10.1097/01.AOG.0000154151.14516.f7
- Pennant ME, Mehta R, Moody P, et al. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG. 2017;124(3):404–411. doi:10.1111/1471-0528.14385
- Epstein E, Fischerova D, Valentin L, et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: prospective multicenter study. Ultrasound Obstet Gynecol. 2018;51(6):818–828. doi:10.1002/uog.18909
- Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28(5):721–739. doi:10.1016/j.bpobgyn.2014.04.007
- Singh S, Best C, Dunn S, Leyland N, Wolfman WL. No. 292-abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can. 2018;40(5):e391–e415. doi:10.1016/j.jogc.2018.03.007
- Fischerova D, Cibula D. Ultrasound in gynecological cancer: is it time for re-evaluation of its uses? Curr Oncol Rep. 2015;17(6):28. doi:10.1007/s11912-015-0449-x
- Getpook C, Wattanakumtornkul S. Endometrial thickness screening in premenopausal women with abnormal uterine bleeding. J Obstet Gynaecol Res. 2006;32(6):588–592. doi:10.1111/j.1447-0756.2006.00455.x
- Ozdemir S, Celik C, Gezginç K, Kıreşi D, Esen H. Evaluation of endometrial thickness with transvaginal ultrasonography and histopathology in premenopausal women with abnormal vaginal bleeding. Arch Gynecol Obstet. 2010;282(4):395–399. doi:10.1007/s00404-009-1290-y
- Bakos O, Heimer G. Transvaginal ultrasonographic evaluation of the endometrium related to the histological findings in pre- and perimenopausal women. Gynecol Obstet Invest. 1998;45(3):199–204. doi:10.1159/000009956
- Minagawa Y, Sato S, Ito M, Onohara Y, Nakamoto S, Kigawa J. Transvaginal ultrasonography and endometrial cytology as a diagnostic schema for endometrial cancer. Gynecol Obstet Invest. 2005;59(3):149–154. doi:10.1159/000083089
- Dueholm M, Jensen ML, Laursen H, Kracht P. Can the endometrial thickness as measured by trans-vaginal sonography be used to exclude polyps or hyperplasia in pre-menopausal patients with abnormal uterine bleeding? Acta Obstet Gynecol Scand. 2001;80(7):645–651.
- Dreisler E, Poulsen LG, Antonsen SL, et al.; European Menopause and Andropause Society. EMAS clinical guide: assessment of the endometrium in peri and postmenopausal women. Maturitas. 2013;75(2):181–190. doi:10.1016/j.maturitas.2013.03.011
- Kim MJ, Kim JJ, Kim SM. Endometrial evaluation with transvaginal ultrasonography for the screening of endometrial hyperplasia or cancer in premenopausal and perimenopausal women. Obstet Gynecol Sci. 2016;59(3):192–200. doi:10.5468/ogs.2016.59.3.192
- Park YR, Lee SW, Kim Y, et al. Endometrial thickness cut-off value by transvaginal ultrasonography for screening of endometrial pathology in premenopausal and postmenopausal women. Obstet Gynecol Sci. 2019;62(6):445–453. doi:10.5468/ogs.2019.62.6.445
- Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206. doi:10.1097/AOG.0b013e318262e320
- Sladkevicius P, Installé A, Van Den Bosch T, et al. International Endometrial Tumor Analysis (IETA) terminology in women with postmenopausal bleeding and sonographic endometrial thickness ≥ 4.5 mm: agreement and reliability study. Ultrasound Obstet Gynecol. 2018;51(2):259–268. doi:10.1002/uog.18813
- Breijer MC, Peeters JA, Opmeer BC, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2012;40(6):621–629. doi:10.1002/uog.12306
- Varner RE, Sparks JM, Cameron CD, Roberts LL, Soong SJ. Transvaginal sonography of the endometrium in postmenopausal women. Obstet Gynecol. 1991;78:195–199.
- Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510–1517. doi:10.1001/jama.280.17.1510
- Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultra-sonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand. 2002;81:799–816. doi:10.1034/j.1600-0412.2001.810902.x
- Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol. 2002;99:663–670. doi:10.1016/s0029-7844(01)01771-9
- Timmermans A, Opmeer BC, Khan KS, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(1):160–167. doi:10.1097/AOG.0b013e3181e3e7e8
- American College of Obstetricians and Gynecologists. ACOG committee opinion, No. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124–e129. doi:10.1097/AOG.0000000000002631
- American College of Obstetricians and Gynecologists. Endometrial cancer. Practice Bulletin No. 149. Obstet Gynecol. 2015;125:1006–1026. doi:10.1097/01.AOG.0000462977.61229.de
- Wong AS, Lao TT, Cheung CW, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG. 2016;123:439–446. doi:10.1111/1471-0528.13342
- Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101:120–125. doi:10.1016/j.ygyno.2005.09.042
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in post-menopausal women. Diagnostics. 2020;10(5):257. doi:10.3390/diagnostics10050257
- Long B, Clarke MA, Morillo ADM, Wentzensen N, Bakkum-Gamez JN. Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: systematic review and meta-analysis. Gynecol Oncol. 2020;157(3):624–633. doi:10.1016/j.ygyno.2020.01.032
- Timmerman D, Verguts J, Konstantinovic ML, et al. The pedicle artery sign based on sonography with color Doppler imaging can replace second-stage tests in women with abnormal vaginal bleeding. Ultrasound Obstet Gynecol. 2003;22:166–171. doi:10.1002/uog.203
- Opolskiene G, Sladkevicius P, Valentin L. Ultrasound assessment of endometrial morphology and vascularity to predict endometrial malignancy in women with postmenopausal bleeding and sonographic endometrial thickness ≥ 4.5 mm. Ultrasound Obstetr Gynecol. 2007;30:332–340. doi:10.1002/uog.4104
- Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound sponsored consensus conference statement. J Ultrasound Med. 2001;20:1025–1036. doi:10.7863/jum.2001.20.10.1025
- Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol. 2010;116:168–176. doi:10.1097/AOG.0b013e3181dfd557
- Gambacciani M, Monteleone P, Ciaponi M, et al. Clinical usefulness of endometrial screening by ultrasound in asymptomatic postmenopausal women. Maturitas. 2004;48:4221–4224. doi:10.1016/j.maturitas.2003.10.006
- American Cancer Society. Cancer Facts & Figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet Gynecol. 2004;24:558. doi:10.1002/uog.1704
- Wolfman W. No. 249-asymptomatic endometrial thickening. J Obstet Gynaecol Can. 2018;40:367–e377. doi:10.1016/j.jogc.2018.03.005
- Linkov F, Edwards R, Balk J, et al. Endometrial hyperplasia, endometrial cancer and prevention: gaps in existing research of modifiable risk factors. Eur J Cancer. 2008;44:1632–1644. doi:10.1016/j.ejca.2008.05.001
- Alcázar JL, Bonilla L, Marucco J, et al. A risk of endometrial cancer and endometrial hyperplasia with atypia in asymptomatic postmenopausal women with endometrial thickness ≥11 mm: a systematic review and meta-analysis. J Clin Ultrasound. 2018;46(9):565–570. doi:10.1002/jcu.22631
- Aggarwal A, Hatti A, Tirumuru SS, Nair SS. Management of asymptomatic postmenopausal women referred to outpatient hysteroscopy service with incidental finding of thickened endometrium - a UK district general hospital experience. J Minim Invasive Gynecol. 2021;28(10):1725–1729. doi:10.1016/j.jmig.2021.02.012
- Goldstein SR, Khafaga A. Ability to successfully image the endometrial echo on transvaginal ultrasound in asymptomatic postmenopausal women. Ultrasound Obstet Gynecol. 2021;58:625–629. doi:10.1002/uog.23667
- Sit AS, Modugno F, Hill LM, Martin J, Weissfeld JL. Transvaginal ultrasound measurement of endometrial thickness as a biomarker for estrogen exposure. Cancer Epidemiol Biomarkers Prev. 2004;13:1459–1465.
- Ragupathy K, Cawley N, Ridout A, Iqbal P, Alloub M. Non-assessable endometrium in women with post-menopausal bleeding: to investigate or ignore. Arch Gynecol Obstet. 2013;288:375–378. doi:10.1007/s00404-013-2746-7
- Kroon CD, Bock GH, Dieben SWM, Jansen FW. Saline contrast hysterosonography in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2003;110:938–947. doi:10.1111/j.1471-0528.2003.02472.x
- Kamel HS, Darwish AM, Mohamed SA. Comparison of transvaginal ultrasonography and vaginal sonohysterography in the detection of endometrial polyps. Acta Obstet Gynecol Scand. 2000;79:60–64.
- Vroom AJ, Timmermans A, Bongers MY, van den Heuvel ER, Geomini P, van Hanegem N. Diagnostic accuracy of saline contrast sonohysterography in detecting endometrial polyps in women with postmenopausal bleeding: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54:28–34. doi:10.1002/uog.20229
- Stock RJ, Kanbour A. Prehysterectomy curettage. Obstet Gynecol. 1975;45:537–541.
- Guido RS, Kanbour-Shakir A, Rulin MC, Christopherson WA. Pipelle endometrial sampling. Sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553–555.
- Elsanabesee D, Greenwood P. The performance of Pipelle endometrial sampling in a dedicated postmenopausal bleeding clinic. J Obstet Gynaecol. 2005;25:32–34. doi:10.1080/01443610400025390
- Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89:1765–1772. doi:10.1002/1097-0142(20001015)89:8<1765::AID-CNCR17>3.0.CO;2-F
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15:87–91. doi:10.1016/j.jmig.2007.10.014
- Gebauer G, Hafner A, Siebzehnrubl E, Lang N. Role of hysteroscopy in detection and extraction of endometrial polyps: results of a prospective study. Am J Obstet Gynecol. 2001;184:59–63. doi:10.1067/mob.2001.108332
- Loffer FD. The time has come to quit relying on a blind endometrial biopsy or dilation and curettage to rule out malignant endometrial changes. J Minim Invasive Gynecol. 2019;26:1207–1208. doi:10.1016/j.jmig.2019.04.011
- Carugno J. Clinical management of vaginal bleeding in postmenopausal women. Climacteric. 2020;23(4):343–349. doi:10.1080/13697137.2020.1739642
- Burbos N, Musonda P, Duncan TJ, Crocker SG, Nieto JJ, Morris EP. Postmenopausal vaginal bleeding in women using hormone replacement therapy. Menopause Int. 2012;18:5–9. doi:10.1258/mi.2011.011111
- Hänggi W, Bersinger N, Altermatt HJ, et al. Comparison of transvaginal ultrasonography and endometrial biopsy in surveillance in postmenopausal HRT users. Maturitas. 1997;27:133–143. doi:10.1016/S0378-5122(97)00037-6
- Langer RD, Pierce JJ, O’Hanlan KA, et al. Transvaginal ultrasonography compared with endometrial biopsy for the detection of endometrial disease. Postmenopausal Estrogen/Progestin Intervention Trial. N Engl J Med. 1997;337:1792–1798. doi:10.1056/NEJM199712183372502
- Mossa B, Imperato F, Marziani R, et al. Hormonal replacement therapy and evaluation of intrauterine pathology in postmenopausal women: a ten-year study. Eur J Gynaecol Oncol. 2003;24:507–512.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion. No. 336: tamoxifen and uterine cancer. Obstet Gynecol. 2006;107(6):1475–1478. doi:10.1097/00006250-200606000-00057
- Clarke MJ. WITHDRAWN: tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2008;8(4):CD000486.
- Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18(11):937–947. doi:10.1046/j.1525-1497.2003.20724.x
- Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86(7):527–537. doi:10.1093/jnci/86.7.527
- Bevers TB, Armstrong DK, Arun B, et al. Breast cancer risk reduction. J Natl Compr Canc Netw. 2010;8(10):1112–1146. doi:10.6004/jnccn.2010.0083
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi:10.3322/caac.20121
- Cheng WF, Lin HH, Torng PL, Huang SC. Comparison of endometrial changes among symptomatic tamoxifen-treated and nontreated premenopausal and postmenopausal breast cancer patients. Gynecol Oncol. 1997;66(2):233–237. doi:10.1006/gyno.1997.4739
- Chalas E, Costantino JP, Wickerham DL, et al. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol. 2005;192(4):1230–7; discussion 1237–9. doi:10.1016/j.ajog.2004.12.083
- Achiron R, Lipitz S, Sivan E, et al. Changes mimicking endometrial neoplasia in postmenopausal, tamoxifen-treated women with breast cancer: a transvaginal Doppler study. Ultrasound Obstet Gynecol. 1995;6(2):116–120. doi:10.1046/j.1469-0705.1995.06020116.x
- Goldstein SR. Unusual ultrasound appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170:447–451. doi:10.1016/S0002-9378(94)70209-8
- Bertelli G, Venturini M, Del Mastro L, et al. Tamoxifen and the endometrium: findings of pelvic ultrasound examination and endometrial biopsy in asymptomatic breast cancer patients. Breast Cancer Res Treat. 1998;47(1):41–46. doi:10.1023/A:1005820115535
- Love CD, Muir BB, Scrimgeour JB, Leonard RC, Dillon P, Dixon JM. Investigation of endometrial abnormalities in asymptomatic women treated with tamoxifen and an evaluation of the role of endometrial screening. J Clin Oncol. 1999;17(7):2050–2054. doi:10.1200/JCO.1999.17.7.2050
- Fung MF, Reid A, Faught W, et al. Prospective longitudinal study of ultrasound screening for endometrial abnormalities in women with breast cancer receiving tamoxifen. Gynecol Oncol. 2003;91(1):154–159. doi:10.1016/S0090-8258(03)00441-4
- Berlière M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40–44. doi:10.1016/S0029-7844(97)00591-7
- Vosse M, Renard F, Coibion M, Neven P, Nogaret JM, Hertens D. Endometrial disorders in 406 breast cancer patients on tamoxifen: the case for less intensive monitoring. Eur J Obstet Gynecol Reprod Biol. 2002;101(1):58–63. doi:10.1016/S0301-2115(01)00516-4
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Tamoxifen and the endometrium (C-Gyn 12), college statement; 2015.
- Fleming CA, Heneghan HM, O’Brien D, McCartan DP, McDermott EW, Prichard RS. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105(9):1098–1106. doi:10.1002/bjs.10899
- Parkash V, Fadare O, Tornos C, McCluggage WG. Committee Opinion No. 631: Endometrial Intraepithelial Neoplasia. Obstet Gynecol. 2015 Oct;126(4):897(Reaffirmed 2020).
- Saccardi C, Gizzo S, Patrelli TS, et al. Endometrial surveillance in tamoxifen users: role, timing and accuracy of hysteroscopic investigation: observational longitudinal cohort study. Endocr Relat Cancer. 2013;20(4):455–462. doi:10.1530/ERC-13-0020
- Pérez-Medina T, Salazar FJ, San-Frutos L, et al. Hysteroscopic dynamic assessment of the endometrium in patients treated with long-term tamoxifen. J Minim Invasive Gynecol. 2011;18(3):349–354. doi:10.1016/j.jmig.2010.12.014
- Garuti G, Sambruni I, Cellani F, Garzia D, Alleva P, Luerti M. Hysteroscopy and transvaginal ultrasonography in postmenopausal women with uterine bleeding. Int J Gynaecol Obstet. 1999;65(1):25–33. doi:10.1016/S0020-7292(98)00224-0
- Ceci O, Bettocchi S, Nappi L, Di Venere R, Pansini MV, Di Fazio F. Comparison of hysteroscopic and hysterectomy findings to assess the diagnostic accuracy of office hysteroscopy in tamoxifen-treated patients with breast cancer. J Am Assoc Gynecol Laparosc. 2003;10(3):392–395. doi:10.1016/S1074-3804(05)60270-8
- Litta P, Merlin F, Saccardi C, et al. Role of hysteroscopy with endometrial biopsy to rule out endometrial cancer in postmenopausal women with abnormal uterine bleeding. Maturitas. 2005;50(2):117–123. doi:10.1016/j.maturitas.2004.05.003
