?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The aim of this study was to evaluate the value of preablative stimulated thyroglobulin (presTg) and thyroglobulin reduction index (TRI) to predict the different responses to second radioactive iodine (RAI) therapy in differentiated thyroid cancer (DTC) patients with structural incomplete response (SIR).
Patients and Methods
A single-center retrospective study analyzed the different clinical outcomes after second RAI therapy in 206 patients with SIR. PresTg1 and presTg2 were measured before first and second RAI management and TRI was the reduction index of presTg1 and presTg2. Cut-off values of presTg and TRI were obtained using receiver operating characteristic analysis. The univariate logistic regression analysis was performed to confirm these parameters as prognostic factors to predict different responses to second RAI therapy.
Results
Only ATA risk stratification, the post-therapy whole-body scanning (Rx-WBS) findings, presTg1, presTg2, TRI, were different in patients with SIR. After second RAI therapy, 28.2% (58/206) of patients with SIR initially were reclassified as excellent response (ER). PresTg1 <6.6 ng/mL, presTg2 <1.2ng/mL, and TRI >74.2% were excellent indications to predict ER from non-ER after second RAI treatment. PresTg1 >14.9 ng/mL, presTg2 >1.8ng/mL and TRI <66.5% were well markers to predict poor outcome (SIR). High risk and distant metastases could still be considered as risk factors.
Conclusion
DTC patients with SIR could benefit through second RAI treatment. PresTg before each RAI therapy and TRI could be considered as effective decision-making markers for second RAI therapy and as predictive indications for clinical outcomes.
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy and accounts for around 90% of all thyroid tumors.Citation1,Citation2 The five-year overall survival rate is as high as 98%, whereas persistent/recurrent diseases still exist in 20–30% of DTC patients following the initial treatment.Citation3–Citation5 In addition, local or regional recurrence and distant metastasis (DM) occur in 20–50% and 10–15% of patients, respectively.Citation6,Citation7 The current treatment modalities for DTC are total or near total thyroidectomy (TT), selective postoperative radioactive iodine (RAI) therapy according to the risk of disease persistence/recurrence and thyroid stimulating hormone (TSH)-suppressive therapy.Citation8 A study for Chinese people has shown that 26% of patients experienced twice or more times of RAI therapy owing to residual thyroid or persistent radioiodine-avid lesion.Citation9 Therefore, it is important to choose appropriate indicators for performing the next RAI management and individualized follow-up strategies for DTC patients.Citation10,Citation11
The 2015 American Thyroid Association (ATA) Guidelines suggested that TNM staging, ATA risk stratification, imaging findings and thyroglobulin (Tg) levels should be considered together when performing RAI therapy.Citation8 Indeed, stimulated thyroglobulin (sTg) has been regarded as an excellent predictor for successful ablation in many studies.Citation12–Citation14 Preablation stimulated thyroglobulin (presTg) measured before RAI treatment reflects the residual disease after surgery and could be incorporated into radioiodine decision-making for tailoring management.Citation15–Citation17 A French study first proposed the concept of thyroglobulin reduction index (TRI) recently, and evaluated the impact of presTg and TRI in all DTC patients to predict an excellent response.Citation13 While, to the best of our knowledge, there is some uncertainty as to what degree of presTg or TRI may be appropriate to prompt another RAI treatment in patients with structural incomplete response (SIR) after initial RAI and no studies have reported it.
In this study, we evaluated the clinical outcomes after second RAI therapy of patients initially classified as SIR. The purpose was to demonstrate the relationship between presTg level, TRI and the different responses to second RAI treatment in patients with SIR, proving whether presTg and TRI could be used as decision-making factors for second RAI therapy and as predictive markers for clinical outcomes.
Patients and Methods
Study Participants
In this retrospective cohort study, we reviewed the electronic medical records of 1275 DTC patients who underwent (near) TT with RAI therapy at Second Hospital of Shandong University between January 2015 and July 2019. 206 patients were finally enrolled. Inclusion criteria were as follows: (1) aged 18 years or older (9 patients were excluded); (2) patients who underwent RAI therapy at our hospital all the time (1 was excluded); (3) patients who were classified as SIR after initial therapy based on 2015 ATA Guidelines (862 were excluded); and (4) patients with second RAI therapy and subsequent follow-up data available (178 were excluded).Citation18 Moreover, 19 patients with positive anti-thyroglobulin antibody (TgAb) levels were excluded considering that the concentration of TgAb could influence the Tg level. The patient data extracted from the clinical electronic medical record system were identified such that all private information was not included. The Institutional Review Board (IRB) of Second Hospital of Shandong University approved the protocol of our study and allowed us to not obtain written consent from each patient. All procedures complied with the Declaration of Helsinki for research involving human subjects.
RAI Therapy and Follow Up
All study participants underwent (near) total TT with or without central/lateral neck dissection and initial RAI therapy, which was performed at least 2 months after surgery to eliminate normal thyroid remnant tissue, suspected microscopic foci of residual disease or known persistent disease in the neck or metastatic sites. Thyroid-stimulating hormone (TSH), presTg, TgAb and imaging examination were tested on or before the day of each RAI therapy to evaluate the patients’ condition and were used to decided therapeutic dosage by clinical doctors. The initial dosage range was 80–150mCi, and most patients received a dose of 100mCi. Meanwhile, TSH levels of >30μIU/mL were required through levothyroxine withdrawal in all patients before each RAI therapy.Citation8,Citation19 Post-therapy whole-body scanning (Rx-WBS) was performed 3–7 days after radioiodine administration to complete disease staging and document the RAI avidity of any lesion. After the RAI therapy, all the patients underwent levothyroxine treatment for TSH suppression.Citation8 Then all patients were instructed to follow a low-iodine diet from the beginning of thyroid hormone withdrawal (THW) to 4 weeks after RAI therapy.
During the first 6–12 months following the initial therapy, neck ultrasonography (US) and whole-body scan (WBS) were conducted with stimulated or suppressed Tg, TgAb, TSH levels to assess the primary responses of RAI treatment and to detect possible persistent or metastatic lesions. Then another RAI treatment (100–200 mCi) was considered based on all available information and the risk of the individual patient at the initial treatment. The response to second RAI therapy was also evaluated after 6–12 months.
Definitions of Response to RAI Therapy, PresTg and TRI
The ongoing disease status of patients after each RAI therapy was assessed by combining the serological results with imaging results according to the 2015 ATA Guidelines.Citation8 Thus, patients were divided into four groups as follows: An excellent response (ER) was defined as negative follow-up imaging and either suppressed Tg <0.2 ng/mL or TSH-stimulated Tg <1 ng/mL. An indeterminate response (IDR) was defined as nonspecific findings on imaging examination, faint uptake in thyroid bed on follow-up WBS, non-stimulated tg detectable but <1 ng/mL, or stimulated Tg detectable but <10 ng/mL, or anti-Tg antibodies stable or declining in the absence of structural or functional disease. A biochemical incomplete response (BIR) was defined as having suppressed Tg ≥1 ng/mL or stimulated Tg ≥10 ng/mL or rising anti-Tg antibody levels with negative imaging. A structural incomplete response (SIR, structural persistent disease) was defined as structural or functional evidence of disease with any Tg level, with or without anti-Tg antibodies. Each cohort patient was classified into one of four categories based on their Tg, TgAb, TSH and imaging findings at each follow-up. A TgAb value exceeding 60 IU/mL was considered to be positive for interfering with the Tg measurement.Citation8,Citation20,Citation21
PresTg was measured at TSH>30μIU/mL before each RAI therapy. The presTg1 means sTg measured after THW and before initial RAI therapy, and presTg2 means sTg tested after THW and before second RAI treatment. TRI was calculated based on presTg before each RAI therapy [TRI=100×(presTg1-presTg2)/presTg1].Citation13
Statistical Analysis
Continuous variables were expressed as the means and standard deviations (SDs) or medians with interquartile ranges (IQRs) when appropriate, and categorical variables were presented as numbers and percentages. The comparisons and associations of categorical variables were performed using the chi-square test and Fisher’s exact test when the number of patient cases was 5. The Wilcoxon–Mann–Whitney U-test was used to compared two group variables. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off points for presTg and TRI to distinguish different responses, and is presented with area under the curve (AUC) and 95% confidence interval (CI). P-value was set at <0.05 for significant results. All of these statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing; www.R-project.org).
Results
Baseline Clinicopathological Characteristics of Patients with SIR
Baseline clinical and pathological characteristics of the 206 DTC patients initially classified as SIR after treatment with (near) TT and initial RAI therapy are presented in . The mean age of patients with SIR at cancer diagnosis was 44.4±13.5 years, and there were 155 (75.2%) patients younger than 55 years of age. 211 (97.7%) patients had papillary thyroid carcinoma (PTC). Of all patients, the ratio of females to males was 1: 0.9. The median primary tumor maximum size was 1.2 cm (IQR 0.7–2.0 cm). Extra-thyroid extension (ETE) was only seen in 54 (26.2%) patients. Cervical lymph node metastasis (LNM) was detected in 178 (86.4%) patients (43.7% were lateral cervical LNM) and, of these patients, the median number of LNM was 4 (IQR 2–8) in the 178 patients with LNM. According to the TNM staging system for DTC, 162, 42, 2 (78.6%, 20.4%, 1.0%) patients were categorized as having stage I, II and III disease, respectively, and no patient was in stage IV. In respect to ATA risk stratification system, 10 (4.8%), 120 (58.3%), and 76 (36.9%) patients were classified as having low, intermediate, and high risk, respectively. The median duration from surgery to first RAI therapy was 2 months (IQR 2.0–2.8). The standard deviations varied greatly for presTg1, presTg2 or TRI. The mean levels for presTg1, presTg2 and TRI were 68.7 ng/mL, 60.5 ng/mL and −12.2%, while the median levels were 8.2 ng/mL, 3.0 ng/mL and 53.2%.
Table 1 Baseline Characteristics of Study Participants and Comparison of These Characteristics in Patients with ER and Non-ER
According to the Rx-WBS at initial RAI therapy, RAI uptake in the thyroid region was present in 39 (18.9%) patients, nodal metastases in 143 (69.4%) patients and DM in 24 (11.7%) patients, including pulmonary metastases in 21 patients, bone metastases in 1, and abdominal or pelvic metastases in 2. According to the Rx-WBS at the second RAI treatment, RAI uptake in the thyroid region was present in 36 (17.5%) patients, lymph node metastases in 133 (64.6%) patients, pulmonary metastases in 31 patients, 3 patients had bone metastasis, 1 patient had brain metastasis and 2 patients had abdominal or pelvic metastases.
Comparing different therapeutic responses (ER vs non-ER, SIR vs non-SIR) of the second treatment, only ATA risk stratification, the Rx-WBS findings at initial RAI, presTg1, presTg2, and TRI were significantly different in two cases ( and S1).
Different Therapeutic Responses After Second RAI Treatment Correlates with presTg Levels
The clinical outcomes of second RAI therapy showed that 25 (43.1%) patients with presTg1 <1 ng/mL, 28 (48.3%) with presTg1 ranging from 1 to 10 ng/mL, and 5 (8.6%) with presTg1 >10 ng/mL achieved ER (p<0.001, ). The proportion of patients with SIR was identified in 12.4% with presTg1 <1 ng/mL, 24.8% with presTg1 from 1 to 10 ng/mL and 62.8% with presTg1 >10 ng/mL (p<0.001, ).
Table 2 Clinical Outcomes of Second RAI Treatment in Terms of presTg Levels
In terms of presTg2, 51 patients of presTg2 below 1 ng/mL, 6 patients between 1 and 10 ng/mL, and 1 above 10 ng/mL achieved ER (p<0.001, ). Among these, 51 patients, 19 patients had presTg2 under 0.1 ng/mL. Patients classified as SIR were 25, 29, and 59 in the three categories of presTg2 (p<0.001, ), respectively. Among them, 7 patients had presTg2 levels under 0.1 ng/mL.
Receiver Operating Characteristic Analysis of presTg Levels with Different Responses
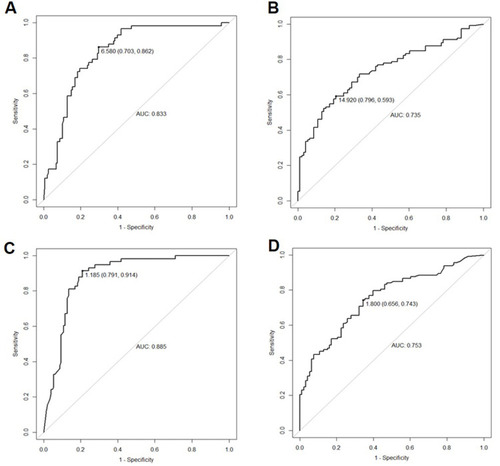
Receiver operating characteristic (ROC) curves were used for evaluating the presTg levels for distinguishing ER from non-ER and separating SIR from non-SIR. The cut-off values of presTg1 were 6.6 ng/mL (sensitivity: 86.2%; specificity: 70.3%; AUC: 0.833, ) for distinguishing ER and non-ER, 14.9 ng/mL (sensitivity: 59.3%; specificity: 79.6%; AUC: 0.735, ) for differentiating SIR from non-SIR. In addition, ROC analysis identified presTg2 <1.2 ng/mL as a sensitive predictor for ER after second treatment with sensitivity of 91.4% and specificity of 79.1% (AUC: 0.885, ). A cut-off value of presTg2 at 1.8 ng/mL was found for differentiating the patients with SIR from non-SIR, with corresponding specificity of 65.6%, sensitivity of 74.3%, and AUC of 0.753 (). No matter what kind of clinical outcome was predicted, the AUC of using presTg2 was larger than that of presTg1.
Figure 1 ROC of presTg level to distinguish different response. (A) ROC in distinguishing ER from non-ER using presTg1 (AUC: 0.833, 95% CI: 0.775–0.891); (B) ROC in distinguishing SIR from non-SIR using presTg1 (AUC: 0.735, 95% CI: 0.667–0.803); (C) ROC in distinguishing ER from non-ER using presTg2 (AUC: 0.885, 95% CI: 0.838–0.932); (D) ROC in distinguishing SIR from non-SIR using presTg2 (AUC: 0.753, 95% CI: 0.688–0.819).
Prognostic Cut-off Value of TRI
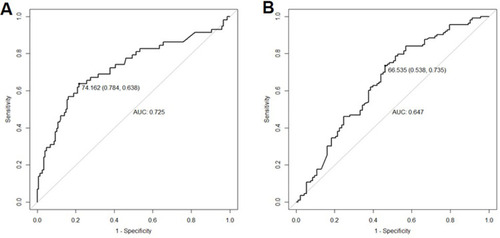
Given that the collinearity logically existed between presTg1 and presTg2, we further analyzed the relationship between TRI and different responses. A cut-off value of 74.2% was identified to predict ER after second RAI treatment with a sensitivity of 63.8% and specificity of 78.4% (AUC: 0.725, ). ROC analysis identified a TRI cut-off of 66.5% (sensitivity: 73.5%; specificity: 53.8%) to discriminate SIR from non-SIR (), while the AUC were only 0.647. Both AUCs of TRI to predict different responses were under 0.750.
Univariate Logistic Regression Analysis of Different Outcomes to Second RAI Treatment
We used univariate analyses to determine the predictive factors for ER after second RAI including ATA risk stratification, the Rx-WBS findings at initial RAI management, presTg1, presTg2, TRI. The results showed that presTg1 <6.6 ng/mL (p<0.001), presTg2 <1.2 ng/mL (p<0.001) and TRI >74.2% (p<0.001, ) were predictors for ER and presTg1 >14.9 ng/mL (p<0.001), presTg2 >1.8ng/mL (p<0.001) and TRI <66.5% (p<0.001, ) were indications for predicting SIR. When presTg2 was used to predict the results (ER/non-ER), the OR values increased from 14.773 to 34.089 (), the phenomenon was not observed when predicting another outcome (SIR/non-SIR, 5.678 vs 5.519, ). The 1–10 ng/mL interval of presTg1 and presTg2 was no longer an excellent predictor for different outcomes (p=0.666, p=0.789, and ). High risk and distant metastases remained good as predictors (p<0.05, p<0.05, and ).
Table 3 Univariate Regression Analysis of Excellent Response and Non-Excellent Response According to Clinical Characteristics
Table 4 Univariate Regression Analysis of Structural Incomplete Response According to Clinical Characteristics
Discussion
In the present study, we evaluated the clinical outcomes of DTC patients after second RAI therapy, who were initially evaluated as structural incomplete responses. Structural persistent disease (SIR) still existed in 54.9% (n=113) of patients. Interestingly, excellent response was present in 28.2% (n=58) of patients initially classified as having structural incomplete response owing to the second RAI treatment. However, at the second evaluation, 18.0% (n=37) of patients classified as SIR had distant metastases. These findings strongly support that early detection and treatment strategies are required for those patients with structural incomplete response.
We evaluated the prognostic value of presTg levels before initial RAI, second RAI and TRI in predicting different responses. PresTg1 <6.6 ng/mL, presTg2 <1.2 ng/mL and TRI >74.2% were good indications to predict excellent responses from other responses for patients with SIR undergoing second RAI. From the results of our analysis, the AUC of using presTg2 to predict ER was larger than that using presTg1 (0.885 vs 0.833) and the OR value of presTg2 was obviously higher than that of presTg1 (34.089 vs 14.773), these phenomena might suggest that presTg before second RAI was a better parameter to predict excellent response and could reflect ongoing disease status. A study from Barres et alCitation13 suggested that presTg <10 ng/mL, sTg <1 ng/mL and TRI >66% were good factors for long-term remission and persistence or recurrence-free survival. While our results suggested lower presTg1 (6.6ng/mL) and higher TRI (74.2%) were needed to predict excellent response, we analyzed the reason for these results was that our study focused on patients with SIR rather than all DTC patients, thus more stringent parameters should be considered. And in the study,Citation13 sTg was measured 72 hours after recombinant TSH (rhTSH) during the evaluation of the initial management, but presTg2 was measured after THW and before second RAI in our analysis, and both usually performed 6 to 12 months after first RAI. We still obtained the similar value of sTg (1 ng/mL and 1.2 ng/mL) to predict excellent response. In a study mentioned previously, presTg threshold of <5 ng/mL was suggested as an indication for initial RAI treatment,Citation22 which was consistent with our analysis. Through the second RAI, the patients with presTg1 <6.6 ng/mL, presTg2 <1.2 ng/mL, TRI >74.2% are more likely to be reclassified as ER in our study. However, one study claimed that patients with presTg <1 ng/mL could avoid RAI therapy.Citation23 One study indicated that 42% of patients with distant metastases could achieve complete remission after single 131I treatment,Citation24 and spontaneous Tg reduction was reported in other studies.Citation5,Citation25 Meanwhile, considering that additional treatment can cause mental and psychological stress, further research may be needed to determine whether it is indispensable for these patients to undergo second RAI therapy or not.
In the study, based on ROC analyses, presTg1 value of 14.9 ng/mL, presTg2 of 1.8 ng/mL, and TRI of 66.5% were obtained to predict SIR from non-SIR. It has been found in some studies that 50–85% of patients continued to have persistent disease despite additional therapy, even death from this disease was seen in 11% of patients with loco-regional metastases and in 57% of patients with structurally identifiable distant metastases up to 15 years of follow-up.Citation25,Citation26 Many studies showed that presTg1 levels of 20~30 ng/mL were the cut-off value to predict recurrent or persistent disease.Citation27,Citation28 A study suggested 13 ng/mL as an indicator of disease recurrence,Citation28 which was similar to our results. Meanwhile the optimal value of post-ablation stimulated thyroglobulin (1 ng/mL) measured after THW was obtained to predict long-term adverse clinical events for DTC patients in a Chinese study.Citation12 The results in our study suggested that patients with presTg1 >14.9 ng/mL, presTg2 >1.8 ng/mL, and TRI <66.5% might still have a poor outcome despite undergoing another RAI management, which leave us adequate time to perform more careful work-up and administrate larger doses of RAI therapy for these patients when another RAI therapy is needed.
In this study, comparisons of different clinical outcomes demonstrated that no difference of TNM staging appeared in the patients with SIR. Previous studies reported that presTg was better than the TNM staging for predicting excellent response to initial therapy in DTC patients,Citation29 even pointing out that the TNM staging system was not appropriate for estimating the risk of recurrence/persistence,Citation30–Citation32 while, as evidenced in other studies, locally advanced disease (T3 to T4) is the main predictor of persistence and recurrence.Citation8,Citation33 Our findings were consistent with the former. The reason for the different results may be the limited number of people in the T3/T4 stages in our study. A study showed that patients with macroscopic distant metastasis were more likely to have progression of disease or death than patients with only loco-regional evidence.Citation26 The results in our study were concordant with previous studies, that is, that high-risk and distant metastases should still be taken into account when directing additional RAI management and predicting adverse outcomes. A study from Kendler et al reported that presTg before the first I-131 remnant ablation was the only independent predictor of ablation success and the cut-off value was 18 ng/mL.Citation34 In our univariate analysis, presTg ranging from 1 ng/mL to 10 ng/mL was no longer a risk factor, which means that we should focus on more accurate cut-off values of presTg, especially before second RAI therapy to instruct more detailed and personalized treatment strategies. Indeed, there is a strong collinearity between presTg1 and presTg2, so we also used TRI to predict response to second RAI. Even though the AUCs were both under 0.75, we still considered that TRI could be a supplemental tool for reflecting tumor response to RAI treatment, which was consistent with the results of a previous study.Citation13 Given that the correlation between ATA risk stratification and Rx-WBS findings, between presTg and TRI, we did not use multivariate analysis to calculate the OR values in verifying which factor could be regarded as the more important predictive factor for different responses.
There are several limitations in this study. First, this study was inevitably limited by its retrospective design at a single center, the possibility of selection bias should be considered and the applicability of the findings in our study needs further external verification. Second, the restricted number of patients could affect the statistical power of this study. Third, although all patients received RAI therapy in our center, the surgery was performed in different centers, which doubtlessly caused the confounding factors, resulting in different strategies of RAI treatment in patients. Fourth, we were not able to use our data to discriminate among four responses at the same time, therefore we will try to pay more attention to this in our following research.
Conclusion
DTC patients with SIR on the initial dynamic evaluation had a high risk of structural persistent disease. Nonetheless, a number of patients can be reclassified from SIR to ER through second RAI treatment. Our data found the corresponding cut-off values to discriminate between different clinical outcomes. PresTg1 <6.6 ng/mL, presTg2 <1.2 ng/mL, and TRI >74.2% could be considered as indications for excellent response to second RAI treatment. PresTg1 >14.8 ng/mL, presTg2 >1.8 ng/mL, and TRI <66.5% were predictors of structural persistent disease. Therefore, our findings suggest that presTg and TRI could be considered as reliable ongoing markers for performing second RAI and as predictive indications for clinical outcomes.
Abbreviations
AJCC, American Joint Committee on Cancer; ATA, American Thyroid Association; AUC, area under the curve; BIR, biochemical incomplete response; CI, confidence interval; DTC, differentiated thyroid carcinoma/cancer; DM, distant metastases; ER, excellent response; ETE, extra-thyroid extension; IDR, indeterminate response; LNM, lymph node metastasis; PresTg, preablative stimulated thyroglobulin; PTC, papillary thyroid carcinoma/cancer; RAI, radioactive iodine; ROC, receiver operating characteristic; Rx-WBS, Post-therapy whole-body scanning; SIR, structural incomplete response; TgAb, anti-thyroglobulin antibody; THW, thyroid hormone withdrawal; TNM, tumor node metastasis; TRI, thyroglobulin reduction index; TSH, thyroid-stimulating hormone; TT, total thyroidectomy.
Ethics Statement
The patient data extracted from the clinical electronic medical record system were identified such that all private information was not included. The Institutional Review Board (IRB) of Second Hospital of Shandong University approved the use of records and allowed us to not obtain written consent from each patient (KYLL-2018[LW]013). All procedures complied with the Declaration of Helsinki for research involving human subjects.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the Jinan clinical medical science and technology innovation plan (Grant No: 202019194). Co-first author: Lihua Wang and Canhua Yun.
References
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–1348. doi:10.1001/jama.2017.271928362912
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.2133826808342
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.2134927253694
- Castagna MG, Maino F, Cipri C, et al. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–446. doi:10.1530/EJE-11-046621750043
- Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349. doi:10.1089/thy.2010.017821034228
- Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S150–S160. doi:10.1017/S002221511600057827841128
- Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. doi:10.1210/jc.2005-283816684830
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.002026462967
- Yang X, Liang J, Li T, Zhao T, Lin Y. Preablative stimulated thyroglobulin correlates to new therapy response system in differentiated thyroid cancer. J Clin Endocrinol Metab. 2016;101(3):1307–1313. doi:10.1210/jc.2015-401626789779
- Raue F, Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res. 2016;22(20):5012–5021. doi:10.1158/1078-0432.CCR-16-048427742787
- Luster M, Weber T, Verburg FA. Differentiated thyroid cancer—personalized therapies to prevent overtreatment. Nat Rev Endocrinol. 2014;10(9):563–574. doi:10.1038/nrendo.2014.10024981455
- Wong KCW, Ng TY, Yu KS, et al. The use of post-ablation stimulated thyroglobulin in predicting clinical outcomes in differentiated thyroid carcinoma - what cut-off values should we use? Clin Oncol (R Coll Radiol). 2019;31(2):e11–e20. doi:10.1016/j.clon.2018.10.00930454940
- Barres B, Kelly A, Kwiatkowski F, et al. Stimulated thyroglobulin and thyroglobulin reduction index predict excellent response in differentiated thyroid cancers. J Clin Endocrinol Metab. 2019;104(8):3462–3472. doi:10.1210/jc.2018-0268030785995
- Prpic M, Franceschi M, Romic M, Jukic T, Kusic Z. Thyroglobulin as a tumor marker in differentiated thyroid cancer - clinical considerations. Acta Clin Croat. 2018;57(3):518–527. doi:10.20471/acc.2018.57.03.1631168186
- Yang X, Liang J, Li TJ, et al. Postoperative stimulated thyroglobulin level and recurrence risk stratification in differentiated thyroid cancer. Chin Med J (Engl). 2015;128(8):1058–1064. doi:10.4103/0366-6999.15508625881600
- Piccardo A, Arecco F, Puntoni M, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med. 2013;38(1):18–24. doi:10.1097/RLU.0b013e318266d4d823242039
- Polachek A, Hirsch D, Tzvetov G, et al. Prognostic value of post-thyroidectomy thyroglobulin levels in patients with differentiated thyroid cancer. J Endocrinol Invest. 2011;34(11):855–860. doi:10.3275/776821646855
- Dessoki N, Nasr I, Badawy A, Ali I. Value of the postablative thyroglobulin measurements for assessment of disease-free status in patients with differentiated thyroid cancer. Indian J Nucl Med. 2019;34(2):118–124. doi:10.4103/ijnm.IJNM_142_1831040522
- Zhao T, Liang J, Guo Z, Li T, Lin Y. In patients with low- to intermediate-risk thyroid cancer, a preablative thyrotropin level of 30 muIU/mL is not adequate to achieve better response to 131I therapy. Clin Nucl Med. 2016;41(6):454–458. doi:10.1097/RLU.000000000000116726914559
- Ahn J, Song E, Kim WG, et al. Long-term clinical outcomes of papillary thyroid carcinoma patients with biochemical incomplete response. Endocrine. 2020;67(3):623–629. doi:10.1007/s12020-019-02142-131776976
- Jeon MJ, Kim WG, Park WR, et al. Modified dynamic risk stratification for predicting recurrence using the response to initial therapy in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2014;170(1):23–30. doi:10.1530/EJE-13-052424088549
- Vaisman A, Orlov S, Yip J, et al. Application of post-surgical stimulated thyroglobulin for radioiodine remnant ablation selection in low-risk papillary thyroid carcinoma. Head Neck. 2010;32(6):689–698. doi:10.1002/hed.2137120187016
- Rosario PW, Mineiro Filho AF, Prates BS, Silva LC, Calsolari MR. Postoperative stimulated thyroglobulin of less than 1 ng/mL as a criterion to spare low-risk patients with papillary thyroid cancer from radioactive iodine ablation. Thyroid. 2012;22(11):1140–1143. doi:10.1089/thy.2012.019023050786
- Avram AM, Rosculet N, Esfandiari NH, et al. Differentiated thyroid cancer outcomes after surgery and activity-adjusted 131I theragnostics. Clin Nucl Med. 2019;44(1):11–20. doi:10.1097/RLU.000000000000232130371575
- Vaisman F, Momesso D, Bulzico DA, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012;77(1):132–138. doi:10.1111/j.1365-2265.2012.04342.x22248037
- Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 2011;21(12):1317–1322. doi:10.1089/thy.2011.023222136267
- Heemstra KA, Liu YY, Stokkel M, et al. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2007;66(1):58–64. doi:10.1111/j.1365-2265.2006.02685.x17201802
- Hall FT, Beasley NJ, Eski SJ, Witterick IJ, Walfish PG, Freeman JL. Predictive value of serum thyroglobulin after surgery for thyroid carcinoma. Laryngoscope. 2003;113(1):77–81. doi:10.1097/00005537-200301000-0001412514386
- Zhang Y, Hua W, Zhang X, Peng J, Liang J, Gao Z. The predictive value for excellent response to initial therapy in differentiated thyroid cancer: preablation-stimulated thyroglobulin better than the TNM stage. Nucl Med Commun. 2018;39(5):405–410. doi:10.1097/MNM.000000000000082729557849
- Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J Clin Endocrinol Metab. 2019;104(9):4087–4100. doi:10.1210/jc.2019-00177
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22(11):1144–1152. doi:10.1089/thy.2012.004323083442
- Cooper DS, Doherty GM, Haugen BR; American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi:10.1089/thy.2009.011019860577
- Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid. 2014;24(2):241–244. doi:10.1089/thy.2012.056723713855
- Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Preablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin Nucl Med. 2012;37(6):545–549. doi:10.1097/RLU.0b013e31824852f822614184