Abstract
Purpose
To further evaluate the efficacy and safety of computed tomography (CT)-guided iodine 125 (125I) brachytherapy to treat locally advanced non-small cell lung cancer (NSCLC) after progression of concurrent radiochemotherapy (CCRT).
Methods
This study obtained written consent from all patients and was approved by our institution. From January 2006 to June 2018, 210 NSCLC patients (progression of first-line CCRT) were retrospectively recruited and then divided into two groups. A total of 116 patients were given CT-guided 125I brachytherapy and second-line chemotherapy (group A), and 94 were treated with second-line chemotherapy alone (group B).
Results
In group A, local response rate (LRR) within 3 years was significantly better (P<0.05). Mean survival time [progression-free survival time (PFST) and overall survival (OS)] was 15.1±1.4 months and 21.2±1.6 months in group A compared with 10.0±1.4 months and 16.2±1.7 months in group B (PFST: P<0.01, HR=1.472, 95% CI 1.097–1.975; OS: P = 0.036, HR=1.342, 95% CI 1.005–1.791). Tumor size and No. of first cycle chemotherapy were independent factors that affected survival, ≤3cm largest tumor diameter and more than 4 first cycles of chemotherapy showed longer PFST and OS (P<0.05). Tumor-related clinical symptoms were relieved in group A (P<0.01). No serious complications occurred in the two groups.
Conclusion
125I brachytherapy is effective and safe in locally advanced NSCLC after progression of CCRT.
Introduction
Clinically, more than 75% of lung cancers is non-small cell lung cancer (NSCLC), and the main causes of death are tumor recurrence, cancer progression, and metastasis.Citation1 In fact, only a few patients are suitable for surgery, the percentage is less for advanced stage III–IV NSCLC.Citation2 As we all know, the first-line treatments for advanced NSCLC with sensitive mutations are epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and anaplastic lymphoma kinase (ALK) inhibitors.Citation3,Citation4 Similarly, for high expression of program death-ligand 1 (PD-L1), the first-line treatment is programmed death-1 (PD-1) inhibitors.Citation5
For patients not suitable for surgery or without sensitive mutations or less than 50% PD-L1 expressions, concurrent radiochemotherapy (CCRT) is seen as the standard treatment.Citation6,Citation7 Many studies have also reported that about 20% of patients could be cured by CCRT alone.Citation8 In addition, the latest research confirmed that CCRT combined with immunotherapy had significantly improved survival rate for advanced NSCLC patients and was a standard treatment.Citation9 However, 80% of patients had recurrence within 1–2 years after CCRT treatment, the long-term survival was disappointing.Citation10 Moreover, due to severe toxicity after radiotherapy and chemotherapy, poor general condition and tumor staging, many patients could also not tolerate the next available treatment, these were considered to be important factors in tumor recurrence.Citation11,Citation12
As a minimally invasive therapy, 125I brachytherapy has been considered a meaningful therapy and a method to achieve remission for many tumors (eg, pancreatic cancer, hepatocellular carcinoma, gynecologic malignancies, and glioma).Citation13–Citation15 Similarly, we also reviewed some reports about 125I brachytherapy in lung cancer, the results showed that CT-guided 125I seed implantation was feasible and safe.Citation16 In 2015, we enrolled 78 locally advanced NSCLC patients from a single center, the local response rate (LRR) was 63.6% in 125I brachytherapy versus 41.5% in second-line chemotherapy (P=0.033), the overall survival (OS, 1-, 2-, 3-year) rates were 56.8%, 16.2%, 2.7% versus 36.6%, 9.8%, 2.4%, respectively (P= 0.059).Citation17 However, this study had a relatively short follow up and small sample; there were no further analyses of prognostic factors affecting survival, which would affect our thinking regarding suitable patients for 125I brachytherapy. Furthermore, in the past 5 years, there have been few reports on 125I brachytherapy in locally advanced NSCLC after progression of CCRT.
Thus, this study aimed to further confirm the efficacy and safety of CT-guided 125I implantation in advanced NSCLC after progression of CCRT.
Materials and Methods
210 patients with stage III–IV NSCLC participated in this study from January 2006 to June 2018. This retrospective study was approved by the institutional review board at our hospital. All patients were previously treated by first cycle of CCRT after the confirmation of NSCLC but had progression of disease, the interval time from the end of first cycle CCRT was 7.6±1.2 months versus 7.3±0.8 months between two groups. After signed consent form regarding CT-guided 125I brachytherapy-related risks, 116 patients underwent 125I seed implantation and second-line chemotherapy for lung lesions (group A), and 94 were only given second-line chemotherapy (group B). The characteristics of patients in the two groups can be found in .
Table 1 Summary of Patient and Tumor Characteristics
The inclusion criteria were: a) treatment for locally advanced NSCLC was CCRT but the disease progressed; b) pathologically confirmed NSCLC, clinical stage: III–IV; c) patient refused external beam re-irradiation; d) no mutation (EGFR or ALK fusion genes) or patient refused targeted therapy; e) ≤3 lesions (unilateral lung), the largest diameter ≤5 cm (single lesion); f) ECOG score was 2 or less.
Exclusion criteria: history of other malignancies; intolerance to percutaneous 125I brachytherapy; cachexia; and severe cardiopulmonary insufficiency.
125I Seed and Radiation Dosimetry
The 125I seed (Zhibo Gaoke Biotechnology Company, Beijing, China) was made into cylindrical nickel-titanium tubes (wall thickness: 0.05 mm, diameter: 0.8 mm, length: 4.5 mm). The center of the tube was a silver rod (3.0 mm length, adsorption of 125I). Parameters of 125I: initial activity, 1.85–2.22×1010 Bq; half life, 59.6 days; average energy, 27–35 keV; and antitumor activity, 1.7 cm. After these seeds were implanted, 95% of low-dose γ-ray and X-ray was released within 8–10 months.
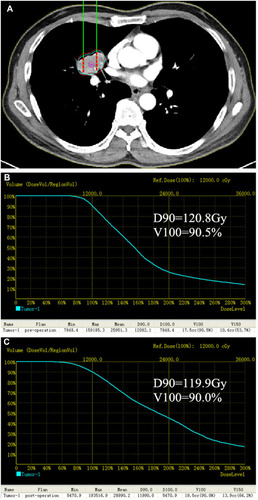
5- mm thickness CT images were obtained and entered into a treatment planning system (TPS, Qilin Company, Beijing, China) to target areas of interest before 125I implantation. Two radiologists (Z.W.X or M.S.H, with 7 and 23 years experience performing 125I brachytherapy) and one physicist (Z.H.Z, with 6 years experience) performed preoperative plan together for each patient. According to recommendations of American Brachytherapy Society (ABS) and previous 125I brachytherapy studies in malignant tumors,Citation18–Citation20 we defined that the prescribed dose was 100–140Gy (average: 120Gy) for lung cancer. Puncture path, number of seeds, dose-volume histogram () were calculated and generated by TPS. The dose prescription of surrounding vital organs or tissues should also be decreased (such as: large vessels, heart, spinal cord, and trachea).
Figure 1 Preoperative treatment planning system (TPS). (A) Red line represented the tumor’s contour, the planning target volume edge was covered by the isodose curve from 70 to 90%. (B) Preoperative dose-volume histograms (DVH). The prescription dose (PD) was 120Gy, a total of 90% of the tumor target (D90) received 120.8Gy and 90.5% of the tumor received 100% of the prescribed dose (V100). (C) Postoperative DVH. After 125I brachytherapy, the dose intensity was verified, D90 = 119.9Gy and V100 = 90.0%.
125I Implantation
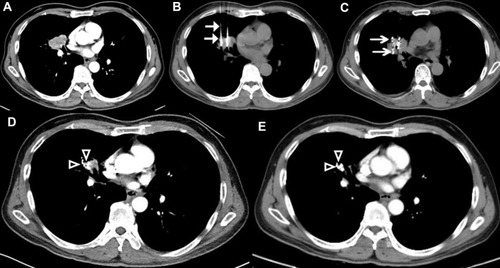
Based on the established treatment plan (TPS), the applicator’s position was marked, then CT scan with 5 mm thickness was performed to confirm the border of the lung lesion (). After 2% lidocaine local infiltration anesthesia (Yichang, China), and avoiding puncture of vital organs, 18-G spinal needles were inserted to reach lung lesion and kept its deepest margin, the distance between every needle was about 10–15 mm (). Then these seeds were released while withdrawing the needles (a distance of 0.5–1.0 cm apart) () from deep to shallow. Postoperative complications (pneumothorax or bleeding) were assessed by the last CT scan, more than 30% pulmonary compression of lung volume should be drained by using a pigtail catheter (Cook Medical, Bloomington, Ind). The position and intensity of 125I seeds was also verified by using the last CT images on TPS ().
Figure 2 Computed tomography (CT)-guided percutaneous 125I seed implantation was performed. (A) CT scan before brachytherapy. (B and C) The procedure of CT-guided 125I brachytherapy was performed according to preoperative TPS, 18 G spinal needles were inserted to reach the tumor (arrows), seeds were released from deep to shallow (arrows). (D) One month after treatment, the lesion apparently shrunk (arrows). (E) Six months after treatment, the tumors disappeared, with only seeds remaining (arrows).
Second-Line Chemotherapy
As second-line chemotherapy, the following medications were administered: single-agent docetaxel was given for squamous cell carcinomas (75 mg/m2/3 weeks); single-agent pemetrexed was given for others (500 mg/m2/3 weeks). Chemotherapy should be delayed or reduced in patients with major complications. All patients had received at least 4–6 cycles of second-line chemotherapy successfully. In group A, the time interval of 125I brachytherapy and first second-line chemotherapy was no more than 2 weeks.
Follow-Up
All postoperative symptoms were recorded. The effectiveness of 125I brachytherapy was recorded by follow-up imaging (). 1 and 3 months after treatment, enhanced chest CT images were taken, and then every 3 months. Local response rate (LRR), survival, complications, and relief of clinical symptoms were recorded.
The response of tumor was evaluated according to the Tumor Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, which defines complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD). LRR was the proportion of patients who achieved CR+PR. Survival time was also defined and calculated (PFST: time from initial 125I brachytherapy or second-line chemotherapy to initial radiological progression or death from any cause; OS: the time from initial 125I brachytherapy or second-line chemotherapy to death from any cause). Chemotherapy-related adverse events were evaluated by Common Terminology Criteria Adverse Events Version 4.0 (CTCAE v4.0).
Statistical Analyses
The characteristics of the patients, complications and relief of clinical symptoms were compared with Pearson’s χ2 test between the two groups. Kaplan-Meier curve (Log rank test) was applied for the analyses of PFST and OS. A stratified Cox proportional hazards regression model was applied for evaluating the relationship between variables (eg, histology, UICC TNM classification, No. of lesions, lesion diameter, etc.) and survival (PFST and OS). SPSS version 20.0 was applied for the statistical analyses, and a p value less than 0.05 was considered as significant.
Results
Local Response Rate
The median follow-up time was 21 months (3–65 months). As shown in , the rate of local control was better in Group A. Although there were no statistically significant differences at 48 and 60 months, the LRR at 1, 2, 4, 6, 12, 18, 24 and 36 months in group A was 58.6%, 53.4%, 50.0%, 41.4%, 22.4%, 18.1%, 16.4%, 11.2% respectively, and was 42.6%, 37.2%, 31.9%, 27.7%, 20.2%, 15.9%, 12.8% and 7.4% in group B (P <0.05).
Table 2 The Clinical Efficacy of Treatment in Two Groups
Progression-Free Survival Time
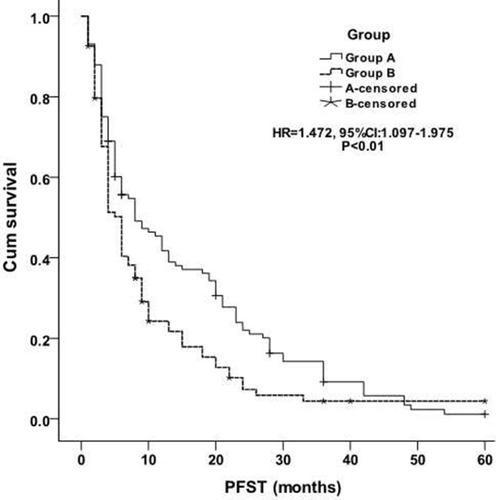
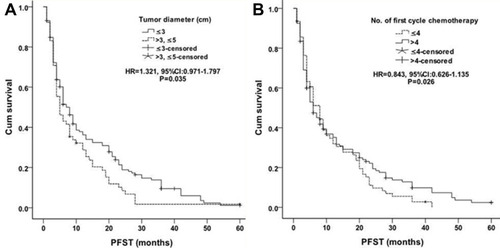
The median PFST was 15.1±1.4 months (95% CI 12.4–17.8) in group A, while 10.0±1.4 months (95% CI 7.3–12.8) in group B [P<0.01, HR=1.472, (95% CI 1.097–1.975), ]. Better PFST was observed in the combined group. Tumor size and No. of first cycle chemotherapy were independent factors, better PFST was found in patients with ≤3cm largest tumor diameter [≤3cm vs >3 to≤5cm: P=0.026, HR=0.843, (95% CI 0.626–1.135), ] and more than 4 number of first cycle chemotherapy [P=0.035, HR=1.321, (95% CI 0.971–1.797), ]. Other factors (eg, age, sex, ECOG score, smoking, TNM classification, histology, No. of tumors, classification of tumor, tumor marker, disease-free interval and distant metastasis) were not related to PFST.
Overall Survival
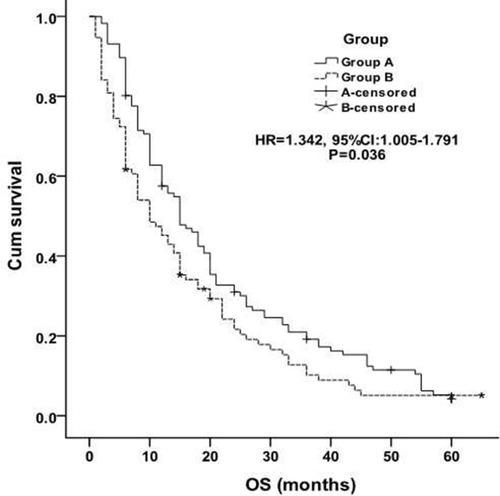
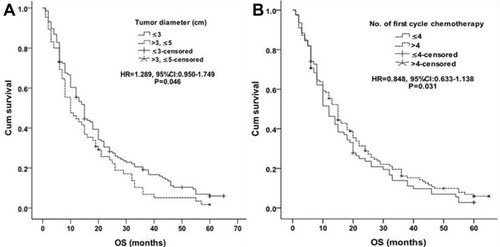
The median OS in group A was significantly better than that in group B [21.2±1.6 months vs 16.2±1.7 months, P = 0.036, HR=1.342, (95% CI 1.005–1.791), ]. The 1-, 2-, 3-, 4- and 5-year OS in group A was 62.1%, 31.9%, 19.8%, 10.3%, 4.3% respectively, and 45.7%, 20.2.4%, 10.6%, 6.4%, 4.3% in group B respectively. Tumor size and No. of first cycle chemotherapy were independent factors, better OS was found in patients with ≤3cm largest tumor diameter [≤3cm vs >3 to≤5cm: P=0.046, HR=1.289, (95% CI 0.950–1.749), ] and more than 4 number of first cycle chemotherapy [P=0.031, HR=0.848, (95% CI 0.633–1.138), ]. Other factors (eg, age, sex, ECOG score, smoking, TNM classification, histology, No. of tumors, classification of tumor, tumor marker, disease-free interval and distant metastasis) were not related to OS.
Relief of Clinical Symptoms
The clinical symptoms of tumors were significantly better in group A (, P < 0.01). The remission rates of chest pain, cough, hoarseness, bloody sputum, and chest tightness (significant remission + partial response) were 88.7%, 84.9%, 78.9%, 77.8%, 79.4% and 21.4%, 24.7%, 28.6%, 30.4%, 25.7% between two groups, respectively.
Table 3 Relief of Clinical Symptoms Associated with the Tumor in Two Groups
Complications
The major complications were summarized in group A, no severe complications occurred during follow-up (). Pneumothorax was found in 44 patients (≤30% unilateral lung volume: 23 patients; ≥30% unilateral lung volume: 11 patients), these patients with more than 30% pulmonary compression volume recovered after drainage. There were no clinical symptoms in three patients with minor displacement of 125I seeds. There was no statistical difference in complications (, P > 0.05).
Table 4 The Complications of 125I Brachytherapy and Chemotherapy in Two Groups
Discussion
According to our study, 125I implantation might be an alternative treatment for advanced NSCLC after progression of CCRT. Our finding is important because 125I brachytherapy had to overcome some difficulties of disease progression on CCRT (eg, poor general condition, severe toxicity). In fact, these patients (more than 50%) were not candidates for re-irradiation and it had a lower success rate than the standard treatment modality and subsequent treatments.Citation21 So, 125I brachytherapy provided an opportunity for remission in advanced NSCLC after progression of CCRT. As we all know, the goal of treatment for these patients was to control local tumor and prolong survival time.Citation22 Brachytherapy 125I seed could inhibit the proliferation and promote the apoptosis by effecting the mitosis of cell cycle.Citation23–Citation25 Similarly, due to the biological effect of 125I seed and full preoperative TPS, many studies have confirmed that it could guarantee a sufficiently high local dose without damaging the surrounding tissue.Citation26 So, 125I brachytherapy could be an alternative therapy for locally advanced NSCLC even if diseased progressed on CCRT.
Another finding was that the combined group achieved better LRR and survival, these results were consistent with previous studies. Zhang et al reported that 125I brachytherapy had good local control and could relieve symptoms of locally advanced NSCLC, the 1-, 2-, and 3-year OS rates were 68.7%, 37.5%, 20.8%, the 3-, 6-,12- and 24-months of LRR and local PFS for 125I brachytherapy was 85%, 77.6%, 64.3%, 39.9% and 100%, 97%, 86.6%, 79.1%, respectively.Citation27 Zhang et al also showed that the RR was higher in 125I implantation (79.2%), the survival rates of 1-, 2-year were 62.5% and 16.7%.Citation28 Wu et al reported 50 patients with stage III or IV NSCLC were treated with 125I brachytherapy and chemotherapy, the symptoms of patients were significantly relieved in 125I brachytherapy group, the OS and PFS were also prolonged (20 months and 13 months).Citation29 The median PFST and OS in group A were 15.1±1.4 months and 21.2±1.6 months in our study, suggesting that 125I brachytherapy with second-line chemotherapy was valuable because the lung lesion was well controlled and survival time was prolonged. For these patients, 125I brachytherapy could be a radical treatment.
Our results also showed that patients with more than 4 number of first cycle chemotherapy and tumor size (≤3 cm) had better OS and PFST, No. of first cycle chemotherapy and tumor size were closely related to survival. Many studies reported that the more chemotherapy cycles received, the better survival in NSCLC patients, number of chemotherapy cycles was one of predictors of metastasis or recurrence.Citation30 Similarly, the tumor size could reflect the invasiveness and metastatic ability of cancer, and it was also the most sensitive marker related to survival.Citation31 However, proportional hazard regression found no association between tumor number and PFST/OS, which could be explained by enrolled indication criteria (more than 65% was a single lesion in this study).Citation32
Clinical symptoms associated with tumors were the most common and severely affected quality of life of NSCLC patients.Citation33 Chest pain could be observed in more than 70% of patients with advanced NSLCLC.Citation34 During follow-up, the clinical symptoms were significantly relieved in group A (P < 0.01), cough and chest pain remission rates (significant remission + partial response) were higher in group A (84.9%, 88.7% versus 24.7%, 21.4%) (P < 0.01). However, we also found that pain and cough were worse in two patients with large lesions after postoperative follow up, while the CT images showed that the tumor had shrunk significantly. We considered that nerve damage caused by repeated puncture or large lesions near the pleura could explain this, this might require further clinical and statistical exploration.
The effectiveness of 125I brachytherapy and rate of related complications were dependent on position of seeds and complete TPS. The American Brachytherapy Society’s “dual 90” guideline showed that 90% of the tumor volume acquired 90% prescription dose for achieving cancer cure.Citation35 According to preoperative TPS plan and 3D images within CT scan, our study could make more than 95% of the tumor get 100% of the prescription dose. Therefore, all patients were successfully treated using CT guidance. The location and boundary of tumor, important blood vessels and organs were well confirmed, no massive bleeding or related deaths occurred due to 125I brachytherapy.
Our study had limitations. First, although more than 200 patients were recruited in our hospital, this was just a retrospective study, more hospitals and prospective clinical studies are needed. Second, it was a little difficult to find accurate position for larger lesion (>5cm) because of the described TPS and applicators. So, we recommend that 125I brachytherapy combined with ablation (eg, radiofrequency) might be a suitable method. Finally, we did not compare 125I brachytherapy with external beam re-irradiation, which would be meaningful for our further study.
In conclusion, as a minimally invasive method, 125I brachytherapy combined with second-line chemotherapy in selected patients with progressive NSCLC following CCRT might improve treatment results without serious treatment-related toxicity.
Abbreviations
CT, computed tomography; NSCLC, non-small cell lung cancer; CCRT, concurrent radiochemotherapy; LRR, local response rate; PFST, progression-free survival time; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Ethics and Consent Statement
This study protocol was approved by the institutional review board of the Third affiliated hospital of Sun Yat-sen University. The study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki. Written informed consent was obtained from all patients before treatment.
Disclosure
The authors declare that there are no conflicts of interest.
References
- Carney DN, Hansen HH. Non-small-cell lung cancer-stalemate or progress? N Engl J Med. 2000;343(17):1261–1262. doi:10.1056/NEJM20001026343171011071679
- Toyokawa G, Takenoyama M, Ichinose Y. Multimodality treatment with surgery for locally advanced non-small-cell lung cancer with N2 disease: a review article. Clin Lung Cancer. 2015;16(1):6–14. doi:10.1016/j.cllc.2014.07.00725220209
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK positive non–small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi:10.1056/NEJMoa170479528586279
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X21783417
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive nonsmall-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa160677427718847
- Planting A, Helle P, Drings P, et al. A randomized study of high-dose split course radiotherapy preceded by high-dose chemotherapy versus high dose radiotherapy only in locally advanced non-small-cell lung cancer. An EORTC lung cancer cooperative group trial. Ann Oncol. 1996;7(2):139–144. doi:10.1093/oxfordjournals.annonc.a0105408777169
- Morton RF, Jett JR, McGinnis WL, et al. Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non-small cell lung cancer. A randomized, Phase III trial. Ann Intern Med. 1991;115(9):681–686. doi:10.7326/0003-4819-115-9-6811656827
- Cardenal F, Nadal E, Jove M, et al. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: a review of the literature. Ann Oncol. 2015;26(2):278–288. doi:10.1093/annonc/mdu22924942274
- Vrankar M, Stanic K. Long-term survival of locally advanced stage III non-small cell lung cancer patients treated with chemoradiotherapy and perspectives for the treatment with immunotherapy. Radiol Oncol. 2018;52(3):281–288. doi:10.2478/raon-2018-000930210037
- Jassem J. Combined chemotherapy and radiation in locally advanced non-small-cell lung cancer. Lancet Oncol. 2001;2(6):335–342. doi:10.1016/S1470-2045(00)00387-911905749
- Ramella S, Fiore M, Silipigni S, et al. Local control and toxicity of adaptive radiotherapy using weekly CT imaging: results from the LARTIA trial in stage III NSCLC. J Thorac Oncol. 2017;12(7):1122–1130. doi:10.1016/j.jtho.2017.03.02528428149
- Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4343–4348. doi:10.1200/JCO.2013.51.535324145340
- Monk BJ, Tewari KS, Puthawala AA, Syed AM, Haugen JA, Burger RA. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. 2002;52(3):806–815. doi:10.1016/S0360-3016(01)02728-611849805
- Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40(04):314–320. doi:10.1055/s-2007-99547618283622
- Hu X, Qiu H, Zhang L, et al. Recurrent gliomas: comparison of computed tomography (CT)-guided 125I seed implantation therapy and traditional radiochemotherapy. Cancer Biol Ther. 2012;13(10):840–847. doi:10.4161/cbt.2083422797010
- Xiang Z, Bai M, Li G, et al. Safety and efficacy of 125I brachytherapy for bilateral lung recurrences from hepatocellular carcinoma after resection or ablation. J Cancer Res Clin Oncol. 2019;145(7):1907–1916. doi:10.1007/s00432-019-02943-x31161374
- Xiang Z, Li G, Liu Z, et al. 125I brachytherapy in locally advanced nonsmall cell lung cancer after progression of concurrent radiochemotherapy. Medicine (Baltimore). 2015;94(49):e2249. doi:10.1097/MD.000000000000224926656370
- Nag S, Beyer D, Friedland J, Grimm P, Nath R. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44(4):789–799. doi:10.1016/S0360-3016(99)00069-310386635
- Yan H, Xiang Z, Zhong Z, et al. CT-guided 125I brachytherapy in the treatment of distant metastases in the oral cavity and maxillofacial region. Transl Oncol. 2017;10(1):90–98. doi:10.1016/j.tranon.2016.11.00727992832
- Mo Z, Zhang T, Zhang Y, et al. Feasibility and clinical value of CT-guided 125I brachytherapy for metastatic soft tissue sarcoma after first-line chemotherapy failure. Eur Radiol. 2018;28(3):1194–1203. doi:10.1007/s00330-017-5036-028956119
- Ruysscher DD, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20(1):98–102. doi:10.1093/annonc/mdn55918718891
- Moreno-Jimenez M, Aristu J, Lopez-Picazo JM, et al. Dosimetric analysis of the patterns of local failure observed in patients with locally advanced non-small cell lung cancer treated with neoadjuvant chemotherapy and concurrent conformal (3D-CRT) chemoradiation. Radiother Oncol. 2008;88(3):342–350. doi:10.1016/j.radonc.2008.05.01918558448
- Qu A, Wang H, Li J, et al. Biological effects of 125I seeds radiation on A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer Invest. 2014;32(6):209–217. doi:10.3109/07357907.2014.90558524745612
- Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67.24974178
- Yang G, Peng S, Zhang Y, et al. Cell-based assay system to estimate the effect of 125I seeds on cancer cells: effect of osteopontin. Recent Pat Anticancer Drug Discov. 2014;9(2):258–263. doi:10.2174/157489280966614033115174724693996
- Yan H, Mo Z, Xiang Z, et al. CT-guided 125I brachytherapy for locally recurrent nasopharyngeal carcinoma. J Cancer. 2017;8(11):2104–2113. doi:10.7150/jca.1907828819412
- Zhang T, Lu M, Peng S, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol. 2014;140(8):1383–1390. doi:10.1007/s00432-014-1655-x24723151
- Zhang S, Zheng Y, Yu P, et al. The combined treatment of CT-guided percutaneous 125I seed implantation and chemotherapy for non-small-cell lung cancer. J Cancer Res Clin Oncol. 2011;137(12):1813–1822. doi:10.1007/s00432-011-1048-321922327
- Wu C, Li B, Sun G, Peng C, Xiang D. Efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in the treatment of advanced NSCLC in the elderly. Onco Targets Ther. 2018;11:6617–6624. doi:10.2147/OTT.S17445730349295
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–1571. doi:10.1016/S0140-6736(13)62159-524576776
- Maeda R, Yoshida J, Hishida T, et al. Late recurrence of non-small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow-up. Chest. 2010;138(1):145–150. doi:10.1378/chest.09-236120382716
- Sen E, Kaya A, Erol S, Savas I, Gonullu U. Kadinlarda akciğer kanseri: klinik özellikler ve sağkalima etkili faktörler [Lung cancer in women: clinical features and factors related to survival]. Tuberk Toraks. 2008;56(3):266–274.18932027
- Smith IE, O’Brien ME, Talbot DC, et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001;19(5):1336–1343. doi:10.1200/JCO.2001.19.5.133611230476
- Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer. 2004;90(12):2288–2296. doi:10.1038/sj.bjc.660181015162156
- Nag S. Brachytherapy for prostate cancer: summary of American Brachytherapy Society recommendations. Semin Urol Oncol. 2000;18(2):133–136.10875454