Abstract
Background
Early incontinence that has great impact on the quality-of-life is one usual drawback after laparoscopic radical prostatectomy (LRP). This prospective study aims at further documenting the improved effect of the “Sandwich” urethra reconstruction technique on continence at the early stage after LRP.
Methods
During the period from October 2017 to December 2018, 130 patients undergoing LRP in our institution were recruited into this prospective study. Sixty-six patients in Group A received LRP with the “Sandwich” technique of urethra reconstruction, while the remaining 64 patients in Group B did not adopt this reconstruction technique. The basic clinical data, perioperative related data, urinary continence, and urodynamic tests were analyzed and evaluated.
Results
There is no statistical difference in patients’ basic clinical data, perioperative related data except urethral reconstruction time, which was 23.49±4.72 minutes in Group A and 20.16±5.75 minutes in Group B (P<0.001). The continence rates in Group A at 2, 4, 8, and 12 weeks were 54.55%, 83.33%, 93.94%, and 96.97%, respectively. The continence rates in Group B were 10.94%, 14.06%, 37.50%, and 71.88%, respectively. The continence rate of Group A was significantly higher after surgery compared with Group B (P<0.001). Maximum flow rates before and after the “Sandwich” procedure for 12 months were 13.2±2.8 m/s and 15.4±3.6 m/s, respectively (P=0.034). In addition, residual volumes before and after the “Sandwich” procedure for 12 months were 15 (0–20) mL and 0 (0–12.5) mL, respectively (P=0.107).
Conclusion
Our prospective study confirms that the “Sandwich” technique of the total urethral reconstruction is safe and feasible. It also very possibly takes the significant advantage in early recovery of urinary continence after LRP. However, multicenter, randomized controlled large sample randomized controlled trials are needed to further confirm this final conclusion.
Introduction
Prostate cancer is one of the most common cancers for men in the world,Citation1 and radical prostatectomy is an optimal standard method for treating patients who suffer from localized prostate cancer. With the increasing aging of the population in China, the number of patients diagnosed with prostate cancer has been continuously increasing recently.Citation2 With the development of minimally invasive techniques, LRP or robot-assisted radical prostatectomy (RALP) has become the preferred surgical method in many large medical centers.Citation3 These minimally invasive techniques have also achieved good oncological outcomes.Citation4,Citation5 Therefore, sexual function and urinary continence after LRP or RALP may be of most concern for both the surgeon and patient. Since the anatomic approach has been introduced to radical prostatectomy (RP), the surgeon has done many surgical innovations to improve urinary continence and sexual function recovery, such as intra-fascial sparing nerve techniqueCitation6,Citation7 and urethral reconstruction technique.Citation8,Citation9 In our study, we designed a prospective study to further verify the effectiveness, especially improving the continence at an early stage, of our “Sandwich” technique of the total urethral reconstruction.
Methods
All patients who have undergone LRP in our institution during the period from October 2017 to December 2018 were recruited for this prospective study. This study included 130 patients, who were divided into two groups. Sixty-six patients in Group A received LRP with “Sandwich” technique of urethra reconstruction, while the remaining 64 patients in Group B did not adopt this reconstruction technique.
The preoperative protocol included the measurement of BMI, prostate-specific antigen (PSA) before the operation, ultrasound for prostate volumes, the examination of the digital rectum (DRE), magnetic resonance imaging (MRI) of the prostate, transrectal prostate biopsy using ultrasound, and bone scintigraphy. The exclusion criteria of the study included the expectation of life being less than 10 years and suffering from metastatic or locally advanced prostate cancer. The study was approved by the Research Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences and Beijing Chaoyang Hospital, Capital Medical University. All the patients in this study provided written informed consent.
Surgical Technique
Under general anesthesia, an extraperitoneal working space was first created via a five-port approach, as described by Stolzenburg et al,Citation10 and the prostate, bladder, and endopelvic fascia were exposed. Then the endopelvic fascia was bilaterally incised and dissected towards the apex of prostate at the beginning. Next the puboprostatic ligament was cut and the dorsal venous complex (DVC) was ligated using a 15 cm and barbed thread and then the bladder neck was carefully transected and preserved. After the dissection of seminal vesicles from vas deferens on both sides, it was easy to incise Denonvillier’s fascia and dissect it down to the apex of the prostate. Next the vascular pedicle (including NVB) was preserved to the maximum extent for selected patients through Hem-O-Lok clip and the cold scissor was used for incising the prostatic fascia sharply and cutting the urethra in the external urethral sphincter center and the prostate apex while the puboprostatic ligaments were kept. Bilateral pelvic lymphadenectomy was carried out for all patients, and the specimens of prostate and lymph nodes were placed in a specimen bag and eventually removed through an umbilical incision.
In Group A, the first step of total urethral reconstruction of the “Sandwich” was started with the reconstruction of the posterior wall via reconstructing Denonvillier’s fascia in two layers. In the first layer, the upper end of the severed fascia was sutured with MDR (median dorsal ralph) (); and, in the second layer, MDR was sutured with the bladder wall at the back of the posterior lip of the bladder neck ().
Figure 1 The first layer of the reconstruction of the posterior wall of total urethral reconstruction of “Sandwich”. (A) The structural location of the medial dorsal raphe (MDR) during the operation. (B) The structural location of the severed end of Denonvillier’s fascia. (C) The severed Denonvillier’s fascia was sutured with MDR.
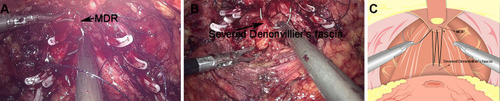
Figure 2 The second layer of the reconstruction of the posterior wall of total urethral reconstruction of “Sandwich”. (A) The structural location of the MDR during the operation. (B) The structural location of the posterior lip of the ladder neck. (C) MDR was sutured with the bladder wall at the back of the posterior lip of the bladder neck.
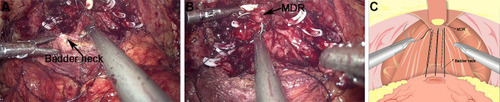
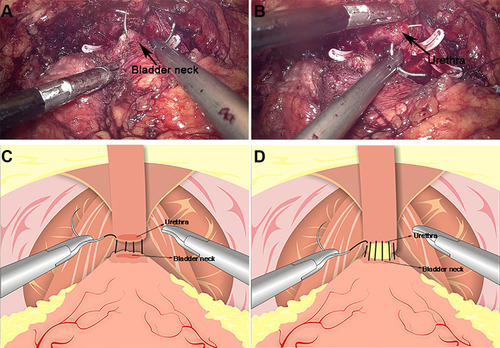
Urethrovesical anastomosis was the second step of the reconstruction. A running urethrovesical anastomosis using an absorbable barbed suture was performed with the posterior wall suturing from 4 o’clock to 8 o’clock and the front wall suturing from 3 o’clock to 9 o’clock (). The first suture was performed from outside–inside in the right posterior of the neck of the bladder, and this suture was put on the urethral stump section from inside to outside correspondingly. Later, the processes from outside–inside at the neck of the bladder and inside–outside at the urethral stump were in the left of the neck of the bladder repeatedly. The 18F Silastic Foley catheter had been inserted into the bladder gently. When it had been sutured continuously around the neck of the bladder with the order from left to right, the urethrovesical anastomosis had been finished.
Figure 3 Anastomosis of bladder and urethra of total urethral reconstruction of the “Sandwich”. (A) The structural location of the bladder neck during the operation. (B) The structural location of the urethra. (C) A running urethrovesical anastomosis was performed with the posterior wall suturing from 4 o’clock to 8 o’clock. (D) A urethrovesical anastomosis was performed with the front wall suturing from 3 o’clock to 9 o’clock.
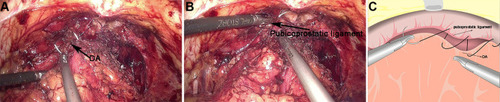
The third step was the reconstruction of the anterior wall of the urethra, including reattaching the arcus tendinous and pubicoprostatic ligament to the bladder neck (). A barbed absorbable suture was applied to approximate the remained arcus tendinous and distal triangular plate anterior to the urethra, which included the residual endopelvic fascia rhabdosphincter, puboprostatic ligaments and dorsal venous complex to the neck of the bladder. In Group B, the urethral was reconstructed in the second step in Group A only.
Figure 4 The reconstruction of the anterior of total urethral reconstruction of the “Sandwich”. (A) The structural location of the detrusor apron (DA) during the operation. (B) The structural location of the pubicoprostatic ligament. (C) The pubicoprostatic ligament is sutured with the bladder wall behind the anterior lip of the bladder neck (equivalent to the position of DA).
According to Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC-CP),Citation11 patients who achieved total control or occasional dribbling or required 0 or 1 pad were considered continent; those who have normal physical activity (walking) requiring 2–3 pads/day were defined as mildly incontinent (stress incontinence); the patients who required for more than 3 pads/day were considered as incontinent.
Statistical Analysis
A parametric continuous variable was presented as a mean ± standard deviation; non-parametric continuous variable was shown in a median and interquartile range (IQR); the t-tests of these two groups were used for comparing numerical variables. SPSS 21.0 Software (IBM Corp, Armonk, NY) was used for processing the data. The statistical significance was P<0.05.
Results
Neither conversion nor re-intervention was found in these 130 cases of Group A and Group B. Similar perioperative data were found in these two groups (). The total operative time, blood loss, duration of the catheter, and post-operative hospital stay were similar to each other in both groups (), but the time of urethral anastomosis in Group A was longer than that in the other group (P<0.01). The pathological stages and positive surgical margin (PSM) rates with a positive value in these two groups were almost the same as each other (). shows the postoperative urinary continence rate of both groups. In Group A, 54.5% of the patients were considered as continent at 2 weeks. Group B was 10.9%. Both groups’ differences were significant statistically (P<0.01). At 4 weeks, the percentages of patients who were continent in both groups were 83.3% and 14.1%, which were statistically significantly different (P<0.01). The continence rates at 8 and 12 weeks in Group A were 93.9% and 96.9%, respectively; and the continence rates in Group B were 37.5% and 71.9%, respectively. The continence rates were significant (P<0.01, respectively). We followed up the patients who had urodynamic tests and found that maximum flow rates before and after the “Sandwich” procedure for 12 months were 13.2±2.8 m/s and 15.4±3.6 m/s, respectively, which have a statistical difference (P=0.034). In addition, residual volumes before and after “Sandwich” procedure for 12 months were 15 (0–20) mL, 0 (0–12.5) mL, which have no statistical difference (P=0.107).
Table 1 Baseline Characteristics of the Patients
Table 2 Operative Date of the Patients
Table 3 Postoperative Data of the Patients
Table 4 Postoperative Urinary Continence Rate of Both Groups
Discussion
Compared with open surgical technique, laparoscopic technique takes the advantage of the hospitalization, blood loss, postoperative complications, and recovery time.Citation12 However, urinary incontinence after LRP may significantly impact patients’ quality-of-life. They may have feelings of low self-esteem, anxiety, and depression.Citation13 UI after LRP is mainly from urethral sphincter deficiency or detrusor overactivity.Citation14 Therefore, the preservation of many anatomic structures of the prostate and the way of urethral reconstruction are important factors for the recovery of urine control and even sexual function.Citation15
The anatomical structures critical to continence were affected to different extents after surgery, which encompassed the muscular components, the urethral components, and the detrusor components.Citation16 Muscular components included the neck of the bladder, internal and external rhabdosphincter, membranous urethra, and puboperinealis as well as pelvic floor muscles, which support structures of the sphincteric complex, such as arcustendinosus, puboprostatic ligament, detrusor apron, denonvilliers’ fascia, and pelvic bones as well as pelvic floor levator ani muscle, and ligaments. The urethral components involved in sphincteric incompetence, which might be caused by the length of the short urethral stump, neural innervations loss, damage to muscle and supporting tissue loss around the potential. The detrusor components involved in denovo bladder instability, detrusor over-activity or hypo-contractility due to damage to nerve or compliance of diminished bladder.Citation17
The preservation of anatomical structures, such as the endopelvic fascia, the detrusor apron nerve through intra-fascial dissecting, and suitable length of urethral membrane contributed to early recovery of urinary continence.Citation18 It was shown that nerve-preserving bilateral processes can retain the function of motor control of the urethral sphincter, thereby improving postoperative urinary control.Citation19 The detrusor apron, extended from the bladder’s interior wall with direct continuity of pubis, had aroused widespread concern recently. The detrusor apron was divided into an anterior, a middle, and a posterior layer anterior to the prostate. The anterior layer passes to the decussated pubococcygeal fibersposteriorly, defuses anteroinferior, and anchors onto the posterior surface of the pubic bone. The middle layer is loose, which connects with the fascial sheath of the dorsal venous complex (DVC). Finally, the posterior layer encloses and extends into the prostate to form the anterior fibromuscular stroma (AFMS) of the prostate.Citation20,Citation21 The urethra is also an important anatomical structure with preservation for continence essentially. Generally, the long urethral stump with lateral supporting tissue is critical to reducing the incontinence risk after the operation. It is shown that the membranous urethra retracts proximally immediately after RP while the urethral stump retracts toward the bladder after the urethrovesical anastomosis. Such processes have a negative impact on the functions of the urethral sphincter and the urethral closure pressure. But with time, such changes can be recovered to their preoperative location, and the urethral closure pressure and urinary continence can be recovered.Citation22
At present, there are a variety of urethral reconstruction techniques, such as posterior wall reconstruction (represented by Roccostitch) and bladder neck anterior wall suspension and total reconstruction of the vesicourethral anastomosis.Citation23,Citation24 In our study, the construction of the “Sandwich” urethra was started with posterior reconstruction. This surgical step mainly targets minimizing urethrosphincteric sliding after RP for supporting VUA, descending the neck of the bladder to the urethral stump closely, and providing enough contraction fulcrum for the rhabdosphincter. It was reported that the rhabdosphincter reconstruction at a posterior position led to continence recovery at an early stage and reduced anastomotic leakage.Citation25
After urethral anastomosis, the anterior suspension was started, which is another important step in sandwich reconstruction. Fixation of the neck of the bladder to the pubis is a proposed technique for anterior suspension through putting a bulbourethral stitch or suspending the neck of the bladder along the VUA with/without the ligated DVC to the pubic symphysis. According to some authors, the continence rates were 53%, 73%, and 100% at 1, 3, and 6 months, respectively, compared with the control group rates of 20%, 47%, and 83%, respectively.Citation26 In a meta-analysis, using this bimodal reconstruction increases short-term (defined as 1–12 weeks) and long-term (up to a year after the operation) urinary continence rates. In our study, comparison between the posterior reconstruction and non-reconstruction, patient’s outcomes of the urinary continence are 49% vs 24% at 3 months, and 92% vs 79% at 1-year post-RP. Once again, it shows the superiority of using sandwich reconstruction technology. It shows once again that the “Sandwich” technique of the total urethral reconstruction is beneficial to the recovery of early urine continence without affecting urodynamic parameters after RP and increasing complications, such as bleeding, positive margins, and prolonged surgical time.
Conclusion
Our prospective study confirms that the “Sandwich” technique of the total urethral reconstruction is safe and feasible. It also very possibly takes the significant advantage in early recovery of urinary continence after LRP. However, multicenter, randomized controlled large sample randomized controlled trials are needed to further confirm this final conclusion.
Abbreviations
RALP, robot-assisted radical prostatectomy; LRP, laparoscopic radical prostatectomy; NVB, neurovascular bundle; RP, radical prostatectomy; PSA, prostate-specific antigen; EDR, an examination of the digital rectum; MRI, magnetic resonance imaging; DVC, dorsal venous complex; DF, Denonvilliers’ fascia; IQR, interquartile range; UI, urinary incontinence; AFMS, anterior fibromuscular stroma; BMI, body mass index; PSM, positive surgical margin; MDR, median dorsal ralph.
Clinical Trial
The trial Registration Number is ChiCTR-IPR-15005903. The name of the registry is “Total Reconstruction” of the Urethrovesical Anastomosis Contributes to Early Urinary Continence in Laparoscopic Radical Prostatectomy. The date of registration is October 19, 2017. The URL of the trial registry record is http://www.medresman.org.cn/uc/project/projectedit.aspx?proj=2912.
Data Sharing Statement
Data used and/or analyzed in the current research can be obtained from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This trial was conducted in accordance with the Declaration of Helsinki. The study was approved by Research Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences and Beijing Chaoyang Hospital, Capital Medical University. All the patients in this study provided written informed consent. Each enrolled patient provided written informed consent.
Consent for Publication
Informed consent was obtained from the patients.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2015;33(9):1078–1085. doi:10.1200/JCO.2014.60.255725667275
- Qi D, Wu C, Liu F, et al. Trends of prostate cancer incidence and mortality in Shanghai, China from 1973 to 2009. Prostate. 2015;75(14):1662–1668. doi:10.1002/pros.2304626184773
- Rassweiler J, Hruza M, Teber D, et al. Laparoscopic and robotic-assisted radical prostatectomy–critical analysis of the results. Eur Urol. 2006;49(4):612–624. doi:10.1016/j.eururo.2005.12.05416442210
- Luzzago S, Rosiello G, Pecoraro A, et al. Contemporary rates and predictors of open conversion during minimally invasive radical prostatectomy for nonmetastatic prostate cancer. J Endourol. 2020;34(5):600–607. doi:10.1089/end.2020.007432178532
- Stolzenbach LF, Knipper S, Mandel P, et al. Oncological outcomes of pathologically organ-confined, lymph node-positive prostate cancer after radical prostatectomy. Urol Oncol. 2020. doi:10.1016/j.urolonc.2020.10.010
- Tewari A, Peabody JO, Fischer M, et al. An operative and anatomic study to help in nerve sparing during laparoscopic and robotic radical prostatectomy. Eur Urol. 2003;43(5):444–454. doi:10.1016/S0302-2838(03)00093-912705985
- Fromont G, Baumert H, Cathelineau X, et al. Intraoperative frozen section analysis during nerve sparing laparoscopic radical prostatectomy: feasibility study. J Urol. 2003;170(5):1843–1846. doi:10.1097/01.ju.0000092081.71167.3414532789
- Liao X, Qiao P, Tan Z, et al. “Total reconstruction” of the urethrovesical anastomosis contributes to early urinary continence in laparoscopic radical prostatectomy. Int Braz J Urol. 2016;42(2):215–222. doi:10.1590/S1677-5538.IBJU.2014.066627256174
- Vis AN, van der Poel HG, Ruiter AEC, et al. Posterior, anterior, and periurethral surgical reconstruction of urinary continence mechanisms in robot-assisted radical prostatectomy: a description and video compilation of commonly performed surgical techniques. Eur Urol. 2019;76(6):814–822. doi:10.1016/j.eururo.2018.11.03530514568
- Stolzenburg JU, Do M, Pfeiffer H, et al. The endoscopic extraperitoneal radical prostatectomy (EERPE): technique and initial experience. World J Urol. 2002;20(1):48–55. doi:10.1007/s00345-002-0265-412088191
- Lourenço DB, Amaral BS, Alfer-Junior W, et al. Portuguese version of the expanded prostate cancer index composite for clinical practice (EPIC-CP): psychometric validation and prospective application for early functional outcomes at a single institution. BMC Urol. 2020;20(1):163. doi:10.1186/s12894-020-00734-y33081748
- Guillonneau B, EI-Fettouh H, Beaument H, et al. Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases a Montsouris Institute. J Urol. 2003;169(4):1261–1266. doi:10.1097/01.ju.0000055141.36916.be12629339
- Da Mata LR, de Carvalho EC, Gomes CR, et al. Post-operative self-efficacy and psychological morbidity in radical prostatectomy. Rev Lat Am Enfermagem. 2015;23(5):806–813. doi:10.1590/0104-1169.0456.261826487129
- Patel MI, Yao J, Hirschhorn AD, et al. Preoperative pelvic floor physiotherapy improves continence after radical retropubic prostatectomy. Int J Urol. 2013;20(10):986–992. doi:10.1111/iju.1209923432098
- Theissen L, Preisser F, Wenzel M, et al. Very early continence after radical prostatectomy and its influencing factors. Front Surg. 2019;25(6):60. doi:10.3389/fsurg.2019.00060
- Walz J, Burnett AL, Costello AJ, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57(2):179–192. doi:10.1016/j.eururo.2009.11.00919931974
- Walz J, Epstein JI, Ganzer R, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70(2):301–311. doi:10.1016/j.eururo.2016.01.02626850969
- Laucirica O, Gomez E, Hajianfar R, Vilanova JC, Muniesa M. Complete puborectalis, puboperinealis muscle and urethral rhabdomyosphincter preservation in laparoscopic radical prostatectomy: anatomical landmarks to achieve early urinary continence. Int J Urol. 2020;27(6):525–536. doi:10.1111/iju.1422832301194
- Nandipati KC, Raina R, Agarwal A, et al. Nerve-sparing surgery significantly affects long-term continence after radical prostatectomy. Urology. 2007;70(6):1127–1130. doi:10.1016/j.urology.2007.07.04218158032
- Xu Z, Chapuis PH, Bokey L, et al. Nature and architecture of the puboprostatic ligament: a macro- and microscopic cadaveric study using epoxy sheet plastination. Urology. 2017;110:263.e1–263.e8. doi:10.1016/j.urology.2017.08.018
- Myers RP. Detrusor apron, associated vascular plexus, and avascular plane: relevance to radical retropubic prostatectomy–anatomic and surgical commentary. Urology. 2002;59(4):472–479. doi:10.1016/S0090-4295(02)01500-511927293
- Kadono Y, Nohara T, Kawaguchi S, et al. Investigating the mechanism underlying urinary continence recovery after radical prostatectomy: effectiveness of a longer urethral stump to prevent urinary incontinence. BJU Int. 2018;122(3):456–462. doi:10.1111/bju.1418129489047
- Grasso AA, Mistretta FA, Sandri M, et al. Posterior musculofascial reconstruction after radical prostatectomy: an updated systematic review and a meta-analysis. BJU Int. 2016;118(1):20–34. doi:10.1111/bju.1348026991606
- Tewari A, Jhaveri J, Rao S, et al. Total reconstruction of the vesico-urethral junction. BJU Int. 2008;101(7):871–877. doi:10.1111/j.1464-410X.2008.07424.x18321319
- Rocco BMC, Bianchi G. Editorial comment on: three-layer two-step posterior reconstruction using peritoneum during robot-assisted radical prostatectomy to improve recovery of urinary continence: a prospective comparative study by Ogawa et al. J Endourol. 2017;31(12):1258. doi:10.1089/end.2017.082329160098
- Noguchi M, Kakuma T, Suekane S, et al. A randomized clinical trial of suspension technique for improving early recovery of urinary continence after radical retropubic prostatectomy. BJU Int. 2008;102(8):958–963. doi:10.1111/j.1464-410X.2008.07759.x18485031
