Abstract
Purpose
The lymph node gross target volume (GTV) delineation in patients with non-small cell lung cancer (NSCLC) is crucial for prognosis. This study aimed to develop a predictive model that can be used to differentiate between lymph nodes micrometastasis (LNM) and non-lymph nodes micrometastasis (non-LNM).
Patients and Methods
A retrospective study involving 1524 patients diagnosed with NSCLC was collected in the First Hospital of Wuhan between January 1, 2017, and April 1, 2020. Duplicated and useless variables were excluded, and 16 candidate variables were selected for further analysis. The random forest (RF) algorithm and generalized linear (GL) algorithm were used to screen out the variables that greatly affected the LNM prediction, respectively. The area under the curve (AUC) was compared between the RF model and GL model.
Results
The RF model revealed that the variables, including pathology, degree of differentiation, maximum short diameter of lymph node, tumor diameter, pulmonary membrane invasion, clustered lymph nodes, and T stage, were more significant for LNM prediction. Multifactorial logistic regression analysis for the GL model indicated that vascular invasion, tumor diameter, degree of differentiation, pulmonary membrane invasion, and maximum standard uptake value (SUVmax) were positively associated with LNM. The AUC for the RF model and GL model was 0.83 (95% CI: 0.75 to 0.90) and 0.64 (95% CI: 0.60 to 0.70), respectively.
Conclusion
We successfully established an accurate and optimized RF model that could be used to predict LNM in patients with NSCLC. This model can be used to evaluate the risk of an individual patient experiencing LNM and therefore facilitate the choice of treatment.
Introduction
Globally, lung cancer cases and deaths remain rising over the past decade. In 2018, the Global Cancer Observatory Organization (GLOBOCAN) estimated more than 2 million new cases and 1.76 million deaths, obviously higher than 2012 reported rates (1.8 million new cases and 1.6 million deaths).Citation1 NSCLC has been always the most common cause of morbidity and mortality worldwide.Citation2 Over the past two decades, important advancements in the treatment of NSCLC have been achieved, such as chemotherapy, molecular-targeted therapy, or immunotherapy.Citation3 However, the overall cure and survival rates for NSCLC remain low, particularly in metastatic disease.Citation3 Therefore, continued research into the optimization of treatment strategy is urgent.
Mediastinal and hilar LNM is one of the recurrence patterns after definitive treatment of lung cancer.Citation4 Lymph node dissection is an important part of this procedure that can improve the prognosis of the patients diagnosed with early stage.Citation5 Although radiotherapy has proven to be effective for LNM, radiation pneumonitis inevitably decreases patients’ performance status and quality of life, which can be related to a worse long-term prognosis.Citation6 Hence, evaluation of regional LNM is important for the clinician to determine the GTV delineation and prognosis.Citation7 Especially, repeated radiotherapy for lymph node recurrence after primary tumors should be evaluated carefully, because irradiation areas are wide and an excess radiation dose can be caused by overlapping radiotherapy.Citation4,Citation8 Additionally, hilar and mediastinal lymph node metastases are frequently located close to organs at risk.Citation4 Therefore, it is an urgent need to develop an accurate model for predicting the risk of LNM that can be used to facilitate the management of clinical treatment.
The rapid evolution of knowledge in artificial intelligence, a new machine learning choice that predicts the risk of LNM with better accuracy has led some clinicians to question the traditional predictive linear model in clinical care at an individualized patient level.Citation7 The RF algorithm allows a machine to be fed with raw data and to automatically screen the useful variables needed for detection with multiple neural layers in the network.Citation9,Citation10 Ensemble predictors such as the RF model are known to have superior accuracy. Besides, a GL model is very interpretable especially when forward feature selection is used to construct a predictive model.Citation11
In the present study, we aimed to develop a predictive model via machine learning that can be used to differentiate between LNM and non-LNM. The validated capability of enabling expeditious and accurate LNM risk stratification of NSCLC may facilitate more responsive radiotherapy that is conducive to high-risk NSCLC patients via early identification, and ensuing instant intervention as well as precise GTV delineation and monitoring, thus, hopefully assisting to manage therapeutic intervention more appropriately.
Patients and Methods
Patients Enrollment
Between January 1, 2017, and April 1, 2020, we retrospectively collated data from consecutive patients who had been diagnosed with NSCLC at the First Hospital of Wuhan. All patients who had received lobectomy or segmentectomy with complete lymph node dissection as per the nomenclature were selected for this study. This study was approved by the Institutional Ethics Committee of the Wuhan No.1 Hospital (Reference: [2020] No.31), in compliance with the Declaration of Helsinki. Written informed consent was obtained from all participants before any treatment. All patients’ information was anonymous. The inclusion criteria were as follows: (1) patient had undergone oncological surgical resections with systematic nodal dissection; (2) patients’ TNM classification (Until then, 6th and 7th edition) were transferred into the definitions of 8th edition of American Joint Committee on Cancer (AJCC) by two pathologists to form unified tumor stage classification. Exclusion criteria were as follows: (1) patient received any neoadjuvant therapy; (2) patient with limited and/or palliative resections; (3) patient with carcinoid tumors.
Evidence of Lymph Node Resection and Staging
Systematic nodal dissection was performed according to the technique as described.Citation12 The mediastinal lymph nodes were dissected systematically within anatomical landmarks and labeled as described according to Mountain&Dresler map (Until then, 2010),Citation13 and thereafter followed the guideline of the International Association for Study of Lung Cancer (IASLC).Citation14 Clinical staging was based on the results of blood tests, Thorax-CT, PET/CT, Brain MRI (mainly for symptomatic patients), and Bronchoscopy. Patients with cN2, cN3 according to the radiological findings received invasive mediastinal staging via the Endobronchial Ultrasound (EBUS) findings. LNM status preoperatively was evaluated as follows: (1) Negative lymph nodes, calcification, and fat in the lymph node; (2) Positive lymph nodes, the short axis of the lymph node ≥10 mm, necrosis in the lymph nodes.Citation15
Data Preparation
Among 46 original variables, we excluded those duplicated variables via correlation matrix analysis. The candidate variables such age, gender, degree of differentiation, histological tumor type, tumor size, and tumor site. Furthermore, the histological and morphological parameters such as pT-category, SUVmax, vascular invasion, pulmonary membrane invasion, clustered lymph nodes, and the maximum short diameter of lymph node.Citation16 We also examined the localization and lobe distribution as to the potential effect on LNM in patients with NSCLC.
Statistical Analysis and Evaluation of Models
For descriptive analysis, continuous variables were presented as the mean with standard deviation (SD). Categorical variables are presented as numbers (%). Bonferroni correction adjusts probability values were used to compare qualitative data.Citation17 The random forest was developed from various decision tree models.Citation18 Random allocated variables were used to evaluate the random forest prediction model. The importance of candidate variables was mirrored by the mean decreased Gini(MDG) score. Besides, cross-validation is performed in the random forest algorithm, which can be merely as useful as depending on a separate test set to evaluate the generalization error of the training set. The stepwise regression based on the Akaike information criterion (AIC) minimum was used to select variables for inclusion in the GL model. The GL model was used to evaluate direct and indirect associations between LNM and candidate variables.Citation19 Concordance index (C-index) and area under the curve (AUC) calculated by bootstrapping plots were used to evaluate calibrating ability. C-index and AUC values vary from 0.5 to 1.0, where 0.5 represents random chance and 1.0 indicates a perfect fit. Typically, C-index and AUC values greater than 0.7 suggest a reasonable estimation. All analyses were performed using the Python programming language (version 3.9.2, Python Software Foundation, https://www.python.org/) and R Project for Statistical Computing (version 4.0.4, http://www.r-project.org/). All P values were two-tailed, and P<0.05 was considered statistically significant.
Results
Patient Demographics and Characteristics
A total of 1524 patients diagnosed with NSCLC were collected in this study. A correlation matrix was spotted, and 16 candidate variables correlated with LNM are summarized in . The whole data set was randomly and automatically divided into a training set (N=991,65%) and a testing set (N=533, 35%). Additionally, the systematic nodal dissection with removal of all ipsilateral hilar and mediastinal lymphatic tissue was carried out for all patients in oncological lung resection. All patients with NSCLC were diagnosed by imaging and pathology, and a total of 5196 groups of mediastinal and hilar small lymph nodes were obtained. Among them, 816 groups were pathologically confirmed, metastasis group. The pathological positive rate of the small lymph node group was 15.7%, mainly distributed in hilar lymph nodes (34.4%), inferior paratracheal lymph nodes (25.1%), and subcarinal lymph nodes (16.2%). The detailed patient demographics and characteristics are shown in .
Table 1 Patients’ Demographics and Clinicopathological Characteristics
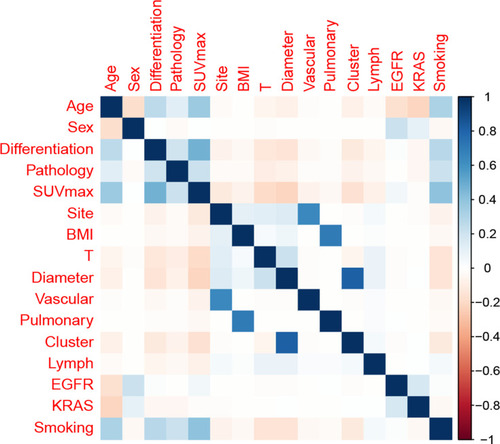
Figure 1 Correlation matrix of candidate features. Values in this matrix demonstrated the correlation coefficient of each corresponding variable.
RF Model Building
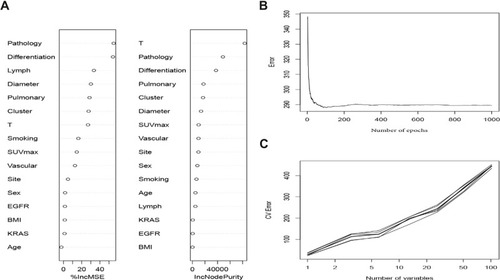
A total of 991 patients from the training set were used to fit the random forest algorithm. The samples were randomly assigned into the non-overlapping training samples for establishing the RF prediction model (). Generally, the 16 candidate variables were ordered according to the MDG score (). The significant variables, such as pathology, degree of tumor differentiation, the maximum short diameter of lymph node, tumor diameter, pulmonary membrane invasion, clustered lymph nodes, and T stage, were more significant for LNM predictive model construction (). The results of internal validation by ten-fold cross-validation showed the stability of the model’s predictive power ().
Figure 2 Random forest model. (A) The candidate factors associated with micrometastasis of lymph nodes were ordered according to the mean decreased Gini index. (B) Relationship of dynamic changes between the prediction error and the number of decision trees. (C) Performance of the prediction model with increasing numbers of features in the tenfold cross-validation.
GL Model Building
The GL model was widely used to extract all neural responses inside an epoch.Citation20 Adjusting for important prognostic factors or baseline covariates in generalized linear models may improve the estimation efficiency.Citation21 Hence, through stepwise regression, the included candidate predictors and the corresponding associations were almost the same with the model developed among the AIC filter, such as tumor diameter, degree of tumor differentiation, pulmonary membrane invasion, SUVmax, and vascular invasion. Besides, the C-index and the Brier score were performed to predict the performance of each model (). According to the AIC filter, the stability and potential utility of model 2 was confirmed for GL model construction, including vascular invasion (OR: 0.31, 95% CI: 0.25–0.40), differentiation (Moderate vs High: OR: 0.97, 95% CI: 0.85–1.10; Poorly vs High: OR: 1.54, 95% CI: 1.39–1.72), tumor site (OR: 2.69, 95% CI: 1.96–3.69), EGFR (OR: 1.07, 95% CI: 0.94–1.22), and SUVmax (OR: 2.49, 95% CI: 2.18–2.84).
Table 2 The Predictive Performances of Different Models Associated with Micrometastasis of Lymph Nodes
RF Model Robustness Verification in the Training and Testing Set
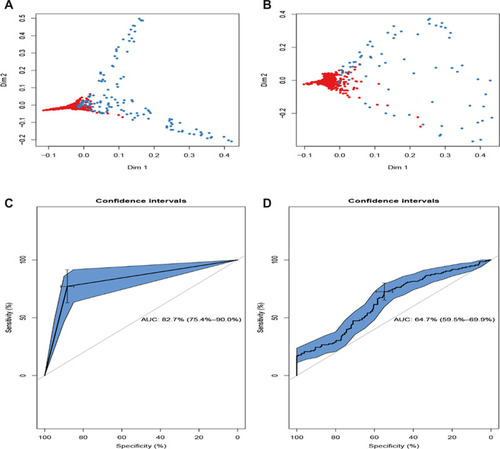
The random forest was commonly used to better distinguish the possibility of LNM in patients with NSCLC. The efficacy of the prediction model was assessed using the training set () and testing set (). As is shown in the multidimensional classification chart of the random forest, which could better distinguish the patient with the possibility of LNM or not.
Figure 3 Validation and comparison of the predictive model. The training set (A) and testing set (B) associated with micrometastasis of lymph nodes were measured via the RF model. The comparison of the ROC curve of the RF model (C) and GL model (D).
Comparison of Two Predictive Models in the Testing Set
The accuracy of probabilistic predictions between the GL model () and RF model () was compared using the testing set. The AUC values were 0.83 (95% CI: 0.75 to 0.90) for the RF model and 0.64 (95% CI: 0.60 to 0.70) for the GL model, which presented the statistical significance of the two prediction models (P<0.05).
Discussion
Precise evaluation of LNM in patients with NSCLC is quite important for clinicians to determine the treatment and prognosis. Accordingly, the IASLC lymph node map is employed in the 8th edition of the TNM staging system.Citation22 Based on the sequence of the IASLC lymph node map, ipsilateral interlobar lymph nodes were first affected, followed by hilar lymph nodes, and then mediastinal lymph nodes. Nagata et al reported that detail of patterns of failures after stereotactic body radiotherapy for stage I lung cancer, and the LNM was 14.2% and isolated LNM without other recurrent site was 3.0%.Citation23 Gorai et al reported that pathological N1 and N2 were incidentally observed in 9.3% and 12.3% in clinical stage IA lung cancer patients who underwent pulmonary resection.Citation24 Kelsey et al reported that Hilar and mediastinal LNM after surgery was detected in 7% of stage I to II lung cancer patients.Citation25 Besides, isolated LNM can be curable by aggressive local treatment including surgery and RT, because the disease site is the only regional.Citation4 In addition, the ideal treatment strategy for LNM remains uncertain. Surgery is the mainstay of LNM, but salvage surgery after the first surgery is not feasible due to the patient burden. Salvage radiotherapy can be a treatment option for LNM. However, the hilar and mediastinal LNM are frequently located close to organs at risk. Detection of disseminated tumor cells is a great technical challenge, and many different technologies have been developed to enhance the sensitivity and specificity of the testing for micrometastasis.Citation26 As for the pathological diagnosis, IHC is the most common staining method used to diagnose suspected micrometastasis. However, micrometastasis is very hard to pathologically diagnose even in permanent sections, even on ample incisional biopsies or resection specimens. Therefore, it is important to establish the treatment planning methods for GTV delineation to avoid organ injury in radiotherapy. In this study, we established an LNM prediction model based on machine deep learning. Compared with traditional linear prediction models, the performance of the LNM prediction model obtained by machine learning is better. To our knowledge, this is the first GTV delineation-assisted LNM prediction model applying a deep learning method.
To date, due to the lack of a “gold standard” for GTV delineation, there is considerable controversy in the delineation of the GTV of the lymph node.Citation27 The International Commission on Radiation Units and Measurements (ICRU) defined the GTV as a tumor area evaluated via clinical examination and imaging. Herth et al reported that Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) contributed to sample enlarged mediastinal lymph nodes in patients with NSCLC.Citation28 Haruki et al observed that having a micropapillary component was one of the significant predictors of unexpected node-positive diseases.Citation29 Nomori et al summarized that although PET was superior to CT scanning in lymph node staging in lung cancer, PET was unable to distinguish metastatic foci smaller than 4 mm, which were not unusual sizes for lymph node metastases in lung cancer. Positive lymph nodes with PET than 9 mm are likely to be true-positive rather than false-positive.Citation30 Collectively, accurate prediction of occult lymph node involvement based on associated risk factors is necessary, which might facilitate pretherapy evaluation and decision-making.
In this study, we developed an RF model for the LNM prediction. When applied clinically, this LNM prediction model might serve as a tool to select patients for precise GTV delineation or provide clinicians with an opportunity to select alternative treatment options. Unlike other established risk assessment methods, the RF model not confined to a small number of risk factors could incorporate all candidate risk factors. With the help of machine learning, we screened out some meaningful variables, including tumor diameter, degree of tumor differentiation, pulmonary membrane invasion, consistent with previous studies.Citation29–Citation32 Meanwhile, we also got some variables that were not developed before, including the maximum short diameter of lymph nodes and clustered lymph nodes. To date, there have been no prospective studies to evaluate the correlation between clustered lymph nodes. Our research demonstrated that there is a correlation between clustered lymph nodes and lymph node metastasis, and clusters of small lymph nodes have a higher risk of metastasis. In other cancer research, the definition of a positive lymph node is also different. In nasopharyngeal carcinoma, clusters of 3 or more lymph nodes with a minimum diameter greater than 0.8 cm are considered positive lymph nodes.Citation33 However, in rectal cancer, 3 or more small lymph nodes less than 0.8 cm in clusters are considered metastatic lymph nodes.Citation34 Our research also suggested that in terms of lymph node size, the risk of tumor metastasis is higher in the small lymph node group with the shortest diameter of the largest lymph node greater than or equal to 0.6 cm. Previous studies have also reported that the short diameter of the largest lymph node for small lymph node metastasis is between 0.6 and 0.9 cm, which is consistent with our research results.Citation35,Citation36 As a difficult point in non-invasive diagnosis, the best cutoff value appears to be more limited in a linear model. However, using a nonlinear model, we can ignore the cut-off value of the short diameter of the largest lymph node, thereby improving the flexibility and accuracy of prediction.
Several studies have demonstrated that the incidence of LNM differs according to individual clinical parameters and histologic components within the tumor.Citation37–Citation40 Appropriate targeted therapy is very effective in patients with advanced NSCLC who have specific genetic alterations.Citation41 Besides, Dong et al reported that patients with high levels of pre-stereotactic body radiotherapy SUVmax had poorer overall survival and local control and higher distant metastases.Citation42 In our correlation matrix analysis, we also found that there is indeed a significant correlation between these variables, which suggests that they can be used as potential predictors of LNM. Consequently, our RF model performs an iterative analysis of candidate variables and shows very robust results in predicting LNM.
Nevertheless, the present study has several limitations that need to be considered. This study was based on data from a single institution. Although our validation was robust, it is now necessary to conduct external validation using data from other centers. Second, the ideal treatment strategy for isolated lymph node metastasis remains uncertain, which might have changed the denominator of truly occult disease. Third, because the model parameters were based on clinical collectable variables, the application of potential variables (such as immunological diagnosis biomarkers, genetical analysis) might improve the accuracy of our prediction model. Future research should be cautious and validated carefully.
Conclusion
To conclude, combinatorial applications of the LNM risk prediction model for NSCLC and electronic health records with readily available features can enable timely and accurate risk stratification of NSCLC patients on admission. The RF model can potentially assist clinicians to promptly target the high-risk patients on admission, and accurately contribute to GTV delineation assessment of patients with NSCLC, although external validation is required.
Disclosure
None of the authors have any conflicts of interest to declare.
Acknowledgments
The authors gratefully acknowledge all of our participants for sharing their medical records. The authors also wish to thank the staff members at the Wuahn No. 1 Hospital for their assistance with data collection. The authors also thank Charlesworth Author Services for their professional English editing service.
References
- Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24. doi:10.1016/j.ccm.2019.10.00132008623
- Cortes J, Perez-García JM, Llombart-Cussac A, et al. Enhancing global access to cancer medicines. CA Cancer J Clin. 2020;70(2):105–124. doi:10.3322/caac.2159732068901
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature2518329364287
- Shirai K, Kubota Y, Ohno T, et al. Carbon-ion radiotherapy for isolated lymph node metastasis after surgery or radiotherapy for lung cancer. Front Oncol. 2019;9:731. doi:10.3389/fonc.2019.0073131448233
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(5):787–798. doi:10.1093/ejcts/ezu02824578407
- Brooks ED, Verma V, Senan S, et al. Salvage therapy for locoregional recurrence after stereotactic ablative radiotherapy for early-stage NSCLC. J Thorac Oncol. 2020;15(2):176–189. doi:10.1016/j.jtho.2019.10.01631712134
- Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.2138828094848
- Lee NK, Moon SH, Kim TH, et al. Prognostic value of gross tumor volume for definitive radiation therapy in patients with locoregionally recurrent non-small-cell lung cancer after surgical resection. Clin Lung Cancer. 2013;14(4):399–406. doi:10.1016/j.cllc.2012.11.00223276823
- Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. 2002;35(5–6):352–359. doi:10.1016/S1532-0464(03)00034-012968784
- Wu S, Roberts K, Datta S, et al. Deep learning in clinical natural language processing: a methodical review. J Am Med Inform Assoc. 2020;27(3):457–470. doi:10.1093/jamia/ocz20031794016
- Song L, Langfelder P, Horvath S. Random generalized linear model: a highly accurate and interpretable ensemble predictor. BMC Bioinform. 2013;14:5. doi:10.1186/1471-2105-14-5
- Graham AN, Chan KJ, Pastorino U, Goldstraw P. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 1999;117(2):246–251. doi:10.1016/S0022-5223(99)70419-89918964
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111(6):1718–1723. doi:10.1378/chest.111.6.17189187199
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-420180029
- Nambu A, Kato S, Motosugi U, et al. Thin-section CT of the mediastinum in preoperative N-staging of non-small cell lung cancer: comparison with FDG PET. Eur J Radiol. 2010;73(3):510–517. doi:10.1016/j.ejrad.2009.01.02119246170
- Feng SH, Yang ST. The new 8th TNM staging system of lung cancer and its potential imaging interpretation pitfalls and limitations with CT image demonstrations. Diagn Interv Radiol. 2019;25(4):270–279. doi:10.5152/dir.2019.1845831295144
- Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–508. doi:10.1111/opo.1213124697967
- Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources, and a solution. BMC Bioinform. 2007;8:25. doi:10.1186/1471-2105-8-25
- Scott M, Flaherty D, Currall J. Statistics: general linear models (a flexible approach). J Small Anim Pract. 2014;55(10):527–530. doi:10.1111/jsap.1226025134691
- Kristensen E, Guerin-Dugué A, Rivet B. Regularization and a general linear model for event-related potential estimation. Behav Res Methods. 2017;49(6):2255–2274. doi:10.3758/s13428-017-0856-z28275950
- Qu Y, Luo J. Estimation of group means when adjusting for covariates in generalized linear models. Pharm Stat. 2015;14(1):56–62. doi:10.1002/pst.165825406099
- Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–577. doi:10.1097/JTO.0b013e3181a0d82e19357537
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. doi:10.1016/j.ijrobp.2015.07.227826581137
- Gorai A, Sakao Y, Kuroda H, et al. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47(4):653–658. doi:10.1093/ejcts/ezu24424957260
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early-stage lung cancer: an 11-year experience with 975 patients. Cancer. 2009;115(22):5218–5227. doi:10.1002/cncr.2462519672942
- Yu Y, Zhao Q, He XP, Wang Z, Liu XY, Zhang ZP. Signal transducer and activator of transcription 3 overexpression promotes lymph node micrometastasis in early-stage non-small cell lung cancer. Thorac Cancer. 2018;9(5):516–522. doi:10.1111/1759-7714.1259829575778
- Vorwerk H, Beckmann G, Bremer M, et al. The delineation of target volumes for radiotherapy of lung cancer patients. Radiother Oncol. 2009;91(3):455–460. doi:10.1016/j.radonc.2009.03.01419339069
- Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133(4):887–891. doi:10.1378/chest.07-253518263680
- Haruki T, Wakahara M, Matsuoka Y, et al. Clinicopathological characteristics of lung adenocarcinoma with unexpected lymph node metastasis. Ann Thorac Cardiovasc Surg. 2017;23(4):181–187. doi:10.5761/atcs.oa.16-0030928539542
- Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg. 2004;127(4):1087–1092. doi:10.1016/j.jtcvs.2003.08.01015052206
- Kaseda K, Asakura K, Kazama A, Ozawa Y. Risk factors for predicting occult lymph node metastasis in patients with clinical stage I non-small cell lung cancer staged by integrated fluorodeoxyglucose positron emission tomography/computed tomography. World J Surg. 2016;40(12):2976–2983. doi:10.1007/s00268-016-3652-527456499
- Murgu SD. Diagnosing and staging lung cancer involving the mediastinum. Chest. 2015;147(5):1401–1412. doi:10.1378/chest.14-135525940251
- van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177(2):379–384. doi:10.1148/radiology.177.2.22177722217772
- de Vries FE, da Costa DW, van der Mooren K, van Dorp TA, Vrouenraets BC. The value of preoperative computed tomography scanning for the assessment of lymph node status in patients with colon cancer. Eur J Surg Oncol. 2014;40(12):1777–1781. doi:10.1016/j.ejso.2014.08.48325260599
- Chen K, Yang F, Jiang G, Li J, Wang J. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg. 2013;96(5):1761–1768. doi:10.1016/j.athoracsur.2013.06.03823998401
- Billé A, Pelosi E, Skanjeti A, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36(3):440–445. doi:10.1016/j.ejcts.2009.04.00319464906
- Zhao F, Zhen FX, Zhou Y, et al. Clinicopathologic predictors of metastasis of different regional lymph nodes in patients intraoperatively diagnosed with stage-I non-small cell lung cancer. BMC Cancer. 2019;19(1):444. doi:10.1186/s12885-019-5632-231088404
- Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg. 2014;98(2):453–458. doi:10.1016/j.athoracsur.2014.04.10824961844
- Ito M, Miyata Y, Kushitani K, et al. Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer. 2014;85(2):270–275. doi:10.1016/j.lungcan.2014.05.01424976332
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Onc. 2014;12:388. doi:10.1186/1477-7819-12-388
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13(5):515–524. doi:10.6004/jnccn.2015.007125964637
- Dong M, Liu J, Sun X, Xing L. Prognostic significance of SUV(max) on pretreatment (18) F-FDG PET/CT in early-stage non-small cell lung cancer treated with stereotactic body radiotherapy: a meta-analysis. J Med Imaging Radiat Oncol. 2017;61(5):652–659. doi:10.1111/1754-9485.1259928266166
