Abstract
Background
Despite the prevalence of pain among patients with cancer and the availability of pertinent guidelines, the clinical management of oncological pain is decisively insufficient. To address this issue, we evaluated current trends in clinical practice and subsequently generated a list of ten corrective actions—five things to do and five things not to do—for the diagnosis, management, and monitoring of cancer pain.
Methods
The survey included 18 questions about clinical practice surrounding background pain and breakthrough cancer pain (BTcP). Survey questions were developed by a scientific board of 10 physician experts and communicated via email to an expanded panel of physicians in Italy. Responses were tabulated descriptively for analysis.
Results
Of 51 invited physicians, 32 (63%) provided complete survey responses. The responses revealed several incongruencies with current guideline recommendations: physicians did not always diagnose or monitor pain using diagnostically validated or disease-specific instruments; frequently based clinical decision-making on time availability or convenience; and pharmacological therapy was often inappropriate (eg, prescribing NSAIDs or corticosteroids for BTcP). The list of corrective actions generated by the scientific board favored a guideline-oriented approach that systematically characterizes oncological pain and implements treatment based on pain characteristics (eg, fast-acting transmucosal opioids for BTcP) and evidence-based recommendations.
Conclusion
Oncologists require better education and training about the diagnosis, treatment, and monitoring of oncological pain. Physicians should be aware of current guideline recommendations as well as available pharmacological tools for BTcP.
Introduction
Pain is a common and feared symptom of cancer that has important implications for patients health-related quality of life (QoL) and adherence to antitumoral treatment. Citation1,Citation2 Cancer pain affects 30–40% of patients at initial presentation, approximately 50% of those in active treatment, and 70–90% of patients with advanced disease. Citation3–5 Oncological pain can be divided into two categories: background pain, which manifests as a persistent pain state usually controlled by long-term analgesic treatment, and breakthrough cancer pain (BTcP), defined as short durations of severe pain intensity that “break through” analgesic coverage. Citation6,Citation7 BTcP affects nearly 60% of oncological patients and is generally unpredictable and difficult to control, associated with higher pain-related distress and poorer QoL compared to background pain. Citation8
Despite the availability of several guidelines for treating and managing cancer pain Citation5,Citation7 common clinical practice surrounding BTcP is decisively inadequate. In one study, 44% of patients were neither satisfied nor dissatisfied with analgesic treatment for cancer pain and 23% were dissatisfied. Citation9 Another review suggested that 43% of cancer patients receive inappropriate care for pain. Citation10 Deficiencies in cancer pain management are characterized by the inadequate use of opioids, Citation10 institutional impediments, and inadequate professional education. Citation11,Citation12 Physicians frequently lack adequate education regarding diagnostically validated BTcP detection and appropriate pharmacological treatment with rapid-onset opioids (ROOs) such as oromucosal and nasal preparations, which show good patient satisfaction in clinical studies. Citation13
Here, we performed a nationwide physician survey to evaluate current trends in clinical practice for cancer pain in Italy. Subsequently, an expert panel was tasked with the generation of ten corrective actions —”five things to do and five things not to do” Citation14 for the diagnosis, management, and monitoring of cancer pain.
Methods
Study Design
The scientific board included 10 physicians with expertise in oncological pain and at least 5 years of clinical experience who currently practice in Italy. Survey questions were by the scientific board in a first face-to-face meeting based on available literature, guidelines, and professional experience. Questions were subjected to revision until all members of the board agreed with their formulation. Following the initial meeting, board members were asked to nominate potential respondents for survey participation. Eligible respondents were physicians in Italy with no limitation on age or years’ experience with oncological pain. The survey was active for a period of 2 weeks (from 4 September to 18 September 2020) and communicated via e-mail using a password-protected web link. Survey responses were anonymized and handled via remote dispersed geographic participation. At the end of the data collection period, the survey results were analyzed and discussed at a final meeting of the scientific board. During this meeting, the results were also used to generate a final list of five “things to do” and five “things not to do” in the detection, management, and monitoring of cancer pain. The present anonymized survey study was not subject to ethics committee approval as per Italian regulations.
Survey
The survey included 18 questions divided into two categories: general cancer pain (including background pain) and BTcP. Questions addressed physician exposure to the target patient population (where patients were seen, number of patients seen per day), the detection and diagnosis of cancer pain, details about pharmacological treatment including opioid use and use of ROOs for BTcP management, and perceived effects of cancer pain on patients. Responses were analyzed descriptively and reported as the percentage and number of responses. No formal statistical analyses were planned for this study. The complete survey is available in the Supplementary Figures 1 and 2 .
Results
Respondent Characteristics
Of 51 physicians who were invited to respond to the survey, 32 (63%) provided complete responses and were included in the analysis. The participants to this survey were Italian oncologists with an age between 36 and 51. Fifty percent of physicians indicated that they see patients during out-patient visits, 6% during in-patient visits, and 44% during both in- and out-patient visits (Q1). Seventy percent of respondents indicated seeing 51–150 patients per month (Q2) and most physicians (55%) indicated that more than half of their patients have cancer pain (Q3). The findings included gaps in the diagnosis of baseline pain and BTcP, as well as the need to implement strategies to improve patient management, pain control, quality of life and continuity of care.
Detection and Diagnosis
Ninety-four percent of physicians usually become aware of their patient’s pain at the first visit by asking targeted questions, while the remainder indicated that they become aware when patients explicitly communicate their pain (Q4). Most physicians reported that they record information about background pain on medical records (45%) or a digital platform (45%; Q4.1). Eighty-seven percent of respondents evaluate background pain at each visit (Q4.2), most frequently using the numerical rating scale (NRS; 81%, Q5). When asked about instruments for evaluating and measuring pain symptoms, the vast majority of respondents agreed that an oncology-specific app would be useful for monitoring and managing cancer pain in their patients (Q9, Q 9.1). Forty-eight percent of physicians think that such an app would be useful to 30–60% of patients (Q9.2), with important obstacles to app use including poor familiarity with app use among patients (77%) and older patient age (63%).
Background Pain
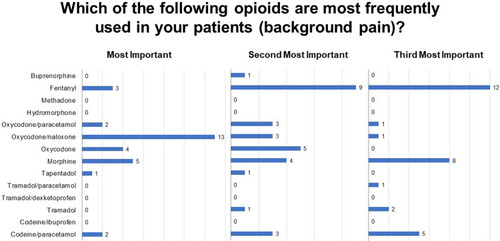
Fifty-four percent of respondents expressed that more half of their patients necessitate opioid therapy to treat cancer pain (Q7). Among these physicians, slow-release opioids (oxycodone/naloxone, followed by fentanyl and oxycodone or morphine) are the treatment of choice for background pain (Q8; ). Physicians who titrate opioids prior to initiating a slow-release opioid regimen typically do so in an in-patient setting (34%), although out-patient titration is also common (Q8.1). The majority of physicians (80%) indicated that very little percentage (10%) of their patients, in the treatment with opioids, were using ≥ 60 mg MMEs for breakthrough pain. (Q11).
Breakthrough Pain
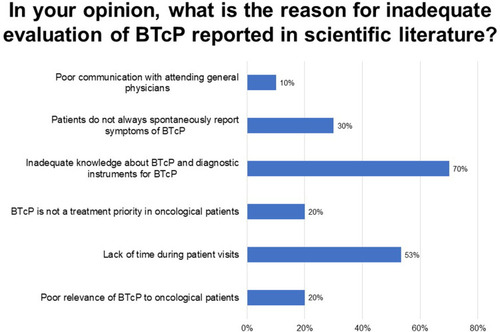
Seventy percent of respondents indicated that inadequate knowledge about BTcP and tools for its diagnosis are a primary cause of “inadequate evaluation of BTcP” described in scientific literature, while 53% attribute it to a lack of time during patient visits; 30% indicated that patients rarely spontaneously report BTcP symptoms; and 20% do not consider BTcP to be a treatment priority among cancer patients (Q10; ).
Figure 2 Perceived motivations for inadequate evaluation of BTcP in the literature. Responses to question 10 (multiple responses permitted). BTcP, breakthrough cancer pain.
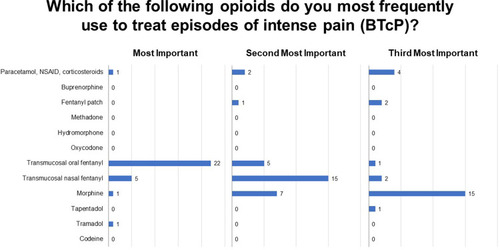
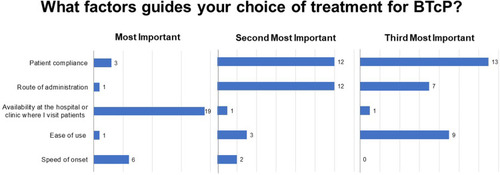
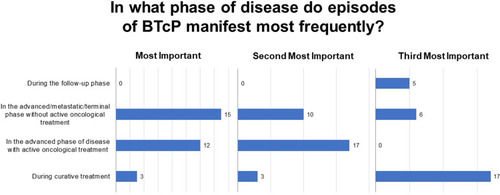
Sixty-four percent of respondents reported that 30–60% of their patients are affected by both background and breakthrough pain (Q 12), most frequently in advanced stages of disease or treatment (Q13; ). Physicians typically evaluate the presence of BTcP using a diagnostically validated algorithm (47%), although 33% base a diagnosis on the number of daily episodes of severe pain and 20% use poor opioid treatment response as an indicator (Q14). Among responding physicians, the treatment of choice for BTcP is transmucosal oral or nasal fentanyl followed by morphine (Q15; ), with physicians vastly preferring transmucosal modes of administration (Q15.2). Fifty-percent of respondents do not titrate the initial dose of a ROO, basing the initial dose on the patient’s opioid dose for background pain, while 47% titrate by initiating with the lowest possible dose of a ROO (15.1). The primary motivations guiding BTcP treatment selection are availability in the hospital or clinic; route of administration; patient compliance; ease of administration; and finally, speed of onset (Q16; ).
Figure 3 Phase of disease during which episodes of BTcP manifest most frequently. Ranked responses to question 13 (n = 30, 30, and 28). BTcP, breakthrough cancer pain.
Influence on Patients
With regard to background pain, 97% percent of respondents agree that cancer pain can influence adherence to antitumoral treatment (Q6) and 90% agree that this was true in up to 50% of their patients. Similarly, 90% of respondents agree that BTcP can negatively influence adherence to antitumoral therapy (Q18). All respondents agree that BTcP interferes with patient QoL to some degree (30%) or significantly (70%; (Q17).
Discussion
The findings of the present survey on cancer pain management revealed a number of important deficits in clinical practice. Physicians did not always diagnose or monitor pain using diagnostically validated or disease-specific instruments and frequently based clinical decision-making on time availability or convenience (eg, availability of a drug formulation at their clinic or hospital). outlines a list of a 10 corrective actions—5 things to do and 5 things not to do—for improving clinical practice surrounding cancer pain proposed by the expert scientific board.
Table 1 What to Do and What Not to Do in the Management of Cancer Pain
Efficient management of cancer pain begins with early detection. The diagnosis of BTcP has historically been complicated by the absence of an international consensus definition, although several specific diagnostic tools are available for detecting BTcP including algorithms published by the Association for Palliative Medicine of Great Britain and Ireland and other groups. Citation7,Citation15–17 A diagnosis of breakthrough pain requires the patient to have adequately controlled background pain; accordingly, physicians must not only be educated about diagnostically validated BTcP detection, but must also be able to recognize and distinguish controlled background pain from BTcP. After adequate background pain control is achieved, BTcP is defined by no more than 4 episodes of severe pain per day. Citation18 In our survey, some physicians diagnosed BTcP based on the number of daily episodes of severe pain alone or excessive pain despite treatment with an opioid for background pain, neither of which systematically distinguish BTcP from controlled or uncontrolled background pain. Systematic use of an algorithm at each assessment can allow physicians to monitor and treat background pain and BTcP separately to ultimately improve patient management. Physician respondents to our survey assessed and documented oncological pain at every patient visit, consistent with national and international guideline recommendations.
In survey responses, the treatment of cancer pain was generally characterized by use of slow-release opioids to cover background pain and the use of ROO formulations for BTcP, with some exceptions. It is noteworthy that speed of onset was the fifth most important factor considered when selecting a pharmacological agent for BTcP. Oral morphine was also a favored agent for BTcP, representing current controversy over the benefits of morphine versus oromucosal and nasal ROOs for BTcP. The most recent guidance from the EAPC identifies oral, immediate‐release opioids and buccal or intranasal transmucosal fentanyl preparations as appropriate for BTcP, favoring the latter in many cases for their rapid onset and shorter duration of effect. Citation19 The use of medications with pharmacokinetic profiles that are incompatible with the temporal nature of BTcP can compromise treatment success. Citation20 Clinical practice can be improved by reducing the numbers of unnecessary prescriptions and instead focusing on guideline-recommended treatments. Preliminary data from the Italian Oncologic Pain Survey (IOPS) study suggests that nasal administration of fentanyl provides faster analgesia and higher patient satisfaction relative to other fentanyl formulations. Citation3
Physician respondents expressed a general consensus about the potential utility of web-based applications for documenting and monitoring cancer pain in their patients. Given that many physicians in our study saw patients in an out-patient setting (ie, without nursing support for pain assessment and documentation) and half used physical records for documenting patient symptoms, introduction of an app specific to cancer pain could significantly improve both documentation and clinical practice. The general objective of a web-based application is to support clinician and nursing/caregiver work-flow and cooperation by facilitating documentation, providing real-time reminders and assessments, and improving the accessibility of patient records. Citation21 In a context of oncology, routine monitoring of patient-reported outcomes with health information technologies improves system-level outcomes (eg, fewer emergency department admissions and hospitalizations) as well as patient-level outcomes (eg, better health-related QoL and longer survival). Citation22–24 Extension of these technologies to cancer pain is a logical next-step to improve patient care and clinical management. Citation25 A patient-facing smartphone application has been developed with the aim to provide a comprehensive cancer pain education spanning pharmacologic and behavioral aspects of self-management, with the help of custom graphics, animated videos, quizzes, and audio-recorded Citation26 . A preliminary testing demonstrated that a digital intervention called “Can-Pain” could promote patient-centred pain management highlighting unrecognised problems, promoting shared understanding about symptoms between patients, caregiver and healthcare professional and supporting shared decision-making. Citation27 A randomized controlled trial of out-patients with stage IV advanced solid tumors demonstrated that standardized pain education and telemonitoring used by nurse practitioners was an efficient way to improve out-patient pain management in the outpatient clinic. Citation28 In another study, telemonitoring improved pain reporting and analgesic prescription among patients with cancer pain, translating to a positive effect on QoL. Citation29 Further essential to patients with chronic pain, continuous monitoring by nursing staff via telemedicine is associated with enhanced feelings of security and safety as well as improved patient outcomes during palliative home care. Citation21,Citation30,Citation31
The present study has some limitations. The survey was completed by a small number of physicians who were identified by members of the scientific board without restriction on years’ experience with oncological pain; this approach aimed to provide a real-world picture of current clinical practice. It is important to note that selection of participants by members of the scientific board may have resulted in selection bias. Self-reporting of clinical practice by physicians was another possible source of bias, as this survey was qualitative in nature. Despite these shortcomings, the survey responses provided an update on clinical cancer pain management in Italy that can facilitate future intervention to improve the quality of care.
In conclusion, current clinical practice for cancer pain and especially BTcP remains inadequate, but can be improved by focusing on physician training and education about diagnostically validated instruments and fast-acting, short duration transmucosal opioid formulations appropriate for treating BTcP. Web-based applications and telemedicine represent important opportunities for innovation and improved patient care that can augment the level of support provided by nursing staff to both physicians and patients, as well as improve pain monitoring and the timely prescription of appropriate therapy.
Abbreviations
BTcP, breakthrough cancer pain; NSAID, non-steroidal anti-inflammatory drug; QoL, quality of life; ROO, rapid-onset opioid.
Data Sharing Statement
The complete dataset is available from the corresponding author upon request.
Ethics Approval and Informed Consent
The study was exempt from informed consent as an anonymized survey. Ethics approval was not required as per Italian regulations.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work”.
Disclosure
PB's is affiliated with both Department of Medical and Surgical Specialties, Radiological Sciences and Public Health University of Brescia and Unit Department Medical Oncology, ASST Spedali Civili di Brescia, Italy. PB also has received support from Merck Serono, Bristol-Myers Squibb, Hinn, Helsinn, and GlaxoSmithKline, Sanofi, Merck Sharp & Dohme, Sun Pharma, Angelini Pharma, and Molteni Farmaceutici. AA reports grants from Bristol-Myers Squibb, Angelini Pharma, Accord, and Kyowa Kirin. GA reports grants from Novartis. JG reports funding from Molteni Farmaceutici. ML reports funding from Eli Lilly. SS reports funding from Ipsen, Novartis, MSD and BMS. FC, RG, AM, and LP have no conflicts of interest to declare. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank Ashley Symons, Ph.D. for draft preparation and editorial assistance on this manuscript.
References
- van den Beuken-van Everdingen MHJ . Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol off J Eur Soc Med Oncol . 2007;18:1437–1449. doi:10.1093/annonc/mdm056
- Portenoy RK . Treatment of cancer pain. Lancet . 2011;377:2236–2247. doi:10.1016/S0140-6736(11)60236-5 21704873
- Mercadante S , Lazzari M , Reale C , et al. Italian Oncological Pain Survey (IOPS): a multicentre Italian study of breakthrough pain performed in different settings. Clin J Pain . 2015;31:214–221. doi:10.1097/AJP.0000000000000161 25654429
- Lesage P , Portenoy RK ; Lesage & Portenoy . Trends in cancer pain management. Cancer Control . 1999;6:136–145. doi:10.1177/107327489900600202 10758542
- Caraceni A , Hanks G , Kaasa S , et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet. Oncol . 2012;13:e58–68.22300860
- Mercadante S , Portenoy RK . Breakthrough cancer pain: twenty-five years of study. Pain . 2016;157:2657–2663. doi:10.1097/j.pain.0000000000000721 27653423
- Fallon M , Giusti R , Aielli F , et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol off J Eur Soc Med Oncol . 2018;29:iv166–iv191. doi:10.1093/annonc/mdy152
- Deandrea S , Corli O , Consonni D , et al. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage . 2014;47:57–76. doi:10.1016/j.jpainsymman.2013.02.015 23796584
- Antón A , Montalar J , Carulla J , et al. Pain in clinical oncology: patient satisfaction with management of cancer pain. Eur J Pain . 2012;16:381–389. doi:10.1002/j.1532-2149.2011.00036.x 22337158
- Deandrea S , Montanari M , Moja L , Apolone G . Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol off J Eur Soc Med Oncol . 2008;19:1985–1991. doi:10.1093/annonc/mdn419
- Zuccaro SM , Vellucci R , Sarzi-Puttini P , et al. Barriers to pain management: focus on opioid therapy. Clin. Drug Investig . 2012;32(Suppl 1):11–19. doi:10.2165/11630040-000000000-00000
- Kwon JH . Overcoming barriers in cancer pain management. J Clin Oncol . 2014;32:1727–1733. doi:10.1200/JCO.2013.52.4827 24799490
- Mercadante S , Adile C , Masedu F , et al. Factors influencing the use of opioids for breakthrough cancer pain: a secondary analysis of the IOPS-MS study. Eur J Pain . 2019;23:719–726. doi:10.1002/ejp.1339 30421474
- Alvaro D , Caraceni AT , Coluzzi F , et al. What to do and what not to do in the management of opioid-induced constipation: a choosing wisely report. Pain Ther . 2020;9:657–667. doi:10.1007/s40122-020-00195-z 32940898
- Portenoy RK , Payne D , Jacobsen P . Breakthrough pain: characteristics and impact in patients with cancer pain. Pain . 1999;81:129–134. doi:10.1016/S0304-3959(99)00006-8 10353500
- Hwang SS , Chang VT , Kasimis B . Cancer breakthrough pain characteristics and responses to treatment at a VA medical center. Pain . 2003;101(1):55–64. doi:10.1016/S0304-3959(02)00293-2 12507700
- Webber K , Davies AN , Cowie MR . Accuracy of a diagnostic algorithm to diagnose breakthrough cancer pain as compared with clinical assessment. J Pain Symptom Manage . 2015;50:495–500. doi:10.1016/j.jpainsymman.2015.05.006 26025280
- Ripamonti CI , Santini D , Maranzano E , et al. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol off J Eur Soc Med Oncol . 2012;23(Suppl 7):vii139–54. doi:10.1093/annonc/mds233
- Hanks GW , Conno FD , Cherny N , et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer . 2001;84:587–593. doi:10.1054/bjoc.2001.1680 11237376
- Bennett D . Consensus panel recommendations for the assessment and management of breakthrough pain PART 2 MANAGEMENT. P T . 2005;30.
- Wright AA , Raman N , Staples P , et al. The HOPE pilot study: harnessing patient-reported outcomes and biometric data to enhance cancer care. JCO Clin Cancer Inform . 2018;2:1–12. doi:10.1200/CCI.17.00149
- Aapro M . Digital health for optimal supportive care in oncology: benefits, limits, and future perspectives. Support Care Cancer . 2020;28:4589–4612. doi:10.1007/s00520-020-05539-1 32533435
- Basch E , Deal AM , Kris MG , et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol . 2016;34:557–565. doi:10.1200/JCO.2015.63.0830 26644527
- Lizée T , Basch E , Trémolières P , et al. Cost-effectiveness of web-based patient-reported outcome surveillance in patients with lung cancer. J. Thorac. Oncol . 2019;14:1012–1020. doi:10.1016/j.jtho.2019.02.005 30776447
- Penedo FJ , Oswald LB , Kronenfeld JP , et al. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet. Oncol . 2020;21:e240–e251. doi:10.1016/S1470-2045(20)30021-8 32359500
- Azizoddin DR , Adam R , Kessler D , et al. Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Support Care Cancer . 2021. doi:10.1007/s00520-021-06146-4
- Adam R , Bond CM , Burton CD , de Bruin M , Murchie P . Can-Pain-a digital intervention to optimise cancer pain control in the community: development and feasibility testing. Support Care Cancer . 2021;29:759–769. doi:10.1007/s00520-020-05510-0 32468132
- Kim HS , Shin SJ , Kim SC , et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support Care Cancer . 2013;21:1751–1759. doi:10.1007/s00520-013-1722-x 23338230
- Knegtmans MF , Wauben LSGL , Wagemans MFM , Oldenmenger WH . Home telemonitoring improved pain registration in patients with cancer. Pain Pract . 2020;20:122–128. doi:10.1111/papr.12830 31419371
- Steindal SA , Nes AAG , Godskesen TE , et al. Patients’ experiences of telehealth in palliative home care: scoping review. J. Med. Internet Res . 2020;22:e16218. doi:10.2196/16218 32369037
- Pavic M . Feasibility and usability aspects of continuous remote monitoring of health status in palliative cancer patients using wearables. Oncology . 2020;98:386–395. doi:10.1159/000501433 31336377