Abstract
Background
As a novel irreversible pan-ErbB inhibitor recently approved in China, pyrotinib has exhibited promising anticancer efficacy and acceptable safety profile in HER2-positive metastatic breast cancer (mBC). The aim of this retrospective study was to estimate the efficacy and safety of pyrotinib treatment in Chinese mBC patients.
Methods
We retrospectively reviewed the real-world clinicopathological and treatment data of HER2-positive mBC patients receiving pyrotinib-based treatment from August 2018 to July 2019 in Qilu Hospital of Shandong University and other medical centers of Shandong Province in China.
Results
A total of 64 patients treated with pyrotinib were included for analysis, and the median follow-up duration was 260 days (interquartile range, 199.0 to 339.0 days). Fifty-nine (92.2%) patients had been previously treated with trastuzumab and/or T-DM1, while 11 (17.2%) patients had been exposed to lapatinib. The objective response rate (ORR) of all patients was 73.4%, and the disease control rate (DCR) was 98.4%, with a clinical benefit rate (CBR) of 87.5%. Patients with exposure to lapatinib responded well to pyrotinib-based treatment, although the ORR was significantly lower compared with that of patients without exposure to lapatinib (44.1% vs 77.5%, p=0.037). Previous lapatinib exposure was negatively associated with the objective response of pyrotinib treatment (odds ratio [OR]=0.248, 95% confidence interval [CI] 0.063–0.970, p=0.045). The median progression-free survival (mPFS) for patients with previous lapatinib exposure and patients with visceral metastasis was 299 days (95% CI 240.1–357.9 days) and 359 days (95% CI 258.3–459.7 days), respectively. But the mPFS of the whole cohort has not been reached until the cut-off date. Cox multivariate analysis revealed that only visceral metastasis was an independent predictor of significantly shorter PFS (p=0.041) but not previous exposure to lapatinib (p=0.092). Diarrhea (28.1%), hand-foot syndrome (17.2%), and neutropenia (9.4%) were the most common grade 3 adverse events associated with pyrotinib treatment.
Conclusion
Pyrotinib is highly beneficial to HER2-positive metastatic breast cancer patients, even in patients with previous lapatinib exposure. Pyrotinib is a feasible replacement of lapatinib in combination with chemotherapeutic drugs or as a monotherapy. Adverse effects are tolerable and easily manageable.
Introduction
Breast cancer is the most common malignant tumor of women both in China and worldwide.Citation1 In 2020, it was estimated that 416,371 patients were diagnosed with breast cancer in China, accounting for 9.1% of all new cancer cases.Citation1 There were 3 million cancer deaths in China with 117,174 deaths from breast cancer, accounting for 3.9% of all cancer deaths.Citation1 Breast cancer is generally categorized to four molecular subtypes based on immunochemistry, including Luminal A, Luminal B, human epidermal growth factor receptor 2 gene (HER2) positive and triple negative subtypes.Citation2 It is estimated that HER2 overexpression was present in approximately 15% to 20% of all breast cancers.Citation2,Citation3 In China, it is reported that 23.3% of breast cancer patients were diagnosed with HER-2 overexpression.Citation4 This molecular subtype of breast cancer exhibited more-aggressive biological behavior and poorer clinical outcome with higher rates of recurrence and metastasis than those without the overexpression of HER2.Citation5 Over the past two decades, the prognosis of patients with HER2-positive breast cancer has been dramatically improved due to the successful development and clinical application of several anti-HER2 therapies including trastuzumab, pertuzumab, lapatinib, as well as ado-trastuzumab emtansine (T-DM1).Citation6–Citation9 However, drug resistance to anti-HER2 therapies still could be frequently observed in clinical practice which emphasized the necessity and urgency for the understanding of drug resistance mechanism and the development of new anti-HER2 therapies.Citation10
In China, a novel orally administrated irreversible pan-ErbB receptor tyrosine kinase inhibitor (TKI), pyrotinib, has shown quite satisfying results in metastatic HER2-positive metastatic breast cancer (mBC) which led to its approval for the treatment of HER2-positive mBC.Citation11–Citation13 Pyrotinib in combination with capecitabine demonstrated significantly higher objective response rate (ORR) (78.5% vs 57.1%) and much longer progression-free survival (PFS) (18.1m vs 7.0m) when compared to lapatinib in combination with capecitabine in previously treated HER2-positive mBC as shown in a Phase II study.Citation12 Another recent randomized Phase III study PHENIX demonstrated that pyrotinib in combination with capecitabine significantly improved PFS (11.1m vs 4.1m) and ORR (68.6% vs 16.0%) than capecitabine monotherapy in previously treated HER2-positive mBC.Citation14 The most frequent grade 3 or 4 adverse events of pyrotinib-based treatment reported in the above two studies were hand-foot syndrome and diarrhea, which were generally tolerable and manageable.Citation12,Citation14 Pyrotinib is currently in Phase I clinical trial in the United States and an ongoing phase III trial is in progress to validate the superiority of pyrotinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive breast cancer previously treated with trastuzumab and taxanes.Citation12
Although above phase II/III trials demonstrated promising efficacy of pyrotinib in HER2-positive mBC, it has to be realized that there were limitations in the generalizability of enrolled patients in terms of previous anti-HER2 treatment history within both trials.Citation15 In real world, HER2-positive mBC patients were usually heavily treated with multiple anti-HER2 therapies, especially in developed countries with more accessible anti-HER2 agents.Citation15 However, almost half of the enrolled patients were trastuzumab naive and no patient with prior exposure to pertuzumab or T-DM1 was enrolled in the above pyrotinib Phase II trial.Citation12 Lapatinib, a reversible HER1 and HER2 receptor TKI, has already been approved for treating HER2-positive in China for several years.Citation7 Nevertheless, both pyrotinib trials mentioned above have excluded the patients previously treated by lapatinib.Citation12 Therefore, the question still remains unresolved whether pyrotinib treatment is efficacious in HER2 positive mBC patients with previous exposure to lapatinib. By now, neither study could sufficiently represent the general population of HER2-positive mBC patients either in China or worldwide. Thus, real-world data is needed to further comprehensively evaluate pyrotinib efficacy in the treatment of HER2-positive breast cancer.
In the current study, we retrospectively reviewed the efficacy data of pyrotinib-based therapy in metastatic or recurrent HER2-positive breast cancer in real-world setting in medical centers of Shandong Province in China. The adverse events associated with pyrotinib treatment were also analyzed in this study.
Methods
Study Population
We retrospectively collected information of HER2-positive metastatic or recurrent breast cancer patients who were treated with pyrotinib-based therapy in Qilu Hospital of Shandong University and other medical centers in Shandong Province from August 2018 to July 2019. This study adopted the following inclusion criteria: (1) Women with metastatic or locally recurrent breast cancer confirmed by histopathology with HER2 positivity defined by immunohistochemistry score of 3+ or 2+ together with HER2 gene amplification verified by fluorescence in situ hybridization (FISH), regardless of the hormone receptors status; (2) Treatment of pyrotinib-based therapy, single agent or in combination with chemotherapy; (3) Adequate hematological, hepatic, and renal functions; (4) The disease must be measurable with at least one unidimensional measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. No limits were set in terms of previously received anti-HER2 agents or previous chemotherapy regimens, however, patients with previous pyrotinib exposure in clinical trial settings were excluded. This study was performed in accordance with the Declaration of Helsinki and was granted with approval by the Ethics Review Board of Qilu Hospital of Shandong University (Shandong Province, China). Written informed consent has been obtained from the patients.
Treatment Methods
Eligible patients were prescribed with pyrotinib single agent or in combination with chemotherapy agents in routine clinical practice. The standard dosage of pyrotinib is 400mg single dose orally per day in 21-day cycles. The specific starting dose, dose modification, dose interruption, treatment discontinuation, combination therapy with cytotoxic drugs were determined by physicians’ choice based on previous clinical trials results, general health status and willingness of individual patients.
Efficacy and Toxicities
Tumor responses were evaluated every two or three cycles of treatment according to criteria in RECIST 1.1 and were evaluated at early time point if significant signs of progressive disease presented quickly. Objective response included complete response (CR) and partial response (PR). The disease control rate (DCR) was defined as the addition of objective response (CR+PR) rate and stable disease (SD) rate. Progression-free survival (PFS) was calculated from the beginning of pyrotinib-based treatment to the time point of progression or death due to any cause. Clinical benefit rate (CBR) was defined as the proportion of patients with a confirmed response of CR and PR or SD lasting at least 6 months. Toxicities were assessed based on the National Cancer Institute Common Toxicity Criteria version 5.0 (CTC5.0). The data cut-off date was Dec 30, 2019.
Statistical Analysis
Pearson’s χ2 test or Kruskal–Wallis test was used to compare the ORR difference between different groups of patients. Spearman Correlation test was recruited to evaluate the correlation between demographic/clinicopathological factors and objective response upon pyrotinib-base treatment. Univariate logistic regression analysis of the demographic/clinicopathological parameters was used to explore factor influencing the efficacy of pyrotinib-base treatment. The PFS curves were constructed with Kaplan–Meier method. Median progression survival time and 95% confidence intervals (CI) were estimated. The Log rank test was used for univariate analysis of PFS between groups. Cox regression estimated the statistically significant factors in univariate analysis. Statistical analysis was carried out using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and p < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 64 female patients with HER2-positive breast cancer treated with pyrotinib were included in this study with a median follow-up duration of 260 days (interquartile range, 199.0 to 339.0 days). The baseline characteristics of patients are shown in . The median age was 54 years (range, 31–75). Thirty-four (53.1%) of the patients were hormone receptor positive while 53 (82.8%) of patients were with distant metastases, among which 37 (57.8%) with visceral metastases and 11 (17.2%) with brain metastases. Sixty (93.8%) of all patients had previously received taxanes-based or anthracyclines-based chemotherapy regimens. Fifty-nine (92.2%) patients had been treated with trastuzumab and/or T-DM1 in the adjuvant or neoadjuvant stage, the metastatic stage, or both. Among the 59 patients, only 2 patients received the treatment of T-DM1. Twenty-six (40.6%) patients were treated with trastuzumab in the adjuvant or neoadjuvant stage, 23 (35.9%) in the metastatic stage and 10 (15.6%) in both stages. Eleven (17.2%) patients were treated with lapatinib after trastuzumab and/or T-DM1 treatment failed.
Table 1 Clinicopathological and Disease Characteristics of 64 HER2-Positive Metastatic Breast Cancer at Baseline
Treatment Administration
The treatment methods and treatment interruption or discontinuation are summarized in . Most patients (58 of 64, 96.5%) were prescribed with pyrotinib in combination with chemotherapy agents, among which capecitabine was the most frequently used one based on previous results of reported clinical trials about pyrotinib and lapatinib. In addition to capecitabine, vinorelbine and taxanes were also commonly used to combine with pyrotinib. All 64 patients but one were initiated pyrotinib treatments at the recommended standard dose of 400mg/day. Nine (11.4%) patients experienced dose reduction and 38 (59.4%) patients experienced treatment interruption, while 1 (1.6%) patients experienced pyrotinib treatment discontinuation due to intolerable adverse events of diarrhea. By the cut-off date, 20 patients among these 64 patients have discontinued pyrotinib treatment due to reasons including: disease progression (n=12), death (n=5), intolerable adverse effects (n=1), economic reason (n=1), ant personal decision (n=1). The other 44 patients are still receiving pyrotinib for treatment at last follow-up visit.
Table 2 Pyrotinib Treatment Modes and Dosage
Clinical Efficacy
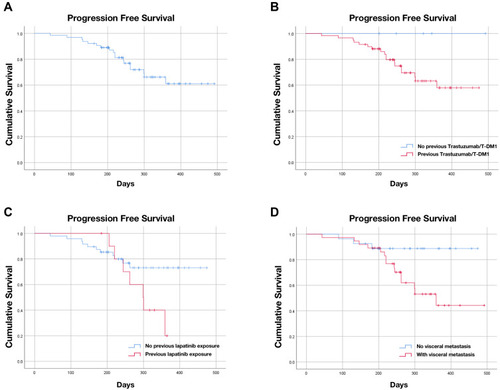
Among the 64 patients, 47 patients reached objective response with 2 CR and 45 PR, 16 patients experienced SD as best response, and 1 patient experienced PD, as shown in . The objective response rate (ORR) of all patients was 73.4% and the disease control rate (DCR) was 98.4%. A total of 56 patients had clinical benefit and the clinical benefit rate (CBR) was 87.5%. The ORR in patient groups with different clinicopathological and disease characteristics are shown in . Patients with previous lapatinib treatment after trastuzumab and/or T-DM1 failure still responded well to pyrotinib, although the ORR of this group of patients was significantly lower compared with that of patients without exposure to lapatinib (45.5% vs 77.1%, p=0.037). However, the ORR differences between patients with or without previous trastuzumab and/or T-DM1 treatment, patients with or without distant metastasis, patients with or without visceral metastasis, or patients between different pyrotinib-based therapies were not statistically significant. The overall ORR is still satisfying in patients with previous trastuzumab and/or T-DM1 treatment (70.0%), as well as in patients with brain metastasis (72.7%, only extracranial lesions considered). In correlation analysis, it is revealed that previous lapatinib exposure and number of visceral metastatic sites were negatively correlated to ORR (p < 0.05). However, Cox univariate analysis demonstrated that previous lapatinib exposure was negatively associated with the objective response of pyrotinib treatment (OR=0.248, 95% CI 0.063–0.970, p=0.045; Table S1), but not the number of visceral metastatic sites (p>0.05). Until the cut-off date, most patients have not progressed on pyrotinib-based treatment and the median PFS has not been reached in the whole cohort () nor in most subgroups of different clinicopathological characteristics, such as the subgroup of patients previously treated with trastuzumab (). In Kaplan–Meier survival analysis, the mPFS for patients with previous lapatinib exposure was 299 days (95% CI 240.1–357.9 days, ) and the mPFS for patients with visceral metastasis was 359 days (95% CI 258.3–459.7 days, ). In log-rank analysis, only previous exposure to lapatinib and visceral metastasis were correlated with significantly shorter PFS (p=0.043 and p=0.028, respectively). However, Cox multivariate analysis revealed that visceral metastasis was an independent predictor of significantly shorter PFS (p=0.041), but not previous exposure to lapatinib (p=0.092), as shown in Table S2.
Table 3 Objective Response Rate in All Patients and in Patients with Prior Exposure to Lapatinib
Table 4 Objective Response Rate in Patients with Different Clinicopathological and Disease Characteristics
Figure 1 Kaplan-Meier plot of progression-free survival (PFS) and log-rank analysis of predictors of pyrotinib-based treatment. (A) Kaplan-Meier plot of PFS of all patients treated with pyrotinib-based treatment. (B) Kaplan-Meier plot of PFS for patients with or without trastuzumab/T-DM1 treatment. (C) Kaplan-Meier plot of PFS for patients with or without exposure to lapatinib; (D) Kaplan-Meier plot of PFS for patients with or without visceral metastasis.
Safety
All 64 patients were assessed for toxicity and the rate of any grade toxicity was 100% (64/64). The detailed adverse events are listed in . Common treatment-related adverse events (AE) of any grade were diarrhea (61/64, 95.3%), hand-foot syndrome (48/64, 75.0%), neutropenia (31/64, 48.4%), nausea and vomiting (24/64, 37.4%). The rate of grade 3 toxicity was 64.1% (39/64), which included diarrhea (n=18), hand-foot syndrome (n=11), neutropenia (n=6), elevated aminotransferase (n=1), nausea and vomiting (n=1), decreased appetite (n=1), and anemia (n=1). No grade 4 or above toxicity was observed. Diarrhea (28.1%), hand-foot syndrome (17.2%), and neutropenia (9.4%) were the most common grade 3 adverse effects associated with pyrotinib treatment, which were generally tolerable and manageable. Most grade 3 diarrhea occurred in the beginning pyrotinib cycle and were improved to grade 1 or 2 after the treatment with loperamide and/or montmorillonite powder without interruption of pyrotinib treatment. Only one patient discontinued pyrotinib treatment due to grade 3 diarrhea. Nine (14.1%) patients experienced dose reduction due to AEs, including diarrhea (n=4), hand-foot syndrome (n=2), elevated aminotransferase (n=1), elevated creatinine (n=1), and decreased appetite (n=1).
Table 5 Pyrotinib Related Adverse Events of All Grades and Grade 3–4
Discussion
The main results of the current study showed that pyrotinib demonstrated a high ORR of 73.4% with acceptable safety profile in HER2 positive mBC patients, which was similar to reported results from previous pyrotinib clinical trials.Citation12 The ORR of hormone receptor negative HER2 positive mBC (83.3%) was higher than that of hormone receptor positive HER2 positive mBC (64.7%), which indicated that the former might benefit more from the treatment of pyrotinib. However, there was no statistically significant difference between the two groups. Importantly, it was shown that mBC patients with previous lapatinib treatment still could benefit from the anti-HER2 treatment of pyrotinib. In survival multivariate analysis, our results indicated visceral metastasis but not previous exposure to lapatinib as an independent predictor of significantly shorter PFS upon pyrotinib treatment.
The key clinical trials regarding the continuous anti-HER2 treatment after trastuzumab failure in HER2 positive breast cancer mainly include the following ones. EGF100151 study enrolled breast cancer patients who progressed after treatment of trastuzumab combined with anthracycline or taxanes. They were randomly divided into lapatinib combined with capecitabine group or single drug capecitabine group and the PFS of the combination group reached 8.4 months.Citation16 The GBG26 study examined the role of continuous trastuzumab treatment beyond progression and found that trastuzumab plus capecitabine prolonged the median time to progression to 8.2m.Citation17 Results from the Emilia study revealed that the PFS of the T-DM1 group after trastuzumab failure could reach as long as 9.6 months.Citation9 In the current study, although the mPFS of patients previously treated with trastuzumab and/or T-DM1 has not reached due to short follow-up time, it could be roughly estimated to be more than 400 days (13.3 months) from which is numerically better that the PFS of above trials, suggesting that pyrotinib exhibited a quite satisfying efficacy in patients previously treated with trastuzumab in real-world settings.
Lapatinib is a reversible HER1/HER2 tyrosine kinase inhibitor, while pyrotinib binds to the intracellular kinase regions of HER1, HER2, and HER4 directly and irrevocably, blocking the formation of HER2 homodimer.Citation7,Citation12,Citation16 In this study, the ORR of those who did not receive lapatinib after failure of previous trastuzumab and/or T-DM1 therapy was higher than that of patients who received lapatinib (77.1% vs 45.5%, P = 0.037). However, this result suggested that pyrotinib treatment was still beneficial after lapatinib treatment with an acceptable ORR of 45.5%. Even more, the mPFS of pyrotinib treatment in patients with previous lapatinib exposure could reach 299 days (9.9 months) in the current study. Our further multivariate survival analysis excluded previous exposure to lapatinib as an independent predictor of shorter PFS (p=0.092). Nevertheless, these results needed to be further confirmed in other clinical studies due to the small sample size in our study.
Brain metastasis (BM) often occurred in mBC especially in HER2-positive subtype, among which BM incidence was reportedly as high as 20–50%.Citation18 It was demonstrated that mBC with HER2 overexpression showed much higher central nervous system metastatic potential with an odds ratio of 5.6 compared with HER2-negative cancers.Citation19 Continuous anti-HER2 treatment after brain metastasis in HER2-positive breast cancer could reduce the risk of death from extracranial metastases by about 50%.Citation18 Anti-HER2 drugs including macromolecule monoclonal antibody and small molecule TKI had different binding sites and action mechanisms on the HER2 signaling pathway. Trastuzumab, as a macromolecular monoclonal antibody, could not easily cross the blood-brain barrier.Citation9 While TKIs such as lapatinib could enter the brain with a higher penetration rate through the blood-brain barrier compared with trastuzumab and thus could reach higher drug concentration around BM.Citation20 As a novel small molecular TKI, theoretically pyrotinib could also penetrate through the blood-brain barrier to effectively control BM. As shown in the phase III PHENIX study, in patients with baseline BM, the mPFS of pyrotinib plus capecitabine treatment could reach 6.9 months versus 4.2 months in capecitabine alone group and the time to intracranial tumor progression was 5.6 months upon pyrotinib-based treatment.Citation14 These results suggested that pyrotinib had a good therapeutic potential for intracranial lesions in HER2 positive mBC. Currently, there are several ongoing clinical trials aimed to confirm the role of pyrotinib in the treatment of BM in HER2 positive mBC.
The standard chemotherapy agent for combination with pyrotinib or lapatinib is capecitabine, which is mainly based on the fact that capecitabine is the most commonly used chemotherapy drug for mBC patients with resistance to anthracycline and taxanes.Citation12,Citation21–Citation23 Moreover, both TKIs and capecitabine are oral drugs with much more convenience for patients to take. In real-world clinical practice, for patients who have progressed on previous capecitabine treatment, other chemotherapy agents such as vinorelbine, gemcitabine and taxanes could also be potentially combined with pyrotinib based on their synergistic effect with trastuzumab. In this study, we found that pyrotinib combined with chemotherapeutic agents other than capecitabine could also achieve satisfying clinical efficacy. The ORR of pyrotinib combined with capecitabine was 77.1%, the ORR of pyrotinib combined with vinorelbine reached 76.9% and the ORR of pyrotinib plus taxanes rechallenge could also reach 55.6%.
As for safety profiles in this study, the incidence of adverse events associated with pyrotinib such as diarrhea, nausea and vomiting was similar to the results reported in previous studies.Citation12,Citation14,Citation23 Diarrhea was the most common AE revealed in this study. Diarrhea, hand foot syndrome, leukopenia and other common adverse reactions could be alleviated by reducing the dosage of pyrotinib or antidiarrheal, using hand cream, granulocyte colony-stimulating factor and other symptomatic treatments. Thus, the real-world adverse effects of pyrotinib were tolerable and easily manageable.
As a retrospective study, the current study has the following limitations. First, the sample size of this study was comparatively small, which makes some subgroup analysis results deviated. Second, the follow-up time of this study was short and the mPFS of the whole cohort had not been reached until the cut-off date. Third, the analysis of adverse events is subjective due to the use of questionnaires and telephone follow-up. Thus, the findings of our study need to be further confirmed in large prospective studies.
In conclusion, pyrotinib was well tolerated with encouraging antitumor activity in second-line or later line HER2 positive metastatic breast cancer patients, even in patients with previous lapatinib exposure. Pyrotinib could be a feasible replacement of lapatinib in combination with chemotherapeutic drugs or as a monotherapy. In addition to capecitabine, pyrotinib could also potentially be combined with other chemotherapeutic drugs such as vinorelbine, which needs further confirmation in future studies.
Data Sharing Statement
Data supporting findings of present study is available from the corresponding author (Dr. Li Li, [email protected]) on request.
Disclosure
The authors report no conflicts of interest for this work.
References
- SungH, FerlayJ, SiegelRL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.2166033538338
- WaksAG, WinerEP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.1932330667505
- SlamonDJ, GodolphinW, JonesLA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi:10.1126/science.24701522470152
- HanX, ShiY, MaL, et al. Comparison of immunohistochemistry with fluorescence in situ hybridization in determining the human epidermal growth factor receptor 2 status of breast cancer specimens: a multicenter study of 3149 Chinese patients. Chin Med J (Engl). 2014;127:246–253.24438611
- ErogluZ, TagawaT, SomloG. Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer. Oncologist. 2014;19:135–150. doi:10.1634/theoncologist.2013-028324436312
- SlamonDJ, Leyland-JonesB, ShakS, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi:10.1056/NEJM20010315344110111248153
- GeyerCE, ForsterJ, LindquistD, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi:10.1056/NEJMoa06432017192538
- SwainSM, MilesD, KimSB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, Phase 3 study. Lancet Oncol. 2020;21:519–530. doi:10.1016/S1470-2045(19)30863-032171426
- DierasV, MilesD, VermaS, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi:10.1016/S1470-2045(17)30312-128526536
- LoiblS, GianniL. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi:10.1016/S0140-6736(16)32417-527939064
- MaF, LiQ, ChenS, et al. Phase I Study and biomarker analysis of pyrotinib, a novel irreversible Pan-ErbB Receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35:3105–3112. doi:10.1200/JCO.2016.69.617928498781
- MaF, OuyangQ, LiW, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a Randomized, Phase II Study. J Clin Oncol. 2019;37:2610–2619. doi:10.1200/JCO.19.0010831430226
- ZhuY, LiL, ZhangG, et al. Metabolic characterization of pyrotinib in humans by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1033–1034:117–127. doi:10.1016/j.jchromb.2016.08.009
- JiangZ, YanM, HuX, et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: a randomized phase III study. J Clin Oncol. 2019;37:suppl.1001. doi:10.1200/JCO.2019.37.15_suppl.1001
- GourdE. Pyrotinib versus lapatinib in HER2-positive breast cancer. Lancet Oncol. 2019;20(10):e562. doi:10.1016/S1470-2045(19)30568-631477452
- CameronD, CaseyM, OlivaC, NewstatB, ImwalleB, GeyerCE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924–934. doi:10.1634/theoncologist.2009-018120736298
- von MinckwitzG, SchwedlerK, SchmidtM, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–2281. doi:10.1016/j.ejca.2011.06.02121741829
- OlsonEM, Abdel-RasoulM, MalyJ, WuCS, LinNU, ShapiroCL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–1533. doi:10.1093/annonc/mdt03623463626
- BuonomoOC, CareddaE, PortarenaI, et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS One. 2017;12(9):e0184680. doi:10.1371/journal.pone.018468028922402
- MehtaAI, BrufskyAM, SampsonJH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39(3):261–269. doi:10.1016/j.ctrv.2012.05.00622727691
- HuangT, LuoX, WuB, et al. Pyrotinib enhances the radiosensitivity of HER2 overexpressing gastric and breast cancer cells. Oncol Rep. 2020;44:2634–2644. doi:10.3892/or.2020.782033125154
- LiY, QiuY, LiH, et al. Pyrotinib combined with vinorelbine in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol. 2021;11:664429. doi:10.3389/fonc.2021.66442933996589
- XuB, YanM, MaF, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–360. doi:10.1016/S1470-2045(20)30702-633581774
