Abstract
Background
This study explored the efficacy of lenalidomide plus rituximab for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) including cases of secondary central nervous system (CNS) involvement and transformed follicular lymphoma (FL) in real-world context because of anti-tumor effect and blood–brain barrier permeability of lenalidomide.
Methods
Twenty-four patients including relapsed or refractory DLBCL (n = 21) including seven patients with secondary CNS involvement and transformed FL (n = 3) were retrospectively analyzed.
Results
Based on the best response, the complete response (CR) rate was 21% (5/24) and the overall response rate (ORR) was 38% (9/24). However, as all cases of transformed FL (n = 3) did not respond, all responders had DLBCL, and the CR and ORR rates of DLBCL were 24% (5/21) and 43% (9/21), respectively. The median number of treatment cycles was only two (range: 1–7) due to frequent occurrence of early progression, and 18 patients died and the cause of death was disease progression. The response rate was not significantly different among patients with and without secondary CNS involvement. The median post-treatment overall and progression-free survival were 7.3 and 1.8 months, respectively. Hematologic toxicities were common adverse events, but most hematologic toxicities were manageable. There were no serious infectious complications or treatment-related mortality.
Conclusion
Lenalidomide plus rituximab might be a salvage therapy for relapsed or refractory DLBCL, especially in case of secondary CNS involvement. However, the addition of other agents should be considered to prolong the duration of response.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma in adults, accounting for more than 30% of all newly diagnosed B-cell lymphomas. Citation1,Citation2 As DLBCL is highly heterogeneous, with variable pathogenesis and cell of origin, DLBCL has been dichotomized into two major subtypes, germinal center B-cell-like (GCB) and activated B-cell-like (ABC) according to the cell of origin. Survival outcomes of GCB type were in general better than those of ABC type. Citation3,Citation4 Currently, immunochemotherapy regimens including rituximab, anti-CD20 monoclonal antibody, such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) have improved the treatment outcome of newly diagnosed DLBCL patients. Citation5,Citation6 Nevertheless, 30% to 40% of patients showed disease relapse in the first two years after completion of R-CHOP, and approximately 10% of patients developed primary refractory disease. Citation7 In particular, patients with high tumor burden frequently showed aggressive disease course including central nervous system (CNS) relapse resulting in poor survival outcome. Citation8,Citation9 Once DLBCL patients relapsed or progressed, salvage chemotherapy followed by autologous stem cell transplantation (ASCT) was planned for responders. However, not all patients respond to salvage chemotherapy, and the risk of treatment-related morbidity could increase due to the intensive nature of salvage chemotherapies. Citation10,Citation11 Furthermore, elderly or frail patients were not eligible for ASCT, and there was no accepted standard of care for patients who failed after ASCT. Citation12 As a result, there has been unmet need for more effective and less toxic salvage treatment than what conventional chemotherapies can offer.
Lenalidomide, an immunomodulatory drug originally developed for multiple myeloma, stimulates T- and natural killer (NK) cell-mediated cytotoxicity increasing functions of tumor-infiltrating lymphocytes and NK cells. Citation13,Citation14 Lenalidomide also induces the ubiquitination of transcription factors Aiolos and Ikaros leading to the inhibition of tumor cell proliferation. Citation15,Citation16 Indeed, lenalidomide has demonstrated single-agent activity for relapsed or refractory DLBCL with response rates of 19–28%. Citation17,Citation18 An observational retrospective study regarding the efficacy of lenalidomide alone (10–25 mg/day of lenalidomide for 21 days of a 28-day cycle) also showed 29.4% response rate in 153 patients with relapsed or refractory DLBCL. Citation19 Lenalidomide was also expected to act synergistically with rituximab by enhancing antibody-dependent cell-mediated cytotoxicity. Citation20 Combined treatment with lenalidomide plus rituximab showed promising activity in patients with relapsed or refractory DLBCL in previous Phase II studies. Citation21,Citation22 However, the outcome of well-designed clinical trials might not reflect the real-world situation since most trials exclude patients with poor organ function or secondary CNS involvement as ineligible for enrollment, the latter of which is a significant event often encountered during the treatment of DLBCL. Likewise, histological transformation to DLBCL can occur in follicular lymphoma (FL) patients with disease progression, and there are also unmet needs for transformed FL because most patients with transformed FL have become refractory to salvage treatments. Citation23
Considering the well-known blood-brain barrier permeability of lenalidomide and its feasibility of use for elderly or frail patients, the efficacy of lenalidomide and rituximab should be studied for heavily pretreated DLBCL patients in real-world context. However, there have been few studies on its real-world use, especially in Asian patients. Thus, we conducted this retrospective study to explore the efficacy of lenalidomide plus rituximab in terms of response rate and survival outcome in heavily pretreated patients with relapsed or refractory DLBCL including cases of secondary CNS involvement and transformed FL.
Methods
This study retrospectively analyzed the outcome of relapsed or refractory patients with DLBCL and transformed follicular lymphoma (FL) who received the combination regimen of lenalidomide and rituximab as a salvage treatment at the Samsung Medical Center, Korea. The treatment was conducted outside of a clinical trial context between January 2019 and December 2020. Since this regimen was not covered by the National Health Insurance, all patients gave written informed consent prior to the initiation of treatment, and paid for treatment out of pocket. The starting dose of lenalidomide was 25 mg per day for 21 days of a 28-day cycle, and the dosage of lenalidomide could be reduced to 20 or 15 mg per day at physician’s discretion. Rituximab was intravenously administered at the dosage of 375 mg/m2 on days 1, 8, 15, and 22 of the 1st cycle. After that, the same dose of rituximab was administered on day one of every cycle, and dose adjustments for rituximab were not performed. Patients continued to receive the treatment until disease progression or the occurrence of unacceptable toxicity. We administrated 100mg of aspirin to all patients for prophylaxis of venous thromboembolism. This study analyzed patients with relapsed or refractory DLBCL and transformed FL. The primary objective was the assessment of overall response rate (ORR) that was defined as the proportion of patients who achieve a complete response (CR) or partial response (PR). The response to treatment was evaluated using computed tomography (CT) and/or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scans. Magnetic resonance imaging (MRI) was utilized for CNS evaluation. The response to treatment was assessed by investigators based on the Lugano Classification. Citation24 The secondary objective was the estimation of post-treatment progression-free survival (PFS) and overall survival (OS), and the occurrence of treatment-related hematologic and non-hematologic adverse events was also assessed by the review of medical records.
Demographic information and patients’ characteristics were obtained by the review of medical records including age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, Ann-Arbor stage, and serum lactate dehydrogenase (LDH). All patient histologic diagnoses were made by the lymphoma pathologist (JC), and the cell of origin was determined by the Hans algorithm. However, we performed the Lymph2Cx assay using five cases for the comparison of two methods, and found the results were same as that of Hans algorithm as previously reported. Citation3 In addition, the association of mutation profiles with outcome was explored in patients who had data available for analysis. After informed consent, targeted sequencing using the HemaSCAN was performed with paraffin-embedded tissue samples containing 425 genes related to hematological malignancies as previously described. Citation25,Citation26 Briefly, genomic DNA was extracted using a QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA). The mean sequencing coverage was greater than 700x. Somatic alterations including mutations, copy number alteration, and structure variants were called using a previously described pipeline: MuTect version 1.1.6, Lowfreq version 0.6.1, Pindel version 0.2.5a4 software, and a custom-built in-house algorithm were used. Citation26–28
For statistical analysis, demographics and patient characteristics were summarized by descriptive statistics, and the chi-square test was used for comparison of characteristics. The Kaplan–Meier method was used for univariate analysis of survival outcomes, and the Log rank test was used for comparisons. The post-treatment OS was defined as the time from initiation of lenalidomide and rituximab treatment to the date of death from any cause, and was censored at the date of last available follow-up. The post-treatment PFS was measured from initiation of treatment to progression, relapse or death from any cause, and was also censored at the date of last available follow-up. All data were analyzed using the Statistical Package for Social Sciences software, version 24.0 (IBM Corp, Armonk, NY, USA).
Results
Patient Characteristics Prior to Lenalidomide and Rituximab Treatment
We analyzed 24 patients including relapsed or refractory DLBCL (n = 21) and transformed FL (n = 3), and they were treated with R-CHOP after diagnosis with the exception of one FL patient receiving bendamustine and rituximab (BR). However, this FL patient showed large cell transformation after the 3rd cycle of BR, and he subsequently received R-CHOP as a salvage treatment. Thus, prior to lenalidomide and rituximab treatment, all patients received R-CHOP. As most patients were heavily pretreated with salvage chemotherapies including seven cases undergoing ASCT, the median number of prior lines of chemotherapy was four (range: 2–9). Among 21 patients with de novo DLBCL, the ABC type (n = 14) was more common than GCB type (n = 7). The median age at the time of lenalidomide and rituximab treatment was 61 years (range: 32–78 years). All but one patient had stage IV disease, and more than 70% of patients had high-intermediate/high risk of International Prognostic Index (IPI) prior to lenalidomide and rituximab treatment (). Disease status prior to the initiation of lenalidomide and rituximab showed 17 patients had disease refractory to primary treatment, which was defined as disease progression during treatment or relapse within 6 months after the completion of primary treatment (). Among the remaining seven patients with relapsed disease, three patients relapsed within 12 months after primary treatment whereas the other four patients relapsed more than 40 months after the first line treatment. Accordingly, the time intervals between the first diagnosis and the initiation of lenalidomide and rituximab treatment were variable among 24 patients (). As 16 patients (67%) were previously treated with more than three chemotherapies before receiving lenalidomide and rituximab treatment, the time intervals between the first relapse and the initiation of lenalidomide and rituximab treatment were also variable (median time interval: 14.8 months, range: 7.6–99.6 months, ). At the time of lenalidomide and rituximab treatment, seven patients had secondary CNS involvement (29%), and the laboratory findings showed 58% of patients had anemia and 71% had decreased platelet count (). In addition, decreased albumin level was also observed in 42% of patients ().
Table 1 Characteristics of Patients Prior to Lenalidomide and Rituximab Treatment
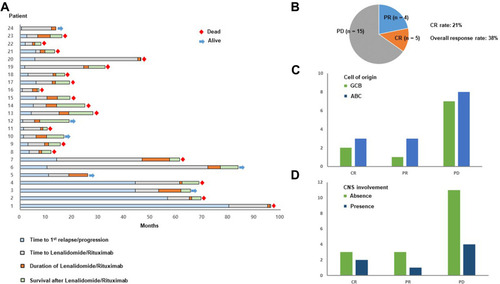
Figure 1 (A) Swimmer plot of 24 patients. The first blue bar represents the time to 1st relapse or progression of each patient whereas the gray bar represents the time between the 1st relapse or progression and the time of beginning lenalidomide and rituximab. Accordingly, the following orange bar represents the treatment duration of lenalidomide and rituximab, and the subsequent green bar represents survival duration after the discontinuation of lenalidomide and rituximab. (B) The response rates of lenalidomide and rituximab based on the best response. CR: complete response; PR: partial response; PD: progressive disease (C) The numbers of responders and non-responders are compared by the cell of origin that was determined by the Hans algorithm. Green bar: patients with germinal center B-cell type; Blue bar: patients with activated B-cell like type (D) Responses are compared between patients who had secondary central nervous system (CNS) involvement (blue bar) and patients without CNS involvement (green bar) at the time of starting lenalidomide and rituximab.
Response to Lenalidomide and Rituximab Treatment
Based on the best response, five patients showed CR and four patients had PR whereas 15 patients showed rapid progression. As a result, the CR rate was 21% (5/24) and the ORR was 38% (9/24) (). However, as all cases of transformed FL (n = 3) did not respond, all responders had DLBCL, and the ORR and CR rate of DLBCL were 43% (9/21) and 24% (5/21), respectively. The median number of treatment cycles was only two (range: 1–7) due to frequent occurrence of early progression; 18 patients died and the cause of death was disease progression (). Of the six living patients, three patients maintained their response whereas the other three patients received different salvage therapies. Although the difference was not significant due to small number of responders, patients with CR or PR were higher in ABC than in GCB type (). The response rate was not significantly different among patients with and without secondary CNS involvement; thus, three patients responded out of seven patients with secondary CNS involvement (). Actually, one patient with both CNS and systemic disease progression responded to lenalidomide and rituximab treatment ().
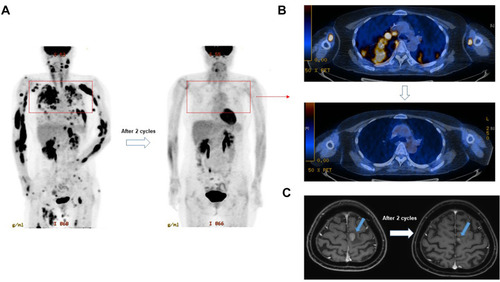
Figure 2 A representative case responding to lenalidomide and rituximab treatment after systemic and CNS relapse. (A and B) The PET/CT scan showed the disappearance of multiple FDG avid lesions after two cycles of lenalidomide and rituximab. (C) The brain MRI also showed the decrease of mass lesion (blue arrows) after two cycles of treatment.
Survival After Lenalidomide and Rituximab Treatment
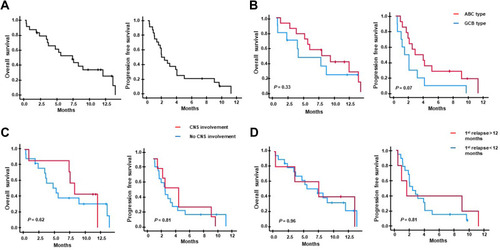
As only nine patients responded, and early progression occurred after lenalidomide and rituximab treatment in 15 patients, the median post-treatment OS and PFS were 7.3 months (95% CI: 3.8–10.8 months) and 1.8 months (95% CI: 0.9–2.8 months), respectively (). Survival outcomes based on the cell of origin showed no difference in comparison between ABC and GCB types although patients with ABC type showed a tendency toward better post-treatment PFS than patients with GCB type (). The survival outcomes of seven patients with secondary CNS involvement were not significantly different from those of patients without secondary CNS involvement (). When we compared survival outcomes of patients with early relapse, defined as the occurrence of relapse within 12 months from the first-line treatment, to those of patients with relapse later than 12 months, there was no significant difference, either ().
Figure 3 (A) Overall and progression-free survival after lenalidomide and rituximab treatment. (B) The patients with activated B-cell like type (ABC type, red line) shows a tendency of better post-treatment overall and progression-free survival compared to the patients with germinal center B-cell type (GCB type, blue line) although their differences are not statistically significant. (C) The post-treatment overall and progression-free survival are not different between patients who had secondary CNS involvement (red line) and patients without secondary CNS involvement (blue line) prior to lenalidomide and rituximab treatment. (D) The post-treatment overall and progression-free survival are not different between patients with early 1st relapse (the occurrence of relapse within 12 months, red line) and late 1st relapse (the occurrence of relapse after 12 months, blue line).
Safety Outcomes
During treatment, 18 patients (75%) experienced any grade of neutropenia and 15 patients were grade 3 or 4. Among 15 patients with grade 3 or 4 neutropenia, six patients (25%) had bacteremia. Although one patient had herpes zoster during treatment, there was no cytomegalovirus infection or pneumocystis carinii pneumonia. The majority of patients with neutropenia had anemia and thrombocytopenia. Grade 3 or 4 anemia and thrombocytopenia was reported in 11 cases (46%) and 12 cases (50%), respectively. Owing to hematologic toxicity, dose adjustment of lenalidomide was done in four patients. There were five cases of renal toxicity. Three patients showed grade 1 of serum creatinine elevation (less than 1.5 times of normal upper limit) whereas two patients experienced grade 2 (less than 3.0 times of normal upper limit). However, there were no cases requiring hemodialysis.
Mutation Profiles and Outcomes
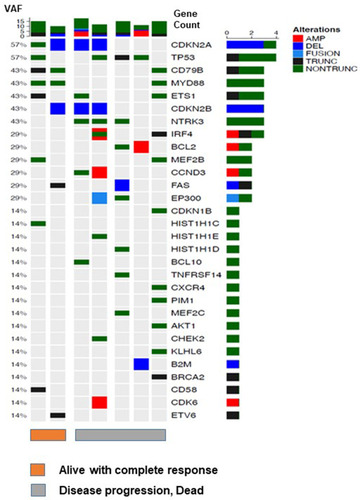
Among 24 patients, targeted sequencing was done in 7 patients who had archived tissue samples available for analysis. Although the number of patients was small, frequently mutated genes were CDKN2A, TP53, CD79B, MYD88, ETS1, CDKN2B and NTRK3 (). When the mutation profiles were compared between two alive patients with CR and five dead patients who were refractory to lenalidomide and rituximab, the number of additional mutated genes such as in IRF4, BCL2, CCND3, and EP300 was higher in patients with disease progression than alive patients with CR ().
Figure 4 Targeted sequencing of seven patients. Among 24 patients, the mutation profiles were compared between two alive patients with complete response (orange bar) and five dead patients who were refractory to lenalidomide and rituximab (gray bar).
Discussion
In this study, we analyzed the real-world, single center data of combination treatment with lenalidomide and rituximab as a salvage therapy for relapsed or refractory DLBCL and transformed FL, and reported 38% of ORR (9/24) with the CR rate of 21% (5/24). Our response rate was comparable to that of a previous retrospective study reporting 41% of ORR in 17 patients with relapsed or refractory DLBCL as well as aforementioned previous phase II studies (). Citation21,Citation22,Citation29 Although the number of transformed FL cases was only three in our study, they all failed to respond. Thus, all responders (5 CR and 4 PR) had DLBCL, and the ORR of DLBCL was 43% (9/21) with CR rate of 24% (5/21). However, the absence of response in our three patients with transformed FL was different from that of the previous phase II study reporting the ORR of 56% in nine patients with transformed FL. Citation22 As the number of transformed FL patients in the current work was too small to comment on this discrepancy, the efficacy of lenalidomide and rituximab in transformed FL cases should be further investigated in future studies with large study populations.
Table 2 Summary of Lenalidomide and Rituximab for Relapsed or Refractory DLBCL
Secondary CNS involvement such as CNS relapse remains a challenging issue in the treatment of DLBCL because damage to the CNS, a chemo-sanctuary site, has negative impact on the prognosis of DLBCL. Citation9 In our study, seven patients with secondary CNS involvement received lenalidomide and rituximab, and three of the seven showed response with decrease of brain lesions (). Thus, the response rate was not significantly different among patients with or without secondary CNS involvement although the number of patients with secondary CNS involvement was relatively small (). These results implied the combined treatment of lenalidomide and rituximab might become a salvage treatment in the case of secondary CNS involvement where treatment options are very limited but should be consistent with proven efficacy for primary CNS DLBCL. Citation30
The cell of origin was also reported to be associated with the response to lenalidomide in DLBCL because lenalidomide could selectively kill tumor cells by targeting interferon regulatory factor-4 in the ABC type of DLBCL. Citation31 Actually, the addition of lenalidomide to R-CHOP showed promising results in single-arm phase II studies, particularly in ABC type of DLBCL. Citation32,Citation33 A recent ECOG randomized phase II study comparing R-CHOP with lenalidomide plus R-CHOP also demonstrated the benefit of lenalidomide in DLBCL patients including the ABC type. Citation34 However, a recent Phase III trial (ROBUST) comparing R-CHOP with lenalidomide plus R-CHOP failed to show positive impact of lenalidomide on the survival outcome of patients with ABC or non-GCB type DLBCL. Citation35 In our study, patients with CR or PR were higher in ABC than GCB type and the ABC type patients showed a tendency to better PFS than those of GCB type although the difference was not significant due to small number of responders ( and ). Nevertheless, our results should be cautiously interpreted because the cell of origin was determined by the Hans algorithm in our study.
Hematologic toxicities such as neutropenia and thrombocytopenia were the common adverse events during treatment. However, most hematologic toxicities were manageable with supportive care. Thus, there were only four patients (16.6%) who underwent dose reduction, and no serious infectious complication or treatment-related mortality occurred. Thus, the combined treatment of lenalidomide with rituximab could be safely used for patients with relapsed or refractory DLBCL, in particular elderly, frail patients.
Nevertheless, the median number of treatment cycles was only two (range: 1–7) due to frequent occurrence of early progression, and 18 patients died due to disease progression at the time of analysis (). Accordingly, the median post-treatment OS and PFS were 7.3 months (95% CI: 3.8–10.8 months) and 1.8 months (95% CI: 0.9–2.8 months), respectively (). These relatively short durations of response and post-treatment survival in our study were consistent with those of the previous phase II study reporting 10 months of post-treatment OS in relapsed or refractory DLBCL. Citation22 Considering this limited role of lenalidomide and rituximab treatment in terms of response duration and survival outcome, the addition of other agents should be explored on the backbone treatment of lenalidomide and rituximab. Indeed, there have been several efforts to increase the response rate and prolong the duration of response by adding nivolumab or ibrutinib to lenalidomide and rituximab treatment. Citation36,Citation37 Our targeted sequencing with seven cases showed commonly occurring mutations related to B-cell receptor signaling pathways, such as CD79B and MYD88, and the number of additional mutated genes such as IRF4, BCL2, and CCND3 was higher in patients with disease progression compared to living patients with CR (). Given this enrichment of mutations in poor responder patients, the addition of novel agents to lenalidomide and rituximab treatment might be a promising approach for the treatment of relapsed or refractory DLBCL.
Taken together, our study shows that the lenalidomide and rituximab treatment might be an effective salvage therapy for relapsed or refractory DLBCL with manageable toxicity, especially in case of secondary CNS involvement. However, lenalidomide and rituximab treatment should be combined with other novel agents or chemotherapy regimens to improve efficacy leading to durable response. Further investigations with larger study populations should be conducted to determine the value of this combination.
Ethics Approval and Consent to Participate
The Institutional Review Board of the Samsung Medical Center approved this study (IRB No: 2020-12-074-001). The requirement for informed consent was waived due to the retrospective nature of the study. We used only anonymized information from patients’ medical charts. All methods were carried out in accordance with relevant guidelines and regulations. All study procedures were approved by the Institutional Review Board and were conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no competing interests to declare.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1C1C1005945).
References
- Teras LR , DeSantis CE , Cerhan JR , Morton LM , Jemal A , Flowers CR . 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin . 2016;66(6):443–459. doi:10.3322/caac.21357 27618563
- Sim J , Takayama T , Cho J , et al. Changing trends in lymphoid neoplasm distribution in South Korea: analysis of 8615 cases from a single institute, 1997–2016: an observational study. Medicine . 2019;98(45):e17641. doi:10.1097/MD.0000000000017641 31702615
- Cho I , Yoon N , Hyeon J , et al. Comparison of the lymph2Cx assay and Hans algorithm in determining the cell-of-origin of diffuse large B-cell lymphomas, not otherwise specified. Appl Immunohistochem Mol Morphol . 2020;28(10):731–740. doi:10.1097/PAI.0000000000000843 32287077
- Wright G , Tan B , Rosenwald A , Hurt EH , Wiestner A , Staudt LM . A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A . 2003;100(17):9991–9996. doi:10.1073/pnas.1732008100 12900505
- Sehn LH , Donaldson J , Chhanabhai M , et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol . 2005;23(22):5027–5033. doi:10.1200/JCO.2005.09.137 15955905
- Coiffier B , Thieblemont C , Van Den Neste E , et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood . 2010;116(12):2040–2045. doi:10.1182/blood-2010-03-276246 20548096
- Maurer MJ , Ghesquieres H , Jais JP , et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol . 2014;32(10):1066–1073. doi:10.1200/JCO.2013.51.5866 24550425
- Hong J , Kim SJ , Chang MH , et al. Improved prognostic stratification using NCCN- and GELTAMO-international prognostic index in patients with diffuse large B-cell lymphoma. Oncotarget . 2017;8(54):92171–92182. doi:10.18632/oncotarget.20988 29190906
- Kim SJ , Hong JS , Chang MH , et al. Highly elevated serum lactate dehydrogenase is associated with central nervous system relapse in patients with diffuse large B-cell lymphoma: results of a multicenter prospective cohort study. Oncotarget . 2016;7(44):72033–72043. doi:10.18632/oncotarget.12459 27713132
- Gisselbrecht C , Glass B , Mounier N , et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol . 2010;28(27):4184–4190. doi:10.1200/JCO.2010.28.1618 20660832
- Hitz F , Connors JM , Gascoyne RD , et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol . 2015;94(11):1839–1843. doi:10.1007/s00277-015-2467-z 26246466
- Salles GA , Pettengell R , Cordoba R , Długosz-Danecka M , Jurczak W , Tilly H . Treatment of aggressive B-cell non-Hodgkin lymphoma beyond frontline therapy in patients not eligible for stem cell transplantation: a structured review. Leuk Lymphoma . 2019;60(7):1610–1625. doi:10.1080/10428194.2018.1564828 30702000
- Wu L , Adams M , Carter T , et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res . 2008;14(14):4650–4657. doi:10.1158/1078-0432.CCR-07-4405 18628480
- Ramsay AG , Clear AJ , Kelly G , et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood . 2009;114(21):4713–4720. doi:10.1182/blood-2009-04-217687 19786615
- Lopez-Girona A , Mendy D , Ito T , et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia . 2012;26(11):2326–2335. doi:10.1038/leu.2012.119 22552008
- Gandhi AK , Kang J , Havens CG , et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol . 2014;164(6):811–821. doi:10.1111/bjh.12708 24328678
- Wiernik PH , Lossos IS , Tuscano JM , et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol . 2008;26(30):4952–4957. doi:10.1200/JCO.2007.15.3429 18606983
- Witzig TE , Vose JM , Zinzani PL , et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol . 2011;22(7):1622–1627. doi:10.1093/annonc/mdq626 21228334
- Broccoli A , Casadei B , Chiappella A , et al. Lenalidomide in pretreated patients with diffuse large B-cell lymphoma: an Italian observational multicenter retrospective study in daily clinical practice. Oncologist . 2019;24(9):1246–1252. doi:10.1634/theoncologist.2018-0603 30940746
- Hernandez-Ilizaliturri FJ , Reddy N , Holkova B , Ottman E , Czuczman MS . Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res . 2005;11(16):5984–5992. doi:10.1158/1078-0432.CCR-05-0577 16115943
- Zinzani PL , Pellegrini C , Gandolfi L , et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a Phase 2 trial. Clin Lymphoma Myeloma Leuk . 2011;11(6):462–466. doi:10.1016/j.clml.2011.02.001 21859554
- Wang M , Fowler N , Wagner-Bartak N , et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia . 2013;27(9):1902–1909. doi:10.1038/leu.2013.95 23545991
- Swerdlow SH , Campo E , Harris NL , et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues . 4th ed. 2017.
- Cheson BD , Fisher RI , Barrington SF , et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol . 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800 25113753
- Hyeon J , Lee B , Shin SH , et al. Targeted deep sequencing of gastric marginal zone lymphoma identified alterations of TRAF3 and TNFAIP3 that were mutually exclusive for MALT1 rearrangement. Mod Pathol . 2018;31(9):1418–1428. doi:10.1038/s41379-018-0064-0 29765142
- Shin HT , Choi YL , Yun JW , et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat Commun . 2017;8(1):1377. doi:10.1038/s41467-017-01470-y 29123093
- Wilm A , Aw PP , Bertrand D , et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res . 2012;40(22):11189–11201. doi:10.1093/nar/gks918 23066108
- Cibulskis K , Lawrence MS , Carter SL , et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol . 2013;31(3):213–219. doi:10.1038/nbt.2514 23396013
- Ivanov V , Coso D , Chetaille B , et al. Efficacy and safety of lenalinomide combined with rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma . 2014;55(11):2508–2513. doi:10.3109/10428194.2014.889822 24506467
- Ghesquieres H , Chevrier M , Laadhari M , et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’ phase II study of the French oculo-cerebral lymphoma (LOC) network and the lymphoma study association (LYSA)†. Ann Oncol . 2019;30(4):621–628. doi:10.1093/annonc/mdz032 30698644
- Yang Y , Shaffer AL , Emre NC , et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell . 2012;21(6):723–737. doi:10.1016/j.ccr.2012.05.024 22698399
- Nowakowski GS , LaPlant B , Macon WR , et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: a phase II study. J Clin Oncol . 2015;33(3):251–257. doi:10.1200/JCO.2014.55.5714 25135992
- Vitolo U , Chiappella A , Franceschetti S , et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol . 2014;15(7):730–737. doi:10.1016/S1470-2045(14)70191-3 24831981
- Nowakowski GS , Hong F , Scott DW , et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. J Clin Oncol . 2021;JCO2001375.
- Nowakowski GS , Chiappella A , Gascoyne RD , et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol . 2021;39(12):1317–1328. doi:10.1200/JCO.20.01366 33621109
- Goy A , Ramchandren R , Ghosh N , et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non–germinal center B-cell–like DLBCL. Blood . 2019;134(13):1024–1036. doi:10.1182/blood.2018891598 31331917
- Sethi T , Kovach AE , Mason EF , et al. Combination of nivolumab, lenalidomide and rituximab in relapsed/refractory non-germinal center diffuse large B cell lymphoma: results from a dose-escalation cohort. Blood . 2019;134(Supplement_1):4100. doi:10.1182/blood-2019-129634
