Abstract
Colorectal cancer (CRC) is a major health burden worldwide, and thus, optimised diagnosis and treatments are imperative. E2F transcription factors (E2Fs) are a family of transcription factors consisting of eight genes, contributing to the oncogenesis and development of CRC. Importantly, E2Fs control not only the cell cycle but also apoptosis, senescence, DNA damage response, and drug resistance by interacting with multiple signaling pathways. However, the specific functions and intricate machinery of these eight E2Fs in human CRC remain unclear in many respects. Evidence on E2Fs and CRC has been scattered on the related regulatory genes, microRNAs (miRNAs), and competing endogenous RNAs (ceRNAs). Accordingly, some drugs targeting E2Fs have been transferred from preclinical to clinical application. Herein, we have systemically reviewed the current literature on the roles of various E2Fs in CRC with the purpose of providing possible clinical implications for patient diagnosis and prognosis and future treatment strategy design, thereby furthering the understanding of the E2Fs.
Introduction
Cancer is a major leading cause of death in the 21st century globally. Despite remarkable advancements that have been made in the diagnosis and treatment of CRC in recent years, the number of new cases of colorectal cancer (CRC) reached 1.8 million across the world in 2018, with 881,000 reported deaths.Citation1 Furthermore, about 30–50% of patients exhibit local recurrence or metastasis after radical resection.Citation2 Since, the principal obstacles to CRC treatment are tumor recurrence, metastasis, and resistance, the 5-year survival rate remains less than 65%.Citation3,Citation4 Unfortunately, the classic biomarkers have limited predictive and clinical value. Thus, there is an urgent need to discover novel diagnostic and prognostic biomarkers for this lethal disease.
In 1986, it was found that the E2F transcription factors (E2Fs) could bind to the promoter of the adenoviral gene E2. Based on their molecular structure and transcriptional properties, the E2F family can be categorized into three groups: transcriptional activators (E2F1, E2F2, and E2F3a), canonical repressors (E2F3b and E2F4-E2F6), and atypical repressors (E2F7 and E2F8).Citation5 The E2Fs are becoming increasingly complex owing to several E2F isoforms, including two splice variants of E2F3a (E2F3c and E2F3d).Citation6 The E2Fs had come to the frontiers of cancer research when they were found to be regulated by the retinoblastoma gene product, composed of pRB (RB1), p107 (RBL1), and p130 (RBL2).Citation7,Citation8 Aberrant E2F transcriptions have been identified in many human malignancies. Mechanistically, dysregulated E2Fs can activate or silence some oncogenes or tumor suppressors at multiple levels, including transcriptional level, post-transcriptional level, translational level, protein-protein interaction level, and transcriptional activity level, and further causing the carcinogenesis in human malignancies, including CRC.Citation9 Importantly, in addition to the classic cell-cycle-intrinsic regulation, E2Fs control apoptosis,Citation10 senescence,Citation11 DNA-damage response,Citation6,Citation12 autophagy,Citation13 metabolism,Citation9 angiogenesis,Citation9 and drug resistance.Citation14 (see ) However, the specific functions and intricate machinery of these eight E2Fs in human CRC remain unclear in many respects. Thus, further studies need to be comprehensively reviewed for a greater understanding of their detailed regulatory mechanisms in CRC. In this review, we have systematically searched Web of Science, EMBASE, PubMed, Wanfang, China National Knowledge Infrastructure (CNKI), VIP databases, and SinoMed databases to investigate the current state of knowledge of the roles of various E2Fs in CRC with the purpose of providing possible clinical implications for patient diagnosis, prognosis, and future treatment strategy design.
Figure 1 Graphical illustration showing that the regulatory mechanisms for E2Fs in CRC. For instance, E2Fs function in CRC is modulated via multiple levels including the transcriptional level (NFYB, KRT23, and ChoKa inhibitors-mediated transcription of the E2F gene), post-transcriptional regulation (E2F mRNA targeted by different miRNAs and ceRNAs), post-translational modifications (deacetylation and acetylation of E2F protein), protein–protein interaction level (phosphorylation and dephosphorylation of RB protein), and transcriptional activity level (XIAPΔRING, TRIP-Br2, cIAP1, NLK, and NPTX1 regulate the transcriptional activity of E2Fs protein). Solid arrows represent promoted effects, while dashed arrows represent inhibitory effects. Different colored lines showed different signaling pathways or targets.
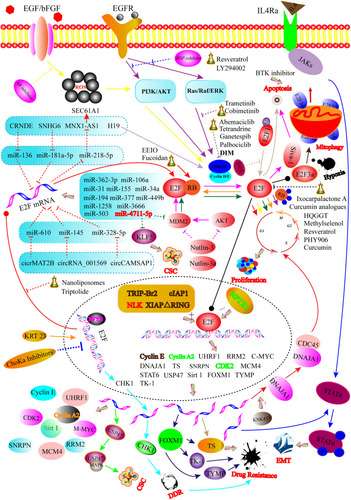
E2F-Related RNAs in CRC
Long non-coding RNAs (lncRNAs) are RNA transcripts with a length of more than 200 nucleotides (nts).Citation15 LncRNAs regulate microRNAs (miRNA) as competing endogenous RNAs (ceRNAs). For instance, colorectal neoplasia differentially expressed (CRNDE) promotes metastasis and oxaliplatin resistance by hijacking miR-136 and regulating E2F1 expression in CRC.Citation16 Similarly, SNHG6, located at chromosome 8q13.1, acts as a ceRNA by sponging miR-181a-5p, promoting E2F5-mediated proliferation of CRC cells.Citation17 Recently, the E2F1-mediated MNX1-AS1-miR-218-5p-SEC61A1 feedback network was discovered to be also pivotal for CRC tumorigenesis.Citation18 Oncogenic H19, another interesting lncRNA, is an independent predictor of CRC survival. It interacts with macroH2A and promotes CRC growth and migration by targeting RB1/E2F1 signaling and cyclin-dependent kinases (CDK)-β-catenin activity.Citation19
Several circular RNAs (circRNAs) also exhibit oncogenic properties by functioning as ceRNAs. For example, MAT2B, a novel circRNA, was found to increase E2F1 expression through sponging miR-610, resulting in tumorigenesis or further development.Citation20 Similarly, cir_001569 upregulates E2F5 by sponging miR-145 and is correlated with the aggressive character of CRC.Citation21 Furthermore, the circCAMSAP1/miR-328-5p/E2F1 axis is also essential for CRC progression.Citation22
MiRNAs are key components of the multi-level regulatory system. They are a class of short (20–22 nts) non-protein-coding endogenous RNAs that regulate CRC oncogenesis by binding to complementary sequences (3ʹ-untranslated regions, 3ʹ-UTRs) of target mRNAs to direct their post-transcriptional repression.Citation23–Citation27 For example, both miR-342-3p and miR-377 target the E2F1 3ʹ-UTRs to inhibit the proliferation of glioma cells.Citation28 Additionally, miR-526b-3p is related to a better prognosis in CRC patients and directly targets the 3ʹ-UTRs of E2F1 mRNA, leading to reduced E2F1 expression.Citation29 It is noteworthy to mention that unique miRNAs expression profiles can be observed in different stages of the CRC progression.Citation30 Similarly, miR-4711-5p dramatically induces G1 arrest by downregulating the downstream molecules of the E2F-TFDP1 complex in HCT-116 cells, including cell division cycle protein 6 (CDC6), CDT1, and MCM7.Citation24 MiR-106a and miR-362-3p are two other promising miRNAs that act as negative upstream regulators of E2F1 and improve patient survival.Citation31,Citation32 It has been well documented that miR-31 and miR-155 drive CRC development by decreasing E2F2.Citation33,Citation34 This suggests that they might become targets for anti-tumour drug design. Furthermore, some known miRNAs, such as miR-194,Citation26 miR-377,Citation35 miR-449b,Citation36 and miR-503,Citation37 play a role in growth suppression through modulating E2F3 in CRC. It has been reported that miR-34a might serve as the key upstream negative regulators of E2F1, E2F3, and E2F5 and enhanced 5-Fluorouracil (5-FU) cytotoxicity in CRC.Citation38–Citation42 The 1,2-diaminocyclohexane carrier ligand-mediated p53-miR-34a-E2F signaling pathway also appears to have an important mechanism.Citation42 Moreover, circulating miR-34a combined with miR-150 has been reported to be capable of distinguishing patients with polyps, adenomas, and advanced cancer.Citation43 The miR-3666 has been identified as a tumor suppressor in breast cancerCitation44 and thyroid carcinoma.Citation45 Specifically, the miR-3666/E2F7 is suggested to play a crucial role in modulating HCT116 cell viability, apoptosis, and migration by inhibiting the signaling activation of mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinases (ERK).Citation46 MiR-1258 negatively controls E2F8 by influencing several cell-cycle factors, including cyclin D1 and cyclin-dependent kinase inhibitor 1A in CRC.Citation47 However, the miRNAs targeting E2F4 and E2F6 in CRC have not yet been discovered.
In cancer cells, the E2F-miRNA regulatory loops have been described.Citation27,Citation48 For instance, miR-26a and E2F7 constitute a reciprocal regulatory network in which miR-26a inhibits E2F7 expression, while E2F7 targets MYC and decreases miR-26a.Citation48 Similarly, E2F7 suppresses miR-199b expression in SW403 cells, and miR-199b targets ubiquitin-specific protease 47 (USP47) that stabilizes MAPK, promoting colon cancer stem cell activity and subsequently accelerating colon cancer progression.Citation49 Furthermore, Gao et al summarized the bidirectional cross-link between E2F3 and 29 miRNAs in human cancers and elucidated how this regulation occurs.Citation27 This review unfolds a series of RNA interaction profiles (see ). Collectively, we think the miRNA-based cancer therapeutic method is a promising next-generation treatment strategy since miRNAs can be readily detected in various biofluids and tissues, such as blood, serum, plasma, saliva, and stools.Citation50,Citation51
Upstream or Downstream Proteins Involved in E2Fs Regulation in CRC
It has been well known that E2Fs can either activate or inhibit gene transcription, depending on the cell type, the target genes, the expression levels of co-regulator partners, and the external environment.Citation52–Citation54 In particular, the CDK-RB-E2F pathway is important for cell fate determination. More specifically, the CDK and cyclin complexes phosphorylate RB and release E2Fs. Re-establishing cell cycle regulation through direct or indirect inhibition of CDK is suggested as an attractive option of the molecularly targeted therapy.Citation55,Citation56 (see ) As reported, the CDK4/6 inhibitors in human CRC are currently being tested, including tetrandrine,Citation56 abemaciclib,Citation57 ganetespib,Citation58 and palbociclib.Citation59 These inhibitors inhibit tumorigenesis, at least partially by reducing the expression of E2F target genes. (see ) Former studies focused on regulatory components modulating the CDK-RB-E2F axis and gained lots of valuable insights.Citation9 Particularly, over-expressed TRIP-Br2 was found to promote anchorage-independent growth of HCT-116 cells by activating the RB/E2F/DP1-mediated transcription through upregulation of cyclin E, cyclin A2, CDC6, and DHFR of the key E2F-responsive partners.Citation60 Likewise, the cellular inhibitor of apoptosis 1 also seems to be crucial for optimal E2F1 mediated-cyclin A and cyclin E expression.Citation54 Another powerful gene, X-linked inhibitor of apoptosis protein (XIAP) with RING (Really Interesting New Gene) domain deletion (XIAPΔRING) translocates into nuclear and promotes cancer cell-autonomous growth by targeting the E2F1/cycle E axis.Citation61 Moreover, Neuronal pentraxin 1 (NPTX1), a member of the long pentraxin family (NPTX1, NPTX2, and NPTXR), suppresses the growth of colon cancer cells through decreasing cyclin A2 and CDK2 expression.Citation62 As reported, histone deacetylases (HDACs) function as the negative regulator of E2F1 through deacetylation. Nemo-like kinase boosts CRC progression by releasing E2F1 from the E2F1/HDAC1 complex.Citation63
Importantly, the cross-talk between the RB/E2F and Wnt/β-catenin signaling pathways in human malignancies has already been characterized.Citation64 Identification of the critical effectors of the cross signaling pathway is beneficial for CRC management. In particular, E2F1 suppresses Wnt/β-catenin activity through inhibitor of β-catenin and TCF4 (ICAT). Phospholipase D1 (PLD1) controls the cross-link among E2F-miR-4496 and Wnt/β-catenin pathways and the tumor-initiating program of CRC cells.Citation65 Furthermore, PLD1 also regulates the Wnt/β-catenin signaling by selectively downregulating ICAT via the Phosphoinositide 3-Kinase (PI3K)/Akt-TopBP1-E2F1 signaling axis.Citation66,Citation67
E2Fs are targeted by different proteins (see ). For instance, Keratin 23 is strongly expressed in colon adenocarcinomas compared to normal colon mucosa, and its depletion leads to a reduced expression of many key molecules including E2F1.Citation68 Spinophilin is a previously recognized novel tumor suppressor gene. Ress et al proposed that spinophilin expression modulates cellular growth, cancer stemness, and 5-FU resistance in CRC cells by inhibiting E2F1 activation.Citation69 ChoKα specific inhibitors, MN58b and TCD-717 modulate the expression levels of TS and TK1 through the inhibition of E2F production.Citation70 Aldose reductase (AR), an NADPH-dependent Aldo ketoreductase, is involved in colon carcinogenesis. Ramana et al reported that inhibition of AR inhibits the related growth factor-induced G1-S phase transition via the AKT/PI3K/E2F1 signaling pathway in human colon cancer cells.Citation71,Citation72
E2Fs activates numerous downstream regulatory genes (see ). E2F1 overexpression has been identified to promote the transformation of aggressive phenotypes in CRC cells by activating the ribonucleotide reductase small subunit M2.Citation73 Ubiquitin-like with PHD and ring-finger domain 1 (UHRF1) expression has been discovered to be upregulated by E2F1 and involved in the cellular proliferation of CRC. Particularly, enhanced UHRF1 expression appears to be involved in carcinogenesis of the right compared to the left hemicolon.Citation74 The MDM2 antagonists nutlin-3 and nutlin-3a can induce cancer cell apoptosis in a p53-dependent manner.Citation42,Citation75,Citation76 Interestingly, they also initiate apoptosis by activating E2F1- and p73-mediated expression of Siva-1 and p53 upregulated modulator of apoptosis (PUMA) regardless of p53 status in CRC.Citation75 Moreover, CDCA3 is referred to as a trigger of mitotic entry, mediates p21-dependent proliferation of CRC by regulating E2F1 expression.Citation77 It has been reported that KNK437 is a heat shock protein inhibitor that inhibits the DNAJA1-induced CRC proliferation and metastasis.Citation78 Mechanistically, DNAJA1 is activated by E2F1 and then promotes the cell cycle by stabilizing CDC45. More importantly, the combined application of KNK437 with 5-FU/L-OHP shows a synergistic inhibitive effect on DNAJA1-mediated liver metastasis. Li et al found that E2F2 acts as a tumor suppressor in CRC by repressing CCNA2, C-MYC, CDK2, and MCM4.Citation33 A recent study also demonstrated that the small nuclear ribonucleoprotein polypeptide N accelerates the malignant progression of CRC regulated by E2F8.Citation79
Immune Microenvironment and E2Fs in CRC
The tumor microenvironment (TME) constitutes immune cells, stromal cells, and extracellular matrix, which functions as an immunologic battleground for tumor cells and the immune system during tumor formation.Citation80 The TME and the related inflammatory response play an imperative role in cancer development and progression. It should be noted that chronic intestinal inflammation such as inflammatory bowel disease promotes pRB hyperphosphorylation and E2F1 activation, directly increasing the CRC risk.Citation81–Citation84 Multiple pro-inflammatory cytokines, including interleukin (IL)-6, IL-8, IL-13, and IL-17 released by diverse infiltrating cells, such as neutrophils, macrophages, and lymphocytes, have been found to induce CRC metastasis.Citation82,Citation85–Citation88 Remarkably, Chen et al proposed a model that the E2F1/SP3/STAT6 axis induced by IL-4 promotes the epithelial-mesenchymal transition (EMT) of CRC cells.Citation89 (see ) The microbiota has been identified as an important part of TME. It has been reported that changes in the microbiota in TME mediate chronic inflammation and CRC initiation.Citation90 Thompson et al found H. influenza to be significantly related to genes in the G2M checkpoint, E2F transcription, and mitotic pathways in breast cancer.Citation91 Similarly, commensal gut microbiota also shapes the colonic immune environment in CRC.Citation92 However, little is known about the relationships between E2Fs and microbiota in CRC.
The human genome is constantly exposed to both endogenous and exogenous stresses, such as hypoxia, ionizing radiation, and acidosis, which can lead to genomic instability and the subsequent increased mutation rate, thereby accelerating the tumorigenesis.Citation93–Citation99 Changes in the TME, such as hypoxia and nutrient deprivation has been discovered to cause mitochondrial damage.Citation100 Araki et al found that a distinctive product, E2F3d triggers the hypoxia-induced fragmentation and mitophagy in cancer cells.Citation6 Furthermore, hypoxia causes elevated mutagenesis,Citation94 diminished capacity of DNA repair,Citation94 reduction in the expression of the key mismatch repair genes, MLH1Citation95 and MSH2,Citation97 and of the homologous recombination (HR) gene.Citation99 Mechanistically, E2Fs could mediate the down-regulation of BRCA1 or RAD51 expression in response to hypoxic stress and consequently suppress HR activity.Citation98,Citation99 Moreover, overexpression of RAD51 is considered to be a poor prognostic predictor in CRC patients.Citation101
Cancer stem cells (CSCs) are a small subset of cells in tumors with the potential of self-renewable, differentiation, and tumor-initiation.Citation102,Citation103 Emerging evidence suggests that adult stem cell populations with high proliferation rates have a higher cancer rate than less proliferative stem cell populations independently of oncogene expression.Citation104,Citation105 Importantly, around two-thirds of mutations in cancer are caused by replicative errors. E2F family members sometimes work in opposition, including copy number gains of E2F1 and E2F3 or copy number deletion of E2F7 and E2F8, inducing cancer in mice.Citation106,Citation107 One promising miRNA, miR-4711-5p, can inhibit CSC properties by downregulating Kruppel-like factor 5 expression and MDM2.Citation24 Similarly, the E2F7-regulated miR-199b/USP47/MAPK axis promotes the stemness of colon CSCsCitation49 (see ).
E2F-Induced Metabolic Dysregulation in CRC
Metabolic reprogramming is considered an emerging hallmark of cancer. Accordingly, several metabolism-targeted therapies have been proven to the promising anti-tumor strategies. It is known that the cellular metabolic changes may precede somatic mutations in CRC. For example, oncogene activation and tumor suppressor loss further reprogram CRC cells and upregulate glycolysis, glutaminolysis, one-carbon metabolism, and fatty acid synthesis.Citation108 Mutated metabolic features occur in CRC at multiple levels, including tumor cells, CSCs, TME, and host–microbiome interactions.Citation108 E2Fs have been reported to contribute to global metabolic homeostasis in a cell-cycle independent manner. E2F1 promotes glycolysis, lipogenesis, bile acid synthesis, and insulin secretion in related normal cells.Citation109 Especially, the mentioned TRIP-Br2 and CDK4-pRB-E2F1 are vital for adipogenesis and maintaining adipocyte function.Citation109,Citation110 Conversely, E2F1 has been reported to repress lipolysis, thermogenesis, and oxidative metabolism of cancerous cells and contribute to the Warburg effect.Citation109,Citation111,Citation112 It is important to note that insulin receptor substrate-4 is overexpressed in CRC cells and increases the RB-cyclin-dependent kinase pathway.Citation113 Although E2Fs is rarely reported in the metabolic signaling pathway of CRC, the above-mentioned studies suggest a possible three-way interaction between E2Fs, metabolism, and CRC.
E2Fs-Target Drugs in CRC
Some pharmacological agents at least partially modulate the CRC progression by targeting E2Fs (see and ). The siE2F1 loaded cationic nanoliposomes (small unilamellar vesicles, SUVs) have been found to exhibit very low cytotoxicity in human CRC cell lines and be effective in silencing E2F1 and in the consequent reduction of cell growth.Citation114 Developing plant-derived products as potential anticancer agents has attracted considerable interest in recent years throughout the world. For instance, resveratrol,Citation115,Citation116 brassinin,Citation117 eguelin,Citation118 tetrandrine,Citation56 ethanol extract of Inonotus obliquus,Citation119 and the non-digestive fraction of beansCitation120 have been identified as antitumor agents. Especially, 3,3ʹ-Diindolylmethane (DIM), as one of the natural indole derivatives originating from broccoli and other cruciferous vegetables, has been shown to exert antitumor effects in both in vivo and in vitro models. Choi et al indicated that DIM restricted CDK2 activity and RB phosphorylation, reducing the levels of the E2F1 protein in HT-29 human colon cells.Citation121 Furthermore, ixocarpalactone A isolated from the Mexican tomatillo was found to manifest potent antiproliferative and apoptotic activity in SW480 cells by modulating E2F1 and Bcl-2 family.Citation122 Recently, a herbal formulation Huang Qin Ge Gen Tang (HQGGT), was discovered to enhance 5-FU cytotoxicity and antitumor activity through the suppression of the E2F1/TS signaling pathway in CRC.Citation123 Generally, curcumin induced reactive oxygen species down-regulation of E2F4 expression and consequently lead to apoptotic cell death in HCT116 colon cancer cells.Citation124 In addition, the curcumin and its analogues EF31 and UBS109 induce apoptosis and inhibit growth by downregulating E2F1 and its target gene thymidylate synthase (TS).Citation125 Similarly, cobimetinib, a MEK inhibitor seems to improve the efficacy of 5-FU by decreasing TS.Citation126 Fucoidan, a natural sulfated polysaccharide that exists in brown seaweed, exerts anticancer effects by inhibiting pRB phosphorylation and enhancing binding pRB with E2Fs in HCT116 cells.Citation127 In general that triptolide can initiate programmed cell death by activating apoptosis or autophagy.Citation128 More interestingly, its water-soluble analogue named minnelide induces cell death by apoptosis at low concentrations and E2F-dependent G1 phase arrest at higher concentrations.Citation129 Likewise, traditional Chinese medicine PHY906,Citation130 methylselenol,Citation131 and irinotecanCitation132 serve oncogenic roles by decreasing the expression of E2Fs.
Table 1 E2Fs-Target Agents Summary in CRC
Resistance and E2Fs in CRC
Currently, surgery and chemoradiotherapy (CRT) are considered to be standard treatment options for CRC. However, the fact that most patients develop resistance to standard therapies poses a significant challenge in the treatment of CRC.Citation133 Therefore, it is crucial to elucidate the underlying mechanisms for CRT in clinical practice.
Mounting evidence suggests that enhanced E2F activity is a key mechanism of the CRT resistance (see ). For instance, E2F1 regulates multiple downstream target genes that are related to DNA synthesis and repairs genes that are involved in resistance, including the BRCA1, RAD51, TS, excision repair genes (ERCC-1), and forkhead box M1 (FOXM1).Citation134–Citation136 It is no exaggeration that 5-FU is the backbone of CRC first-line therapy and exerts crucial anti-tumor activities, at least partially, via E2F1/TS downregulation.Citation134,Citation136–Citation139 This signaling pathway can be interrupted by diverse stimuli, including glycogen synthase kinase 3β (GSK-3β) inhibitor (2ʹZ,3ʹE)-6-bromo- indirubin-3ʹ -oxime (BIO),Citation140 curcumin analogues (EF31 and UBS109),Citation125 as well as RB-reactivating agents (trametinib (MEK inhibitor), fenofibrate (PPARα agonist), and LY294002 (PI3K inhibitor)).Citation141 Of note, the combined E2F1+TS+immunophenotype in CRC manifests a poor prognosis.Citation139 These findings suggest that the downregulation of TS expression might be a promising method of improving the efficacy of 5-FU. Recently, Lavitrano et al identified a novel oncogenic isoform of Bruton’s tyrosine kinase, namely p65BTK.Citation142 Silencing p65BTK was found to overcome the 5-FU resistance of CRC cell lines and restore the E2F-dependent apoptosis. The aberrant activation of nuclear transcription factor Y subunit beta (NFYB)-E2F1-checkpoint kinase 1 (CHK1) was identified to maintain the tumorigenicity in oxaliplatin-resistant CRC and significantly related to a poor prognosis.Citation143 Chen et al revealed that antagonism of CDK8 inhibits the fractional survival of CRC cells and increases radiotherapy-induced apoptosis in vivo and in vitro through potentiating the transcription of E2F1 target gene apaf1.Citation144 Apart from their roles in CRC carcinogenesis, MiRNAs may also be involved in affecting the chemosensitivity by targeting E2Fs in CRC. For example, miR-329 attenuates the chemoresistance of CRC to 5-FU by degrading E2F1.Citation145 Similarly, miR-34a enhances the sensitivity of human CRC cells to 5-FU by inhibiting Sirt1 and E2F3, which is correlated with inactive PI3K/AKT signaling pathway.Citation39,Citation40 The liposomal miR-34a mimic, MRX34, is the first synthetic miRNA that has been already entered into clinical trials, providing a proof-of-concept for mi-RNA-based cancer therapy.Citation51 Some miRNAs, such as miR-200b, miR-21, and miR-192, were successively found to induce apoptosis and restore chemosensitivity in an E2F-dependent manner. Recently, Lin et al have constructed an adenoviral vector (AdCMVE2F-1) to transfected an ectopic E2F1 into human CRC cells. The findings showed that the upregulated E2F1 exerts a synergistic anticancer effect with gemcitabine.Citation146 It was suspected that the apoptotic effect of E2F1 is due to its unscheduled entry into the S phase. Importantly, the exogenous E2F1 exhibits clinical chemosensitizing effects in CRC cells by inducing pro-apoptotic behavior.Citation147,Citation148 In conclusion, targeting the inhibition of E2F or killing oncogenes that drive E2F activity could be a good complement to current treatment strategies.Citation9
Conclusions and Perspectives
Taken together, the E2Fs are a quite complex family of the transcription factor. They have been found to appear in many emerging fields of CRC in addition to classic cell-cycle regulation, such as CSCs, TME, and metabolism. Importantly, they can exert different biological functions depending on context. Some pharmacological agents indirectly or directly regulate the CRC progression by targeting E2Fs. Accumulating evidence has shown that enhanced E2F activity is a critical mechanism of CRT resistance in CRC. Therefore, targeting the inhibition of E2F or killing oncogenes that drive E2F activity could be a good complement to current treatment strategies.
However, further studies are warranted to more thoroughly examine the effect and mechanisms further of E2Fs in CRC.
The endogenous carcinogenic and exogenous pro-apoptotic effects of E2F1 are an ongoing paradox for the scientific community. How to explain the pro-apoptotic molecular mechanism of E2Fs in CRC?
How E2Fs specifically mediate metabolism and TME in CRC?
More clinical agents can be designed for targeting E2Fs, and the related CRT mechanisms can be further studied.
Collectively, E2Fs are thought to be a promising target in CRC. More prospective research is needed to verify this conclusion.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest for this work.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. doi:10.1053/j.gastro.2012.06.00122763141
- Hohla F, Winder T, Greil R, et al. Targeted therapy in advanced metastatic colorectal cancer: current concepts and perspectives. World J Gastroenterol. 2014;20(20):6102–6112. doi:10.3748/wjg.v20.i20.610224876732
- Colvin H, Mizushima T, Eguchi H, et al. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1(1):5–10. doi:10.1002/ags3.1200829863129
- Xanthoulis A, Tiniakos DG. E2F transcription factors and digestive system malignancies: how much do we know? World J Gastroenterol. 2013;19(21):3189–3198. doi:10.3748/wjg.v19.i21.318923745020
- Araki K, Kawauchi K, Sugimoto W, et al. Mitochondrial protein E2F3d, a distinctive E2F3 product, mediates hypoxia-induced mitophagy in cancer cells. Commun Biol. 2019;2(1):3. doi:10.1038/s42003-018-0246-930740539
- Liu ZL, Bi XW, Liu PP, et al. Expressions and prognostic values of the E2F transcription factors in human breast carcinoma. Cancer Manag Res. 2018;10:3521–3532. doi:10.2147/CMAR.S17233230271201
- Wu L, Timmers C, Maiti B, et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414(6862):457–462. doi:10.1038/3510659311719808
- Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19(6):326–338. doi:10.1038/s41568-019-0143-731053804
- Ginsberg D. E2F1 pathways to apoptosis. FEBS Lett. 2002;529(1):122–125. doi:10.1016/S0014-5793(02)03270-212354623
- Dimri GP, Itahana K, Acosta M, et al. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14 ARF tumor suppressor. Mol Cell Biol. 2000;20(1):273–285. doi:10.1128/MCB.20.1.273-285.200010594030
- Stevens C, La Thangue NB. The emerging role of E2F-1 in the DNA damage response and checkpoint control. DNA Repair. 2004;3(8–9):1071–1079. doi:10.1016/j.dnarep.2004.03.03415279795
- Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24(17):2810–2826. doi:10.1038/sj.onc.120861215838517
- Kovesdi I, Reichel R, Nevins JR. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986;45(2):219–228. doi:10.1016/0092-8674(86)90386-72938741
- Ma H, Hao Y, Dong X, et al. Molecular mechanisms and function prediction of long noncoding RNA. Sci World J. 2012;2012:541786. doi:10.1100/2012/541786
- Gao H, Song X, Kang T, et al. Long noncoding RNA CRNDE functions as a competing endogenous RNA to promote metastasis and oxaliplatin resistance by sponging miR-136 in colorectal cancer. Onco Targets Ther. 2017;10:205–216. doi:10.2147/OTT.S11617828115855
- Yu C, Sun J, Leng X, et al. Long noncoding RNA SNHG6 functions as a competing endogenous RNA by sponging miR-181a-5p to regulate E2F5 expression in colorectal cancer. Cancer Manag Res. 2019;11:611–624. doi:10.2147/CMAR.S18271930666158
- Ye Y, Gu B, Wang Y, et al. E2F1-mediated MNX1-AS1-miR-218-5p-SEC61A1 feedback loop contributes to the progression of colon adenocarcinoma. J Cell Biochem. 2019;120(4):6145–6153. doi:10.1002/jcb.2790230362161
- Ohtsuka M, Ling H, Ivan C, et al. H19 noncoding RNA, an independent prognostic factor, regulates essential Rb-E2F and CDK8-β-catenin signaling in colorectal cancer. EBioMedicine. 2016;13:113–124. doi:10.1016/j.ebiom.2016.10.02627789274
- Zhao JP, Chen LL. Circular RNA MAT2B induces colorectal cancer proliferation via sponging miR-610, resulting in an increased E2F1 expression. Cancer Manag Res. 2020;12:7107–7116. doi:10.2147/CMAR.S25118032848465
- Xie H, Ren X, Xin S, et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7(18):26680–26691. doi:10.18632/oncotarget.858927058418
- Zhou C, Liu HS, Wang FW, et al. circCAMSAP1 promotes tumor growth in colorectal cancer via the miR-328-5p/E2F1 axis. Mol Ther. 2020;28(3):914–928. doi:10.1016/j.ymthe.2019.12.00831951832
- Ungerbäck J, Belenki D, Jawad ul-Hassan A, et al. Genetic variation and alterations of genes involved in NFκB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis. 2012;33(11):2126–2134. doi:10.1093/carcin/bgs25622843550
- Morimoto Y, Mizushima T, Wu X, et al. miR-4711-5p regulates cancer stemness and cell cycle progression via KLF5, MDM2 and TFDP1 in colon cancer cells. Br J Cancer. 2020;122(7):1037–1049. doi:10.1038/s41416-020-0758-132066912
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.00219167326
- Nakaoka T, Saito Y, Shimamoto Y, et al. Cluster microRNAs miR-194 and miR-215 suppress the tumorigenicity of intestinal tumor organoids. Cancer Sci. 2017;108(4):678–684. doi:10.1111/cas.1316528092415
- Gao Y, Feng B, Lu L, et al. MiRNAs and E2F3: a complex network of reciprocal regulations in human cancers. Oncotarget. 2017;8(36):60624–60639. doi:10.18632/oncotarget.1736428947999
- Huang Y, Chi C. Glioma cell proliferation is inhibited by miR-342-3p, miR-377/E2F1 signaling pathway. Neoplasma. 2019;66(4):524–531. doi:10.4149/neo_2018_180805N57430868897
- Fang Z, Yang H, Chen D, et al. YY1 promotes colorectal cancer proliferation through the miR-526b-3p/E2F1 axis. Am J Cancer Res. 2019;9(12):2679–2692.31911854
- Kanaan Z, Rai SN, Eichenberger MR, et al. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum Mutat. 2012;33(3):551–560. doi:10.1002/humu.2202122241525
- Christensen LL, Tobiasen H, Holm A, et al. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int J Cancer. 2013;133(1):67–78. doi:10.1002/ijc.2801023280316
- Huang Q, Ma Q. MicroRNA-106a inhibits cell proliferation and induces apoptosis in colorectal cancer cells. Oncol Lett. 2018;15(6):8941–8944. doi:10.3892/ol.2018.851629805629
- Li T, Luo W, Liu K, et al. miR-31 promotes proliferation of colon cancer cells by targeting E2F2. Biotechnol Lett. 2015;37(3):523–532. doi:10.1007/s10529-014-1715-y25362258
- Li T, Yang J, Lv X, et al. miR-155 regulates the proliferation and cell cycle of colorectal carcinoma cells by targeting E2F2. Biotechnol Lett. 2014;36(9):1743–1752. doi:10.1007/s10529-014-1540-324793496
- Yang B, Du K, Yang C, et al. CircPRMT5 circular RNA promotes proliferation of colorectal cancer through sponging miR-377 to induce E2F3 expression. J Cell Mol Med. 2020;24(6):3431–3437. doi:10.1111/jcmm.1501932020730
- Fang Y, Gu X, Li Z, et al. miR-449b inhibits the proliferation of SW1116 colon cancer stem cells through downregulation of CCND1 and E2F3 expression. Oncol Rep. 2013;30(1):399–406. doi:10.3892/or.2013.246523674142
- Chang SW, Yue J, Wang BC, et al. miR-503 inhibits cell proliferation and induces apoptosis in colorectal cancer cells by targeting E2F3. Int J Clin Exp Pathol. 2015;8(10):12853–12860.26722476
- Jiang H, Ge F, Hu B, et al. rs35301225 polymorphism in miR-34a promotes development of human colon cancer by deregulation of 3′UTR in E2F1 in Chinese population. Cancer Cell Int. 2017;17(1):39. doi:10.1186/s12935-017-0402-128293146
- Zhang Q, Wang J, Li N, et al. miR-34a increases the sensitivity of colorectal cancer cells to 5-fluorouracil in vitro and in vivo. Am J Cancer Res. 2018;8(2):280–290.29511598
- Akao Y, Noguchi S, Iio A, et al. Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer Lett. 2011;300(2):197–204. doi:10.1016/j.canlet.2010.10.00621067862
- Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17(2):193–199. doi:10.1038/cdd.2009.5619461653
- Kiyonari S, Iimori M, Matsuoka K, et al. The 1,2-diaminocyclohexane carrier ligand in oxaliplatin induces p53-dependent transcriptional repression of factors involved in thymidylate biosynthesis. Mol Cancer Ther. 2015;14(10):2332–2342. doi:10.1158/1535-7163.MCT-14-074826208523
- Aherne ST, Madden SF, Hughes DJ, et al. Circulating miRNAs miR-34a and miR-150 associated with colorectal cancer progression. BMC Cancer. 2015;15(1):329. doi:10.1186/s12885-015-1327-525924769
- Li D, Li L. MicroRNA-3666 inhibits breast cancer cell proliferation by targeting sirtuin 7. Mol Med Rep. 2017;16(6):8493–8500. doi:10.3892/mmr.2017.760328944911
- Wang G, Cai C, Chen L. MicroRNA-3666 regulates thyroid carcinoma cell proliferation via MET. Cell Physiol Biochem. 2016;38(3):1030–1039. doi:10.1159/00044305426937629
- Liu W, Song Y, Zhang C, et al. The protective role of all-transretinoic acid (ATRA) against colorectal cancer development is achieved via increasing miR-3666 expression and decreasing E2F7 expression. Biomed Pharmacother. 2018;104:94–101. doi:10.1016/j.biopha.2018.05.01529772445
- Zhang Z, Li J, Huang Y, et al. Upregulated miR-1258 regulates cell cycle and inhibits cell proliferation by directly targeting E2F8 in CRC. Cell Prolif. 2018;51(6):e12505. doi:10.1111/cpr.1250530144184
- Liu J, Li X, Wang M, et al. A miR-26a/E2F7 feedback loop contributes to tamoxifen resistance in ER-positive breast cancer. Int J Oncol. 2018;53(4):1601–1612. doi:10.3892/ijo.2018.449230066905
- Guo X, Liu L, Zhang Q, et al. E2F7 transcriptionally inhibits MicroRNA-199b expression to promote USP47, thereby enhancing colon cancer tumor stem cell activity and promoting the occurrence of colon cancer. Front Oncol. 2020;10:565449. doi:10.3389/fonc.2020.56544933489876
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi:10.1073/pnas.080454910518663219
- Hong DS, Kang YK, Borad M, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122(11):1630–1637. doi:10.1038/s41416-020-0802-132238921
- Wang C, Chen L, Hou X, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8(9):1025–1031. doi:10.1038/ncb146816892051
- Ianari A, Gallo R, Palma M, et al. Specific role for p300/CREB-binding protein-associated factor activity in E2F1 stabilization in response to DNA damage. J Biol Chem. 2004;279(29):30830–30835. doi:10.1074/jbc.M40240320015123636
- Cartier J, Berthelet J, Marivin A, et al. Cellular inhibitor of apoptosis protein-1 (cIAP1) can regulate E2F1 transcription factor-mediated control of cyclin transcription. J Biol Chem. 2011;286(30):26406–26417. doi:10.1074/jbc.M110.19123921653699
- Johnson J, Thijssen B, McDermott U, et al. Targeting the RB-E2F pathway in breast cancer. Oncogene. 2016;35(37):4829–4835. doi:10.1038/onc.2016.3226923330
- Meng LH, Zhang H, Hayward L, et al. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004;64(24):9086–9092. doi:10.1158/0008-5472.CAN-04-031315604277
- Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–753. doi:10.1158/2159-8290.CD-16-009527217383
- Nagaraju GP, Alese OB, Landry J, et al. HSP90 inhibition downregulates thymidylate synthase and sensitizes colorectal cancer cell lines to the effect of 5FU-based chemotherapy. Oncotarget. 2014;5(20):9980–9991. doi:10.18632/oncotarget.248425296971
- Zhang J, Zhou L, Zhao S, et al. The CDK4/6 inhibitor palbociclib synergizes with irinotecan to promote colorectal cancer cell death under hypoxia. Cell Cycle. 2017;16(12):1193–1200. doi:10.1080/15384101.2017.132000528486050
- Cheong JK, Gunaratnam L, Zang ZJ, et al. TRIP-Br2 promotes oncogenesis in nude mice and is frequently overexpressed in multiple human tumors. J Transl Med. 2009;7(1):8. doi:10.1186/1479-5876-7-819152710
- Cao Z, Li X, Li J, et al. X-linked inhibitor of apoptosis protein (XIAP) lacking RING domain localizes to the nuclear and promotes cancer cell anchorage-independent growth by targeting the E2F1/Cyclin E axis. Oncotarget. 2014;5(16):7126–7137. doi:10.18632/oncotarget.222725216527
- Peng X, Pan K, Zhao W, et al. NPTX1 inhibits colon cancer cell proliferation through down-regulating cyclin A2 and CDK2 expression. Cell Biol Int. 2018;42(5):589–597. doi:10.1002/cbin.1093529345391
- Li SZ, Zeng F, Li J, et al. Nemo-like kinase (NLK) primes colorectal cancer progression by releasing the E2F1 complex from HDAC1. Cancer Lett. 2018;431:43–53. doi:10.1016/j.canlet.2018.05.03229803790
- Wu Z, Zheng S, Li Z, et al. E2F1 suppresses Wnt/β-catenin activity through transactivation of β-catenin interacting protein ICAT. Oncogene. 2011;30(37):3979–3984. doi:10.1038/onc.2011.12921532622
- Kang DW, Choi CY, Cho YH, et al. Targeting phospholipase D1 attenuates intestinal tumorigenesis by controlling β-catenin signaling in cancer-initiating cells. J Exp Med. 2015;212(8):1219–1237. doi:10.1084/jem.2014125426122663
- Kang DW, Lee BH, Suh YA, et al. Phospholipase D1 inhibition linked to upregulation of ICAT blocks colorectal cancer growth hyperactivated by Wnt/β-catenin and PI3K/Akt signaling. Clin Cancer Res. 2017;23(23):7340–7350. doi:10.1158/1078-0432.CCR-17-074928939743
- Kang DW, Lee SW, Hwang WC, et al. Phospholipase D1 acts through Akt/TopBP1 and RB1 to regulate the E2F1-dependent apoptotic program in cancer cells. Cancer Res. 2017;77(1):142–152. doi:10.1158/0008-5472.CAN-15-303227793841
- Birkenkamp-Demtröder K, Hahn SA, Mansilla F, et al. Keratin23 (KRT23) knockdown decreases proliferation and affects the DNA damage response of colon cancer cells. PLoS One. 2013;8(9):e73593. doi:10.1371/journal.pone.007359324039993
- Ress AL, Stiegelbauer V, Schwarzenbacher D, et al. Spinophilin expression determines cellular growth, cancer stemness and 5-flourouracil resistance in colorectal cancer. Oncotarget. 2014;5(18):8492–8502. doi:10.18632/oncotarget.232925261368
- de la Cueva A, Ramírez de Molina A, Alvarez-Ayerza N, et al. Combined 5-FU and ChoKα inhibitors as a new alternative therapy of colorectal cancer: evidence in human tumor-derived cell lines and mouse xenografts. PLoS One. 2013;8(6):e64961. doi:10.1371/journal.pone.006496123762272
- Tammali R, Ramana KV, Singhal SS, et al. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66(19):9705–9713. doi:10.1158/0008-5472.CAN-06-210517018629
- Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9(4):813–824. doi:10.1158/1535-7163.MCT-09-079520354121
- Fang Z, Gong C, Liu H, et al. E2F1 promote the aggressiveness of human colorectal cancer by activating the ribonucleotide reductase small subunit M2. Biochem Biophys Res Commun. 2015;464(2):407–415. doi:10.1016/j.bbrc.2015.06.10326093293
- Kofunato Y, Kumamoto K, Saitou K, et al. UHRF1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol Rep. 2012;28(6):1997–2002. doi:10.3892/or.2012.206423023523
- Ray RM, Bhattacharya S, Johnson LR. Mdm2 inhibition induces apoptosis in p53 deficient human colon cancer cells by activating p73- and E2F1-mediated expression of PUMA and Siva-1. Apoptosis. 2011;16(1):35–44. doi:10.1007/s10495-010-0538-020812030
- Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi:10.1126/science.109247214704432
- Qian W, Zhang Z, Peng W, et al. CDCA3 mediates p21-dependent proliferation by regulating E2F1 expression in colorectal cancer. Int J Oncol. 2018;53(5):2021–2033. doi:10.3892/ijo.2018.453830226575
- Yang S, Ren X, Liang Y, et al. KNK437 restricts the growth and metastasis of colorectal cancer via targeting DNAJA1/CDC45 axis. Oncogene. 2020;39(2):249–261. doi:10.1038/s41388-019-0978-031477839
- Ji M, Ren L, Lv Y, et al. Small nuclear ribonucleoprotein polypeptide N accelerates malignant progression and poor prognosis in colorectal cancer transcriptionally regulated by E2F8. Front Oncol. 2020;10:561287. doi:10.3389/fonc.2020.56128733224876
- Hu ZQ, Xue H, Long JH, et al. Biophysical properties and motility of human mature dendritic cells deteriorated by vascular endothelial growth factor through cytoskeleton remodeling. Int J Mol Sci. 2016;17:11. doi:10.3390/ijms17111756
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114.e2105. doi:10.1053/j.gastro.2010.01.05820420949
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–1816. doi:10.1053/j.gastro.2011.01.05721530747
- Shawki S, Ashburn J, Signs SA, et al. Colon cancer: inflammation-associated cancer. Surg Oncol Clin N Am. 2018;27(2):269–287. doi:10.1016/j.soc.2017.11.00329496089
- Ying L, Marino J, Hussain SP, et al. Chronic inflammation promotes retinoblastoma protein hyperphosphorylation and E2F1 activation. Cancer Res. 2005;65(20):9132–9136. doi:10.1158/0008-5472.CAN-05-135816230367
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8(9):1248–1253. doi:10.7150/ijbs.461423136553
- Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106(11):1833–1841. doi:10.1038/bjc.2012.17722617157
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72(11):2780–2790. doi:10.1158/0008-5472.CAN-11-409022505647
- Hyun YS, Han DS, Lee AR, et al. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33(4):931–936. doi:10.1093/carcin/bgs10622354874
- Chen J, Gong C, Mao H, et al. E2F1/SP3/STAT6 axis is required for IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int J Oncol. 2018;53(2):567–578. doi:10.3892/ijo.2018.442929901191
- Keku TO, Dulal S, Deveaux A, et al. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G351–G363. doi:10.1152/ajpgi.00360.201225540232
- Thompson KJ, Ingle JN, Tang X, et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS One. 2017;12(11):e0188873. doi:10.1371/journal.pone.018887329190829
- Parekh PJ, Balart LA, Johnson DA. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin Transl Gastroenterol. 2015;6(6):e91. doi:10.1038/ctg.2015.1626087059
- Choi EH, Kim KP. E2F1 facilitates DNA break repair by localizing to break sites and enhancing the expression of homologous recombination factors. Exp Mol Med. 2019;51(9):1–12. doi:10.1038/s12276-019-0307-2
- Yuan J, Narayanan L, Rockwell S, et al. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60(16):4372–4376.10969780
- Mihaylova VT, Bindra RS, Yuan J, et al. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23(9):3265–3273. doi:10.1128/MCB.23.9.3265-3273.200312697826
- Bindra RS, Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res. 2005;569(1–2):75–85. doi:10.1016/j.mrfmmm.2004.03.01315603753
- Koshiji M, To KK, Hammer S, et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17(6):793–803. doi:10.1016/j.molcel.2005.02.01515780936
- Bindra RS, Gibson SL, Meng A, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65(24):11597–11604. doi:10.1158/0008-5472.CAN-05-211916357170
- Bindra RS, Schaffer PJ, Meng A, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24(19):8504–8518. doi:10.1128/MCB.24.19.8504-8518.200415367671
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi:10.1038/nrc298121258394
- Tennstedt P, Fresow R, Simon R, et al. RAD51 overexpression is a negative prognostic marker for colorectal adenocarcinoma. Int J Cancer. 2013;132(9):2118–2126. doi:10.1002/ijc.2790723065657
- Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23(1):675–699. doi:10.1146/annurev.cellbio.22.010305.10415417645413
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi:10.1158/0008-5472.CAN-06-312616990346
- Zhu L, Finkelstein D, Gao C, et al. Multi-organ mapping of cancer risk. Cell. 2016;166(5):1132–1146.e1137. doi:10.1016/j.cell.2016.07.04527565343
- Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355(6331):1330–1334. doi:10.1126/science.aaf901128336671
- Kent LN, Bae S, Tsai SY, et al. Dosage-dependent copy number gains in E2f1 and E2f3 drive hepatocellular carcinoma. J Clin Invest. 2017;127(3):830–842. doi:10.1172/JCI8758328134624
- Thurlings I, Martínez-López LM, Westendorp B, et al. Synergistic functions of E2F7 and E2F8 are critical to suppress stress-induced skin cancer. Oncogene. 2017;36(6):829–839. doi:10.1038/onc.2016.25127452520
- Brown RE, Short SP, Williams CS. Colorectal cancer and metabolism. Curr Colorectal Cancer Rep. 2018;14(6):226–241. doi:10.1007/s11888-018-0420-y31406492
- Denechaud PD, Fajas L, Giralt A. E2F1, a novel regulator of metabolism. Front Endocrinol. 2017;8:311. doi:10.3389/fendo.2017.00311
- Liew CW, Boucher J, Cheong JK, et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat Med. 2013;19(2):217–226. doi:10.1038/nm.305623291629
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi:10.1016/j.tibs.2015.12.00126778478
- Wu M, Seto E, Zhang J. E2F1 enhances glycolysis through suppressing Sirt6 transcription in cancer cells. Oncotarget. 2015;6(13):11252–11263. doi:10.18632/oncotarget.359425816777
- Sanmartín-Salinas P, Lobo M, Noguerales-Fraguas F, et al. Insulin receptor substrate-4 is overexpressed in colorectal cancer and promotes retinoblastoma–cyclin-dependent kinase activation. J Gastroenterol. 2018;53(8):932–944. doi:10.1007/s00535-018-1432-829353348
- Bochicchio S, Dapas B, Russo I, et al. In vitro and ex vivo delivery of tailored siRNA-nanoliposomes for E2F1 silencing as a potential therapy for colorectal cancer. Int J Pharm. 2017;525(2):377–387. doi:10.1016/j.ijpharm.2017.02.02028189855
- Lee SR, Jin H, Kim WT, et al. Tristetraprolin activation by resveratrol inhibits the proliferation and metastasis of colorectal cancer cells. Int J Oncol. 2018;53(3):1269–1278. doi:10.3892/ijo.2018.445329956753
- Kumazaki M, Noguchi S, Yasui Y, et al. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J Nutr Biochem. 2013;24(11):1849–1858. doi:10.1016/j.jnutbio.2013.04.00623954321
- Bakar-Ates F, Ozkan E. The combined treatment of brassinin and imatinib synergistically downregulated the expression of MMP-9 in SW480 colon cancer cells. Phytother Res. 2019;33(2):397–402. doi:10.1002/ptr.623330450754
- Varughese RS, Lam WS-T, Marican A, et al. Biopharmacological considerations for accelerating drug development of deguelin, a rotenoid with potent chemotherapeutic and chemopreventive potential. Cancer. 2019;125(11):1789–1798. doi:10.1002/cncr.3206930933320
- Lee HS, Kim EJ, Kim SH. Ethanol extract of innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr Res Pract. 2015;9(2):111–116. doi:10.4162/nrp.2015.9.2.11125861415
- Haydé VC, Ramón GG, Lorenzo GO, et al. Non-digestible fraction of beans (Phaseolus vulgaris L.) modulates signalling pathway genes at an early stage of colon cancer in Sprague-Dawley rats. Br J Nutr. 2012;108(Suppl 1):S145–S154. doi:10.1017/S000711451200078522916810
- Choi HJ, Lim DY, Park JH. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,3ʹ-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009;9:39. doi:10.1186/1471-230X-9-3919480695
- Choi JK, Murillo G, Su B-N, et al. Ixocarpalactone a isolated from the Mexican tomatillo shows potent antiproliferative and apoptotic activity in colon cancer cells. FEBS J. 2006;273(24):5714–5723. doi:10.1111/j.1742-4658.2006.05560.x17212786
- Liu H, Liu H, Zhou Z, et al. Herbal formula Huang Qin Ge Gen Tang enhances 5-fluorouracil antitumor activity through modulation of the E2F1/TS pathway. Cell Commun Signal. 2018;16(1):7. doi:10.1186/s12964-018-0218-129458395
- Kim K-C, Lee C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010;14(6):391–397. doi:10.4196/kjpp.2010.14.6.39121311680
- Rajitha B, Belalcazar A, Nagaraju GP, et al. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016;373(2):227–233. doi:10.1016/j.canlet.2016.01.05226850372
- Gong S, Xu D, Zhu J, et al. Efficacy of the MEK inhibitor cobimetinib and its potential application to colorectal cancer cells. Cell Physiol Biochem. 2018;47(2):680–693. doi:10.1159/00049002229794421
- Park HY, Park S-H, Jeong J-W, et al. Induction of p53-independent apoptosis and G1 cell cycle arrest by fucoidan in HCT116 human colorectal carcinoma cells. Mar Drugs. 2017;15(6):6. doi:10.3390/md15060154
- Mujumdar N, Mackenzie TN, Dudeja V, et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139(2):598–608. doi:10.1053/j.gastro.2010.04.04620434451
- Oliveira A, Beyer G, Chugh R, et al. Triptolide abrogates growth of colon cancer and induces cell cycle arrest by inhibiting transcriptional activation of E2F. Lab Invest. 2015;95(6):648–659. doi:10.1038/labinvest.2015.4625893635
- Su Z, Zhou C, Qin S, et al. The significant pathways and genes underlying the colon cancer treatment by the traditional Chinese medicine PHY906. Evid Based Complement Alternat Med. 2017;2017:8753815. doi:10.1155/2017/875381528588641
- Zeng H, Cheng W-H, Johnson LK. Methylselenol, a selenium metabolite, modulates p53 pathway and inhibits the growth of colon cancer xenografts in Balb/c mice. J Nutr Biochem. 2013;24(5):776–780. doi:10.1016/j.jnutbio.2012.04.00822841391
- Fukushima M, Sakamoto K, Ohshimo H, et al. Irinotecan overcomes the resistance to 5-fluorouracil in human colon cancer xenografts by down-regulation of intratumoral thymidylate synthase. Oncol Rep. 2010;24(4):835–842. doi:10.3892/or.2010.83520811661
- Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84. doi:10.1177/175883401561453026753006
- Kasahara M, Takahashi Y, Nagata T, et al. Thymidylate synthase expression correlates closely with E2F1 expression in colon cancer. Clin Cancer Res. 2000;6(7):2707–2711.10914714
- Intuyod K, Saavedra-García P, Zona S, et al. FOXM1 modulates 5-fluorouracil sensitivity in cholangiocarcinoma through thymidylate synthase (TYMS): implications of FOXM1-TYMS axis uncoupling in 5-FU resistance. Cell Death Dis. 2018;9(12):1185. doi:10.1038/s41419-018-1235-030538221
- Varghese V, Magnani L, Harada-Shoji N, et al. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9(1):1505. doi:10.1038/s41598-018-38017-030728402
- Cardinale D, Guaitoli G, Tondi D, et al. Protein-protein interface-binding peptides inhibit the cancer therapy target human thymidylate synthase. Proc Natl Acad Sci U S A. 2011;108(34):E542–E549. doi:10.1073/pnas.110482910821795601
- Banerjee D, Gorlick R, Liefshitz A, et al. Levels of E2F-1 expression are higher in lung metastasis of colon cancer as compared with hepatic metastasis and correlate with levels of thymidylate synthase. Cancer Res. 2000;60(9):2365–2367.10811110
- Sulzyc-Bielicka V, Domagala P, Bielicki D, et al. E2F1/TS immunophenotype and survival of patients with colorectal cancer treated with 5FU-based adjuvant therapy. Pathol Oncol Res. 2016;22(3):601–608. doi:10.1007/s12253-016-0043-z26831819
- Liu KP, Luo F, Xie SM, et al. Glycogen synthase kinase 3β inhibitor (2ʹZ,3ʹE)-6-bromo-indirubin- 3ʹ-oxime enhances drug resistance to 5-fluorouracil chemotherapy in colon cancer cells. Chin J Cancer Res. 2012;24(2):116–123. doi:10.1007/s11670-012-0116-923359767
- Watanabe M, Sowa Y, Yogosawa M, et al. Novel MEK inhibitor trametinib and other retinoblastoma gene (RB)-reactivating agents enhance efficacy of 5-fluorouracil on human colon cancer cells. Cancer Sci. 2013;104(6):687–693. doi:10.1111/cas.1213923438367
- Lavitrano M, Ianzano L, Bonomo S, et al. BTK inhibitors synergise with 5-FU to treat drug-resistant TP53-null colon cancers. J Pathol. 2020;250(2):134–147. doi:10.1002/path.534731518438
- Fang Z, Gong C, Yu S, et al. NFYB-induced high expression of E2F1 contributes to oxaliplatin resistance in colorectal cancer via the enhancement of CHK1 signaling. Cancer Lett. 2018;415:58–72. doi:10.1016/j.canlet.2017.11.04029203250
- Chen B, Wen P, Hu G, et al. Antagonizing CDK8 sensitizes colorectal cancer to radiation through potentiating the transcription of e2f1 target gene apaf1. Front Cell Dev Biol. 2020;8:408. doi:10.3389/fcell.2020.0040832596239
- Yin J, Shen X, Li M, et al. miR-329 regulates the sensitivity of 5-FU in chemotherapy of colorectal cancer by targeting E2F1. Oncol Lett. 2018;16(3):3587–3592. doi:10.3892/ol.2018.912130127965
- Lin Z, Ren N, Jiang Y, et al. Adenovirus-mediated E2F-1 gene transfer augments gemcitabine-induced apoptosis in human colon cancer cells. Clin Lab. 2015;61(10):1435–1444. doi:10.7754/Clin.Lab.2015.15010426642705
- Dong YB, Yang HL, McMasters KM. E2F-1 overexpression sensitizes colorectal cancer cells to camptothecin. Cancer Gene Ther. 2003;10(3):168–178. doi:10.1038/sj.cgt.770056512637937
- De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Biol Ther. 2008;7(6):833–841. doi:10.4161/cbt.7.6.583918360108
