Abstract
Introduction
Circular RNA (CircRNA) SCARB1 plays an oncogenic role in renal cell carcinoma, while its role in other cancers is unclear. The aim of this study was to explore the role of circRNA SCARB1 in hepatocellular carcinoma (HCC).
Methods
The expression of circRNA SCARB1, mature miR-497 and miR-497 precursor in HCC and paired non-tumor tissues from 64 HCC patients were analyzed by RT-qPCR. CircRNA SCARB1 was overexpressed in HCC cells, followed by the measurement of the expression levels of both mature miR-497 and miR-497 precursor to evaluate the effects of overexpression of circRNA SCARB1 on the maturation of miR-497. The effects of circRNA SCARB1 and miR-497 on the proliferation and migration of HCC cells were assessed by CCK-8 assay and Transwell assay, respectively.
Results
We found that circRNA SCARB1 was upregulated in HCC. In addition, mature miR-497 and miR-497 were downregulated in HCC. Correlation analysis showed that circRNA SCARB1 was inversely correlated with mature miR-497 but not miR-497 precursor. Consistently, in HCC cells, downregulated mature miR-497, but not miR-497 precursor, was observed in HCC cells transfected with circRNA SCARB1 expression vector. Analysis of cellular behaviors showed that overexpression of circRNA SCARB1 increased the proliferation and migration of HCC cells, while overexpression of miR-497 decreased cell proliferation and migration. Moreover, overexpression of miR-497 reduced the effects of overexpression of circRNA SCARB1.
Discussion
Therefore, circRNA SCARB1 is upregulated in HCC and promotes HCC cell proliferation and migration by suppressing the maturation of miR-497.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and accounts for about 85% of all cases. Citation1 The annual incidence of HCC is about 2.5 to 6.6 per 100, 000 with significantly higher incidence observed in North America and lower incidence in south-central Asia. Citation2–4 After diagnosis, the median survival time of HCC patients is only 6–20 months, and the overall 5-year survival rate is only as low as 10% Citation5 The poor prognosis of HCC is mainly due to the low early diagnostic rate and the lacking of effective treatments for advanced HCC. Citation6 Therefore, novel therapeutic approaches and sensitive diagnostic biomarkers are needed to improve the survival of HCC patients.
It is believed that more than 70% of HCC cases are attributed to chronic infections of HBV and HCV. Citation7,Citation8 However, infections of HBV and HCV are not sufficient for the development of HCC, Citation7,Citation8 suggesting the involvement of other factors, such as molecular factors, in the pathogenesis of HCC. Citation9 Increased understanding of the molecular mechanisms of HCC facilitated the development of targeted therapy for HCC, which aims to suppress HCC by regulating gene expression. Citation10,Citation11 However, effective targets for HCC targeted therapy remain lacking. Circular RNAs (circRNAs) are covalently closed RNA transcripts (single-strand) that are not directly involved in protein synthesis but participate in cancer biology by regulating transcription and translation, Citation12,Citation13 indicating that circRNAs are promising targets for the treatment of HCC. On the other hand, circRNAs are involved in the regulation of gene expression and specific biological functions by sponging targeted miRNAs. Citation14 A recent study reported that circRNA SCARB1 plays an oncogenic role in the development and progression of renal cell carcinoma. Citation15 Another study showed that circRNA SCARB1 promoted cell proliferation, invasion and migration of renal carcinoma via sponging miR-510-5p. Citation15 However, the expression and regulatory network of circRNA SCARB1 in HCC are unknown. In this study, our preliminary deep-sequencing analysis results revealed aberrant expression of circRNA SCARB1 in HCC. In addition, circRNAs can regulate gene expression and specific biological roles of HCC cells by sponging targeted miRNAs. Our bioinformatics analysis results showed that there was a potential correlation between circRNA SCARB1 and miR-497. As a tumor suppressor, miR-497 has been reported to suppress malignant phenotype of HCC, including cell proliferation, invasion, migration and angiogenesis. Citation16–18 Therefore, this study was carried out to investigate the biological roles of circRNA SCARB1 and its regulatory relationship with miR-497 in the development and progression of HCC.
Materials and Methods
Tissue Acquisition
HCC and paired adjacent (within 3 cm around tumors) non-tumor tissues were collected from a total of 64 HCC patients (40 males and 24 females) who were admitted at Xuzhou Cancer Hospital between May 2018 and May 2020. Tissue samples were stored in liquid nitrogen before use. The age range of patients was 44–68 years old, with a median age of 55.8 years old. All patients were newly diagnosed cases and patients with other clinical disorders were excluded. No therapy for any clinical disorder was performed within 3 months before the admission of patients. HBV and HCV infections were observed in 24 and 31 cases, respectively. These patients were grouped into 27 cases at AJCC stage I or II, and 37 cases at AJCC stage III or IV. This study was approved by the Ethics Committee of aforementioned hospital. All patients signed the informed consent.
HCC Cells and Transfections
Two human HCC cell lines SNU-423 and SNU-387 (ATCC, USA) were used as the cell models of HCC. Cells were cultivated in RPMI supplemented with FBS (10%) to in a 5% CO2 incubator at 37°C with 95% humidity. To overexpress circRNA SCARB1, pcDNA3.1(+) CircRNA Mini Vector (Addgene) was used as the backbone to construct the expression vector of circRNA SCARB1. Mimic of miR-497 and negative control (NC) miRNA were purchased from Sigma-Aldrich. HCC cells were seeded into a 6-well plate with 1.0×106 cells/well, followed by transfection with 1 ug vector and 40 nM synthetic miRNA oligonucleotides using Lipofectamine 2000 (Invitrogen). NC miRNA- or empty vector-transfected cells were included to serve as NC groups. The control (C) cells were cells without transfections. Cells were cultivated in fresh medium for another 48 h prior to the subsequent experiments.
RNA Preparations
Direct-zol™ RNA Miniprep Plus (Zymo Research) was used to isolate total RNAs from both tissues and cells. RNA integrity was checked by 5% urea-PAGE gel electrophoresis. The OD 260/280 ratio was checked to determine RNA purity.
RT-qPCR
The SS-IV-RT system (Invitrogen) was used to perform reverse transcriptions (RTs) with RNA samples as template to prepare cDNA samples. SYBR Green Master Mix (Bio-Rad) was used to prepare qPCR reactions with cDNA samples as template. The expression of circRNA SCARB1 was determined with 18S rRNA as an internal control. The expression of mature miR-497 and miR-497 precursor was determined using All-in-One™ miRNA qRT-PCR Reagent Kit (GeneCopoeia). To determine the expression of mature miR-497, RTs and qPCRs were performed using sequence-specific forward and reverse primers. The sequences of primers used in qRT-PCR assay were shown as follows:
CircRNA SCARB1 (Forward: 5ʹ-TCATCCTTCTAAGGTAGCAGCAG-3ʹ; Reverse: 5ʹ-CTTCGACGCTGTAGCCTTGT-3ʹ);
mature miR-497 (Forward: 5ʹ-CCTTCAGCAGCACACTGTGG-3ʹ; Reverse: 5ʹ-CAGTGCAGGGTCCGAGGTAT-3ʹ);
miR-497 precursor (Forward: 5ʹ-ACCAGCAGCACACTGTGGTTTGT-3ʹ; Reverse: 5ʹ-ATCCAGTGCAGGGTCCGAGG-3ʹ). All reactions were performed in 3 technical replicates. The 2−ΔΔcq method was used to normalize Ct values of target genes to internal controls.
CCK-8 Assay
Cells with transfections were transferred to a 96-well cell culture plate with 3,000 cells in 0.1 mL fresh medium per well. Cells were cultivated at 37°C and OD values at 450 nm were measured every 24 h for a total of 4 d. CCK-8 solution (Sigma-Aldrich) was added to 10% prior to the measurement of OD values.
Transwell Assay
Transwell inserts (8 μm pore, Corning) were used to evaluate the migration ability of HCC cells. Cells in serum-free medium were transferred to the upper Transwell chamber with 3,000 cells per well. Medium supplemented with 20% FBS was used to fill the lower chamber. Cells were cultivated at 37°C for 12 h to allow cell migration. The upper surface of membrane was cleaned with a cotton swab. The lower surface was stained with 0.5% crystal violet (Sigma-Aldrich) for 26 min in dark. Migration cells were observed and counted in 3 randomly selected fields per well under a light microscope as previously described. Citation15
Statistical Analysis
The mean values of 3 technical replicates were used to express gene expression data in HCC and paired non-tumor tissues. Data comparisons were performed by paired t-test. Mean ± standard deviation (SD) values were used to express data of 3 biological replicates of in vitro cell experiments. Data comparisons were performed by ANOVA Tukey’s test. Correlations were analyzed by Pearson’s correlation coefficient. P < 0.05 was statistically significant.
Results
The Expression of circRNA SCARB1, Mature miR-497 and miR-497 Precursor Were Altered in HCC
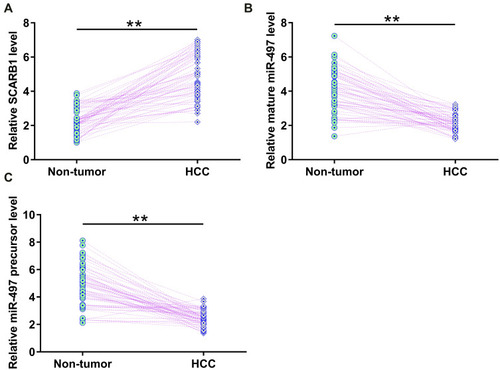
HCC and paired non-tumor tissues were collected from the 64 HCC patients included in this study, and the expression of circRNA SCARB1, mature miR-497 and miR-497 precursor in these tissue samples were determined by RT-qPCR. It was observed that circRNA SCARB1 was significantly upregulated in HCC tissues compared to that in non-tumor tissues (, p < 0.01). Based on the median expression levels of circRNA SCARB1, all patients were divided into high (n = 32) and low expression group (n = 32). The correlations between the expression levels of circRNA SCARB1 and clinicopathological parameters of HCC patients were analyzed. As shown in , the high expression levels of circRNA SCARB1 were significantly correlated with higher level of AFP (P = 0.024), larger tumor size (P = 0.021), vascular invasion (P = 0.048) and TNM stage III (P = 0.045). In addition, mature miR-497 (, p < 0.01) and miR-497 precursor (, p < 0.01) were significantly downregulated in HCC. It is worth noting that the expression of circRNA SCARB1, mature miR-497 and miR-497 precursor were not significantly different among patients with or without HBV or HCV infections. Therefore, altered expression of circRNA SCARB1 and miR-497 may participate in HCC.
Table 1 The Correlations Between Clinicopathological Variables and the Expression of circRNA SCARB1 in HCC
Figure 1 The expression of circRNA SCARB1, mature miR-497 and miR-497 precursor was altered in HCC. HCC and paired non-tumor tissues were collected from the 64 HCC patients included in this study, and the expression of circRNA SCARB1 (A), mature miR-497 (B) and miR-497 precursor (C) in these tissue samples was determined by RT-qPCR. Average values of three technical replicates were used to express gene expression data in paired HCC and non-tumor tissues, **p < 0.01.
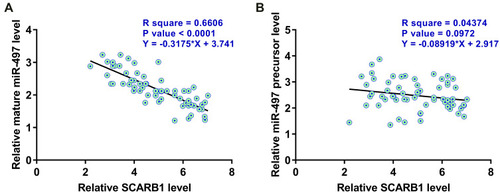
CircRNA SCARB1 and Mature miR-497 Were Inversely Correlated Across HCC Samples
Pearson’s correlation coefficient analysis was used to analyze the correlations between circRNA SCARB1 and mature miR-497 or miR-497 precursor across HCC tissue samples. It was observed that circRNA SCARB1 was significantly and inversely correlated with mature miR-497 (), but not miR-497 precursor (), across HCC samples. Therefore, circRNA SCARB1 may affect the maturation of miR-497 in HCC.
Overexpression of circRNA SCARB1 Downregulated Mature miR-497 in HCC Cells
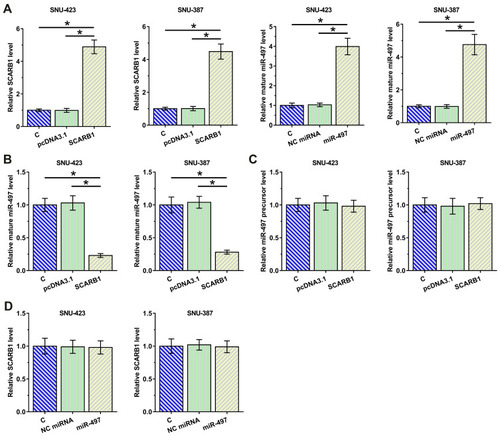
To explore the effects of overexpression of circRNA SCARB1 on the maturation of miR-497, SNU-423 and SNU-387 cells were transfected with either circRNA SCARB1 expression vector or miR-497 mimic, followed by the confirmation of transfections at 48 h post-transfection by RT-qPCR (, p < 0.05). Significantly downregulated mature miR-497 (, p < 0.05), but not miR-497 precursor () were observed in HCC cells transfected with circRNA SCARB1 expression vector. Moreover, transfection of miR-497 mimic did not affect the expression of circRNA SCARB1 (). Therefore, overexpression of circRNA SCARB1 suppressed the maturation of miR-497 from precursor to mature miRNA.
Figure 3 Overexpression of circRNA SCARB1 downregulated mature miR-497 in HCC cells. To explore the effects of overexpression of circRNA SCARB1 on the maturation of miR-497, SNU-423 and SNU-387 cells were transfected with either circRNA SCARB1 expression vector or miR-497 mimic, followed by the confirmation of transfections at 48 h post-transfection by RT-qPCR (A). The effects of overexpression of circRNA SCARB1 of the expression of mature miR-497 (B) and miR-497 precursor (C), as well as the effects of the transfection of miR-497 mimic on circRNA SCARB1 expression (D) were also analyzed by RT-qPCR. Mean ± SD values were used to express data of 3 biological replicates of in vitro cell experiments. *p < 0.05.
Overexpression of circRNA SCARB1 Increased HCC Cell Proliferation and Migration Through miR-497
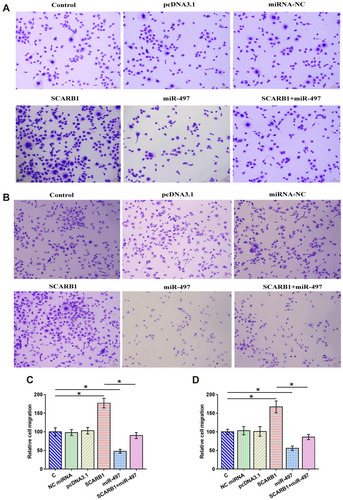
CCK-8 assay and Transwell assay were performed to explore the effects of overexpression of circRNA SCARB1 and miR-497 on the proliferation and migration of HCC cells. It showed that overexpression of circRNA SCARB1 increased the proliferation of SNU-423 (, p < 0.05) and SNU-387 cells (, p < 0.05), while overexpression of miR-497 decreased cell proliferation and migration. Similarly, the migration of SNU-423 (, p < 0.05) and SNU-387 cells (, p < 0.05) was significantly promoted by overexpression of circRNA SCARB1 and inhibited by overexpression of miR-497. To further determine the promoting effects of overexpression of circRNA SCARB1 on the proliferation and migration of HCC cells by regulating the expression of miR-497, a rescue assay was further performed. The results indicated that overexpression of miR-497 partially reversed the oncogenic role of circRNA SCARB1 in HCC cells ( and ). These data suggested that circRNA SCARB1 might accelerate the proliferation and migration of HCC cells by downregulating miR-497.
Figure 4 Overexpression of circRNA SCARB1 increased HCC cell proliferation through miR-497. CCK-8 assay was performed to analyze the effects of overexpression of circRNA SCARB1 and miR-497 on the proliferation of SNU-423 (A) and SNU-387 cells (B). Mean ± SD values were used to express data of three biological replicates of in vitro cell experiments. *p < 0.05.
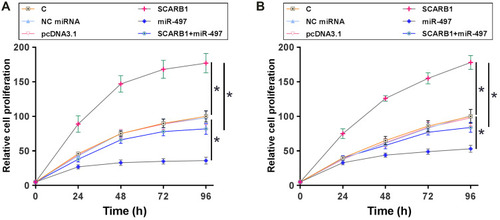
Figure 5 Overexpression of circRNA SCARB1 increased HCC cell migration through miR-497. Transwell assay was conducted to analyze the effects of overexpression of circRNA SCARB1 and miR-497 on the migration of SNU-423 (A and C) and SNU-387 cells (B and D). Mean ± SD values were used to express data of three biological replicates of in vitro cell experiments. *p < 0.05.
Discussion
This study investigated the function of circRNA SCARB1 in HCC and its interaction with miR-497. We found that circRNA SCARB1 was upregulated in HCC and it may suppress the maturation of miR-497 to promote HCC cell proliferation and migration.
To the best of our knowledge, the functionality of circRNA SCARB1 has only been investigated in renal cell carcinoma. Citation15 CircRNA SCARB1 is upregulated in renal cell carcinoma and targets miR-510-5p to upregulate SDC3, thereby promoting cancer cell proliferation, invasion and migration, as well as suppressing cell apoptosis. Citation15 In this study, we reported the upregulation of circRNA SCARB1 in HCC. In addition, the expression of circRNA SCARB1 in HCC tissues was not affected by the infection of HBV and HCV, which are major contributors to HCC. Citation7,Citation8 Therefore, the altered expression of circRNA SCARB1 in HCC is unlikely a consequence of HBV and HCV infections. Moreover, overexpression of circRNA SCARB1 significantly decreased the migration and proliferation of HCC cells. Therefore, circRNA SCARB1 may play an oncogenic role in HCC by increasing cancer cell migration and proliferation through HBV and HCV infection-independent pathways.
MiR-497 plays a tumor suppressive role in many types of cancer including HCC. Citation16–18 MiR-497 is significantly downregulated in HCC and targets multiple genes, such as VEGFA, AEG-1 and genes involved in the Rictor/Akt signal pathway to suppress tumor metastasis and angiogenesis. Citation16,Citation17 Consistently, our study also observed the downregulation of miR-497 in HCC and its inhibitory effects on cancer cell migration and proliferation.
The upstream regulators of miR-497 in cancer biology remain largely unknown. It has been well established that circRNAs can serve as the endogenous sponges of miRNAs to suppress their functions. Citation12 Interestingly, our study showed that circRNA SCARB1 could suppress the maturation of miR-497 from precursor to mature miRNA. In addition, the promoting effect of overexpression of circRNA SCARB1 on the cell proliferation and migration of HCC could be partially inhibited by upregulating the expression of miR-497. These findings suggested that circRNA SCARB1 might be involved in the tumorigenesis and progression of HCC by downregulating miR-497. Therefore, these findings further enriched our understandings of the interactions between circRNAs and miRNAs. However, the mechanism is still unknown. To be cleaved into mature miRNAs, miRNA precursors should be transported out of nucleus to cytoplasm. Citation19 Therefore, circRNA SCARB1 may suppress the transportation of miR-497 precursor. Our future studies will explore this possibility.
In conclusion, circRNA SCARB1 is upregulated in HCC and it may suppress the maturation of tumor suppressive miR-497 to promote HCC cell proliferation and migration.
Data Sharing Statement
The data during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Xuzhou Cancer Hospital. All the 64 patients signed informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors declare that they have no competing interests.
References
- Yang JD , Roberts LR . Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol . 2010;7:448–458. doi:10.1038/nrgastro.2010.100 20628345
- Njei B , Rotman Y , Ditah I , et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology . 2015;61:191–199. doi:10.1002/hep.27388 25142309
- Altekruse SF , Henley SJ , Cucinelli JE , et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol . 2014;109:542–553. doi:10.1038/ajg.2014.11 24513805
- Venook AP , Papandreou C , Furuse J , et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist . 2010;15:5–13. doi:10.1634/theoncologist.2010-S4-05
- Altekruse SF , McGlynn KA , Dickie LA , et al. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology . 2012;55:476–482. doi:10.1002/hep.24710 21953588
- Tsuchiya N , Sawada Y , Endo I , et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol . 2015;21:10573–10583. doi:10.3748/wjg.v21.i37.10573 26457017
- Arzumanyan A , Reis HM , Feitelson MA . Pathogenic mechanisms in HBV-and HCV-associated hepatocellular carcinoma. Nat Rev Cancer . 2013;13:123–135. doi:10.1038/nrc3449 23344543
- El-Serag HB . Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology . 2012;142:1264–1273. e1. doi:10.1053/j.gastro.2011.12.061 22537432
- Alqahtani A , Khan Z , Alloghbi A , et al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina . 2019;55:526. doi:10.3390/medicina55090526
- Ding XX , Zhu QG , Zhang SM , et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget . 2017;8:55715–55730. doi:10.18632/oncotarget.18382 28903454
- Thillai K , Ross P , Sarker D . Molecularly targeted therapy for advanced hepatocellular carcinoma – a drug development crisis? World J Gastrointest Oncol . 2016;8:173–185. doi:10.4251/wjgo.v8.i2.173 26909132
- Patop IL , Kadener S . circRNAs in cancer. Curr Opin Genet Dev . 2018;48:121–127. doi:10.1016/j.gde.2017.11.007 29245064
- Meng S , Zhou H , Feng Z , et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer . 2017;23:94. doi:10.1186/s12943-017-0663-2
- Hansen TB , Jensen TI , Clausen BH , et al. Natural RNA circles function as efficient microRNA sponges. Nature . 2013;495:384–388. doi:10.1038/nature11993 23446346
- Sun J , Pan S , Cui H , et al. CircRNA SCARB1 promotes renal cell carcinoma progression via miR-510-5p/SDC3 axis. Curr Cancer Drug Targets . 2020;20(6):461–470. doi:10.2174/1568009620666200409130032 32271695
- Yan JJ , Zhang YN , Liao JZ , et al. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget . 2015;6:29527–29542. doi:10.18632/oncotarget.5012 26336827
- Zhang M , Wu J , Zhang R , et al. miR-497 inhibits the carcinogenesis of hepatocellular carcinoma by targeting the Rictor/Akt signal pathway. Int J Clin Exp Pathol . 2019;12:1992–2000.31934021
- Ding Q , He K , Luo T , et al. SSRP1 contributes to the malignancy of hepatocellular carcinoma and is negatively regulated by miR-497. Mol Ther . 2016;24:903–914. doi:10.1038/mt.2016.9 26755331
- Achkar NP , Cambiagno DA , Manavella PA . miRNA biogenesis: a dynamic pathway. Trends Plant Sci . 2016;21:1034–1044. doi:10.1016/j.tplants.2016.09.003 27793495