Abstract
Purpose
Patients with T3-4N0M0 nasopharyngeal carcinoma (NPC) are a unique subgroup of locoregional advanced NPC, which generally have a better prognosis than others and are often excluded in most randomized controlled clinical trials focusing on locoregional advanced NPC. The management of this population is still controversial. This study aims to evaluate the outcomes of T3-4N0M0 NPC patients treated with sequential induction chemotherapy and concurrent chemoradiotherapy (IC+CCRT) or chemoradiotherapy (CCRT) alone.
Patients and Methods
We included 362 patients diagnosed with T3-4N0M0 NPC from two hospitals between December 2005 and December 2014. All patients were received IC + CCRT (n=146) or CCRT (n=216). Locoregional failure-free survival (LRFFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) were retrospectively estimated.
Results
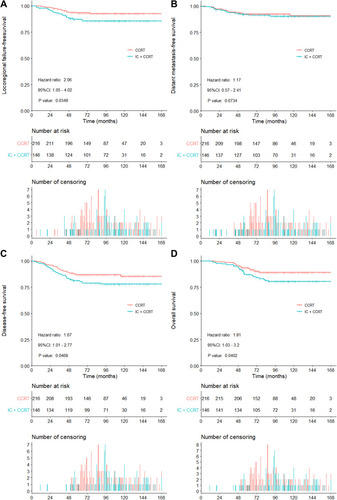
The median follow-up was 95 (range: 11–168) months. Univariable analyses have shown that 5-year LRFFS, DFS and OS in the IC+CCRT group and the CCRT group were 87.4% vs 93.4% (P = 0.035), 80.4% vs 87.0% (P = 0.047) and 86.3% vs 93.0% (P = 0.040). Multivariate analyses demonstrated that only the T stage was the independent prognostic factor for LRFFS, DFS, and OS in the entire group analysis. Subgroup analysis revealed that patients with T3 tumors who received IC+CCRT had significantly lower LRFFS, DFS, and OS than those treated with CCRT. For T4 patients, the outcomes had no significant difference between the two groups.
Conclusion
This retrospective study showed that T3N0M0 patients who received CCRT had better prognosis than those treated with IC+CCRT. In terms of T4N0M0 disease, treatment outcomes are similar in both treatment groups. However, these results require further confirmation of large sample size, prospectively, randomized controlled trials.
Introduction
In 2018, approximately 129,000 new nasopharyngeal carcinomas (NPCs) were identified worldwide. Over 70% of the cases were reported in East and Southeast Asia.Citation1,Citation2 NPC is quite distinct from other head and neck cancers. It is associated with Epstein-Barr virus (EBV) infection, radiotherapy and chemotherapy susceptibility, and specific geographic distribution.Citation2 Over two-thirds of NPC patients present with locally advanced (LA) disease at diagnosis. Chemoradiotherapy (CCRT) is now the primary treatment for NPC patients. The induction-concurrent sequence further improved treatment efficacy. Several multicenter, Phase 3, randomized controlled trials showed induction chemotherapy (IC) plus CCRT significantly improved prognosis in LA-NPC patients compared with CCRT alone.Citation3–Citation7 IC plus CCRT is now a category 2A recommendation in the latest NCCN guidelines based on the above trials.Citation8 However, what is noteworthy is that T3-4N0M0 and/or T3N0-1M0 NPC patients are ruled out in the mentioned studies.Citation3–Citation7 As is known to all, patients with T3-4N0M0 NPC are a unique subgroup of locoregionally advanced NPC, which generally have a favorable prognosis. Our previous study showed that adding IC to CCRT had a negative effect on patients with low risk (stage II) NPC.Citation9 The effectiveness of IC on T3-4N0M0 NPC patients remains indistinct, and data for this group of patients are even limited in the intensity-modulated radiotherapy (IMRT) era. Whether IC + CCRT is a better choice than CCRT for this group of patients requires further confirmation. Therefore, this retrospective research aims to assess the outcomes of T3-4N0M0 NPC patients treated with sequential IC+CCRT or CCRT alone in the IMRT era.
Patients and Methods
Patients
The present study integrated clinic data from two hospitals. We included 362 patients diagnosed with T3-4N0M0 NPC between December 2005 and December 2014. Inclusion criteria were as follows: (1) histopathology confirmed nonkeratinizing NPC, (2) T3-4N0M0 disease restaged by using the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system, (3) completion of IC+CCRT or CCRT, (4) 18–75 years old. The flow diagram of patients selection was shown in Figure S1 (Supplement Data).
Chemotherapy and Radiotherapy
IC schemes were as follows: cisplatin + fluorouracil (PF), gemcitabine + cisplatin (GP), docetaxel + cisplatin + fluorouracil (TPF), or docetaxel + cisplatin (TP), repeated every three weeks, 2–3 cycles. CCRT scheme was cisplatin, repeated every three weeks, 2–3 cycles. Chemotherapy could be postponed or suspended for patients who suffered from severe adverse reactions. Radiation was performed 3 weeks after IC. All patients received IMRT, and the IMRT projects were designed based on previous researches.Citation10–Citation12
Follow-Up
The methods we used to track and monitor patients were described in the previous research.Citation13 The last follow-up time of this study was October 31, 2020.
Statistical Analysis
Locoregional failure-free survival (LRFFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) were defined as the duration from the start of treatment to the first failure at the locoregional site, distant organs, at any site or death of any cause and the death for any reason, respectively. Kaplan–Meier method was conducted for the analysis of the time-to-event endpoints. A Log rank test was performed in the comparison of the differences between the two groups. Multivariate analyses were used to identify factors associated with the above endpoints. Categorical and continuous variables were compared using the Chi-squared test and t-test. R software (R version 4.0.2) was used for data analysis.
Results
Characteristics of Patients
The median follow-up was 95 (range: 11–168) months (m). The male-to-female ratio was 2.93:1. The mean age of all the patients in this study was 47.1 (19–77) years. Among the 362 T3-4N0M0 NPC patients, 146 patients received IC + CCRT and 216 patients received CCRT. Detailed baseline characteristics of the two groups are well balanced, as shown in .
Table 1 Baseline Characteristics of the whole group analysis
Failure Patterns
Twenty-two (10.2%) deaths occurred in the CCRT group, while 26 (17.8%) in the IC + CCRT group (P = 0.036). The incidence of relapse at the locoregional site was significantly lower in the CCRT group than IC + CCRT group (6.9% vs 13.7%, P = 0.033). The incidence of distant metastasis was not significantly different in both groups (7.9% vs 8.9%, P = 0.726). Elaborate failure modes of all patients are shown in .
Table 2 Failure Modes and Survival Outcomes in the Whole Group
Survival Outcomes
Overall, LRFFS rates at 5- and 10-year in the CCRT group were 93.4% and 92.7%, while 87.4% and 85.7% in the IC+CCRT group (HR: 2.06, 95% CI: 1.05 ~ 4.02, P = 0.035; , ). DMFS rates at 5- and 10-year in the CCRT group were 92.5% and 90.8%, while 92.2% and 91.3% in the IC+CCRT group (HR: 1.17, 95% CI: 0.57 ~ 2.41, P = 0.673; , ). DFS rates at 5- and 10-year in the CCRT group were 87.0% and 85.4%, while 80.4% and 77.9% in the IC+CCRT group (HR: 1.67, 95% CI: 1.01 ~ 2.77, P = 0.047; , ). OS rates at 5 and 10 years in the CCRT group were 93.0% and 89.1%, while 86.3% and 80.5% in the IC+CCRT group (HR: 1.81, 95% CI: 1.03 ~ 3.20, P = 0.040; , ). Multivariate analysis indicated that only the T stage was the independent prognostic factor for LRFFS (HR: 2.12, 95% CI: 1.09 ~ 4.13, P = 0.027), DFS (HR: 1.92, 95% CI: 1.15 ~ 3.21, P = 0.012), and OS (HR: 1.80, 95% CI: 1.02 ~ 3.20, P = 0.044), as is shown in . The effect of IC+CCRT on LRFFS (HR: 1.93, 95% CI: 0.99 ~ 3.77, P = 0.055), DFS (HR: 1.58, 95% CI: 0.95 ~ 2.62, P = 0.079) and OS (HR: 1.75, 95% CI: 0.99 ~ 3.09, P = 0.056) did not reach statistical significance in the entire group analysis. Thus, we proceeded to subgroup analysis of these outcomes in patients with T3N0M0 and T4N0M0 disease.
Table 3 Multivariate Analyses of Clinical Outcomes for the Whole Group and T3N0M0 Subgroup
Subgroup Analysis
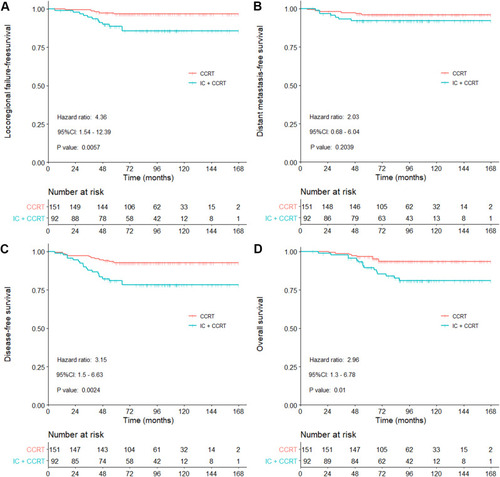
There were 243 patients with T3 tumor and 119 patients with T4 tumor. Among patients with T3N0M0 disease, the 5-year LRFFS, DFS and OS rates were remarkably lower in the IC+CCRT group than the CCRT group (88.6% vs 96.6%, P = 0.006; 81.0% vs 92.7%, P = 0.002; 89.5% vs 96.7%, P = 0.010; , Supplement Data: Table S1), DMFS was similar between the two groups (92.1% vs 96.0%, P = 0.204; , Supplement Data: Table S1). Meanwhile, multivariate analysis demonstrated that therapeutic patterns were independent prognostic factor for LRFFS (HR: 4.66, 95% CI: 1.61 ~ 13.50, P = 0.005), DFS (HR: 3.03, 95% CI: 1.41 ~ 6.50, P = 0.005) and OS (HR: 3.02, 95% CI: 1.29 ~ 7.08, P = 0.011, ). In terms of T4N0M0 NPC patients, the 5-year LRFFS, DMFS, DFS, and OS rates did not significantly differ between the two treatment groups (All P > 0.05, Supplement Data: Figure S2 and Table S1), multivariate analysis revealed different treatment patterns had no significant difference in prognosis as well (all P > 0.05, Supplement Data: Table S2).
Toxicity Profiles
In all the patients, the most common grade 3–4 hematologic side-effect during IC was neutropenia (34.2%). Besides, 21.9% of patients experienced grade 1–2 impaired liver function, and 2.1% experienced grade 1–2 impaired kidney function during IC. During CCRT period, there were significant more grade 3–4 hematologic side-effects occurred in the IC + CCRT group than CCRT group (Anemia: 10.3% vs 1.4%, P = 0.002; Thrombocytopenia: 16.4% vs 1.4%, P < 0.001; Neutropenia: 43.8% vs 6.9%, P < 0.001). Grade 1–2 impaired liver function and grade 1–2 impaired kidney function were significantly more frequently seen in the IC + CCRT group than the CCRT group, 30.8% vs 7.4% (P < 0.001) and 12.3% vs 1.0% (P < 0.001), respectively. shows details of side-effects and cycle(s) of concurrent chemotherapy completed during the treatment period in the two groups.
Table 4 Adverse Events
Discussion
This study compared the treatment outcomes of IC + CCRT with CCRT alone in T3-4N0M0 NPC patients. Our results found that patients with T3N0M0 NPC receiving IC+CCRT had significantly lower 5-year LRFFS, DFS, and OS rates than those receiving CCRT. For patients with T4N0M0 NPC, outcomes were similar between the two treatment groups. As expected, the IC + CCRT group had a significantly higher incidence of treatment-associated side-effects during CCRT than the CCRT group.
It has been reported that treatment outcomes of T3-4 NPC patients by using two-dimensional conformal radiation (2D-CRT) or three-dimensional conformal radiation (3D-CRT) with or without chemotherapy are inferior to the IMRT.Citation14,Citation15 Tatsuya Ohno et alCitation16 reviewed a total of 70 T3-4N0-1M0 NPCs (21% were T3-4N0) who received 2D-CRT plus weekly concurrent cisplatin. The 3-year local relapse-free survival (LRFS), regional relapse-free survival (RFS), DMFS, and OS rates were 80%, 75%, 74%, and 80%, respectively. Sun et alCitation17 reported that in 610 TanyN0M0 NPCs (46.1% were T3-4N0) treated with 2D-CRT (52.8%) or 3D-CRT (47.2%), 10.7% of patients received CCRT, the 5-year OS, DFS, LRFFS and DMFS rates were 78.7%, 68.8%, 78.3% and 88.5%. Compared with 2D/3D-CRT, IMRT is a milestone in the management of NPC. IMRT improves the treatment ratio due to the highly conformal dose distributions in the tumor target volume and sharp-dose gradients at the transition to the adjacent normal structures. Potential benefits of IMRT were investigated in a series of studies. Peng et alCitation18 reviewed 251 patients with TanyN0M0 NPC (46.6% were T3-4N0) who received CCRT alone or in combination with IC or adjuvant chemotherapy (AC) in the IMRT era. The 4-year DFS and OS were 91.4% and 95.3% for the entire cohort. Wu et alCitation19 retrospectively reported treatment outcomes of a matched group of 236 pairs of T3-4N0-1 NPC patients (15% were T3-4N0). All patients received CCRT alone or in combination with IC or AC. The 3-year OS, DFS, and LRFFS rates for the entire cohort were 90.6%, 88.4%, and 94.2%. Sun et alCitation20 retrospectively reviewed 506 NPC patients. Treatment outcomes of T3N0 disease in their study were similar to T1-2N0 disease by using the IMRT technique. However, the T4N0 disease remained challenging, though many of them had combined with chemotherapy. In the study by Su et alCitation21, 865 NPC patients treated with IMRT alone or in combination with chemotherapy were included. The 5-year DMFS, DFS, and OS rates for the small groups of T3N0 disease (n=84) and T4N0 disease (n=43) were 96.6%, 88.2%, 98.8%, and 90.6%, 59.1%, 68.2%, respectively. Our results are consistent with the outcomes of the above studies in the IMRT era. Although many studies focused on LA-NPC, T3-4N0M0 were often analyzed as a subgroup or excluded in RCTs. The appropriate management for T3-4N0M0 NPC is still controversial. Zhang et alCitation22 demonstrated that the 3-year DFS rate of T3N0M0 NPC patients treated with IMRT-based CCRT was only 91.1%, which still can be improved. IC + CCRT or CCRT + AC sequence are considered as the possible strategies to improve the prognosis of T3N0M0 NPC patients. However, Li’s studyCitation19 comparing CCRT±IC/AC with CCRT in patients with T3N0-1M0 disease, no benefit was found on OS (90.8% vs 90.3%, P = 0.820), DMFS (87.3% vs 89.4%, P = 0.896), and LRFFS (95.4% vs 93.0%, P = 0.311) by adding IC or AC. Our study revealed that the 5-year LRFFS, DFS, and OS were remarkably worse in the IC+CCRT group than the CCRT group, which was confirmed by multivariable analysis in the T3N0M0 subgroup. The result did not accord with expectations, and the following reasons might explain the results of this study. First, approximately 20% of patients were insensitive to induction chemotherapy due to chemoresistance.Citation23,Citation24 The delayed delivery of definitive radiotherapy would result in missing the best treatment occasion and allow time for tumor progression. Secondly, selective killing of radiosensitive subpopulations or accelerated repopulation of tumor clones induced by induction chemotherapy might compromise the treatment effectiveness of RT. An additional dose of RT may be given to compensate for this phenomenon of accelerated tumor repopulation.Citation25 Besides, one of the effects of IC is the early killing of micro-metastases. However, as we know, the 5-year DMFS of this subgroup is more than 93%,Citation20 which narrowed the potential therapeutic gain in distant metastasis control offered by IC, the ceiling effect. Fourthly, the extra severe acute hematologic and nonhematologic side effects result from IC will directly or indirectly harm the patient’s immune system, which has become the focus of researchers’ attention in the study of tumor treatment. Lastly, one important thing that cannot be ignored is that patients with bulk tumor volume or suspicion of a positive node or distant metastasis even at the same stage are more likely to be given induction chemotherapy. Patients at relatively high risk might be assigned to the IC+CCRT group.
For T4N0M0 NPC patients, it was often analyzed together with patients with T4N1M0 NPC. Cao et alCitation26 showed that the 5-year OS and DMFS rates of patients with T4N0-1M0 NPC were only 74% and 82%, which still requires improvement. IC used in the advanced primary tumor is to shrink the lesion, improve hypoxia, and enhance the sensitivity of the following radiotherapy. And it is also a possible strategy for the early eradication of micro-metastases. Yang et alCitation6 carried out a randomized Phase III trial comparing IC+CCRT with CCRT in III–IVB NPC patients (except T3N0-1), of which nearly 20% of patients were T4N0-1 NPC. They suggested that IC + CCRT improved DMFS, DFS, and OS compared with CCRT in LA-NPC. Therefore, IC is recommended for LA-NPC patients. Nevertheless, other retrospective studies did not get the same conclusion. Luo et alCitation27 retrospectively reported outcomes of T4 NPC patients, and 5-year DMFS and OS for T4N0-1 patients were 75.8% and 79.4%. No significant benefit was found by adding IC to the whole group analysis. The exact treatment outcome of T4N0M0 NPC patients was undefined. Our study revealed that the 5-year LRFFS, DMFS, DFS, and OS rates of T4N0M0 NPC patients in the IC+CCRT group did not significantly differ from the CCRT group. However, this study demonstrated that IC + CCRT group had a significantly higher treatment-related side effect rate than the CCRT group, mainly in grade 3–4 anemia, thrombocytopenia, neutropenia, and grade 1–2 impaired kidney and liver function. The purposes of IC before CCRT were to reduce tumor volume, enhance radiotherapy feasibility, and protect normal organs around the tumor. But T3-4 disease is different from T1-2 because IC could not lead to a centripetal reduction in tumor volume due to skull base bone invasion and/or intracranial extension. Generally, doctors delineate patients’ tumor targets with T3-4 NPC refers to the MRI before IC. Thus, although radiotherapy-related non-hematological side-effects could not be wholly collected for analysis in this study, we can still conjecture that IC scarcely makes a difference in organ at risk protection between IC + CCRT group and CCRT group. However, the Kaplan–Meier survival curve of DMFS and DFS in the T4N0M0 subgroup separated after a point of about 2 years, even though without statistical significance (Supplement Data: Figure S2). Therefore, the role of IC in patients with T4N0 NPC is worthy of further discussion in prospective trials or combined with other systematic treatments. Zhang et alCitation28 retrospectively reviewed 49 T4N0-1 NPC patients treated with CCRT plus nimotuzumab and found that the 3-year OS, DFS, LRFFS, and DMFS rates were 89.7%, 85.7%, 87.8%, and 97.9%, respectively. For the T4N0M0 NPC patients, the optimal treatment is worth exploring in various fields, such as combining target treatment and improving the dose of tumor target based on precision radiation.
The limitations of the present study are as follows: First, the retrospective nature of this study; second, evaluation of non-hematological side-effects was not included; last, the practical risk stratification factor, like pre-treatment EBV DNA, could not be wholly collected for analysis. Nevertheless, our report is noteworthy because of the large population of T3-4N0M0 NPC patients, the long-term follow-up, the adoption of multivariate analysis and subgroup analysis to evaluate IC’s contribution in patients with T3-4N0M0 disease.
In conclusion, this retrospective study showed that T3N0M0 patients who received CCRT had better prognosis than those treated with IC+CCRT. In terms of T4N0M0 disease, the outcomes are similar in both treatment groups. Treatment-related toxicities are more common in IC + CCRT group. However, our results require further confirmation of large sample size, prospectively, randomized controlled trials.
Data Sharing Statement
All data analyzed during this study are available from the first or corresponding author by request.
Ethics Approval
This study gained approval from the clinical research Ethics Committee of Zhejiang Cancer Hospital and Sun Yat-sen University Cancer Center. The requirement for informed consent was waived because the medical records used in this study were obtained from previous clinical treatments and it will not adversely affect the rights and health of the subject. We declare that we would protect the confidentiality of personal information of research subjects. This research was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Ting Jin and Xiaozhong Chen are joint senior authors.
Disclosure
We declare no conflict of interest regarding the publication of this article.
Acknowledgments
We thank Doctor Hui-Zhang Li from the Zhejiang Cancer Hospital for his excellent advice in study design and statistical analysis.
References
- BrayF, FerlayJ, SoerjomataramI. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- ChenYP, ChanATC, LeQT, BlanchardP, SunY, MaJ. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-031178151
- SunY, LiWF, ChenNY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-727686945
- LiWF, ChenNY, ZhangN, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145(1):295–305. doi:10.1002/ijc.3209930613964
- CaoSM, YangQ, GuoL, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. doi:10.1016/j.ejca.2016.12.03928214653
- YangQ, CaoSM, GuoL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. doi:10.1016/j.ejca.2019.07.00731425966
- ZhangY, ChenL, HuGQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–1135. doi:10.1056/NEJMoa190528731150573
- PfisterDG, SpencerS, AdelsteinD, et al. NCCN clinical practice guidelines: head and neck cancers.Version 3.2021. Natl Compr Cancer Netw. 2021.
- JinT, ZhangQ, LuoD-H, et al. Concurrent chemoradiotherapy with or without induction chemotherapy for patients with stage II nasopharyngeal carcinoma: an update. Transl Oncol. 2020;13(1):25–31. doi:10.1016/j.tranon.2019.08.00731743830
- YuZ, LuoW, ZhouQC, ZhangQH, KangDH, LiuMZ. Impact of changing gross tumor volume delineation of intensity-modulated radiotherapy on the dose distribution and clinical treatment outcome after induction chemotherapy for the primary locoregionally advanced nasopharyngeal carcinoma. Ai Zheng. 2009;28(11):1132–1137. Chinese.19895731
- LinS, LuJ, HanL, ChenQ, PanJ. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10(1):39. doi:10.1186/1471-2407-10-3920146823
- LeeAWM, LauKY, HungWM, et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol. 2008;87(2):204–210. doi:10.1016/j.radonc.2008.02.00318329742
- LiPJ, MoHY, LuoDH, HuWH, JinT. The efficacy of induction chemotherapy in the treatment of stage II nasopharyngeal carcinoma in intensity modulated radiotherapy era. Oral Oncol. 2018;85(September):95–100. doi:10.1016/j.oraloncology.2018.08.01630220326
- ZhangMX, LiJ, ShenGP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi:10.1016/j.ejca.2015.08.00626318726
- LeeAWM, NgWT, ChanLLK, et al. Evolution of treatment for nasopharyngeal cancer - success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi:10.1016/j.radonc.2014.02.00324630534
- OhnoT, WakatsukiM, ThinhDHQ, et al. Concurrent chemoradiotherapy for T3-4 and N0-1 nasopharyngeal cancer: Asian multicenter trial of the Forum for Nuclear Cooperation in Asia. J Radiat Res. 2016;57(1):44–49. doi:10.1093/jrr/rrv04626254458
- SunJD, ChenCZ, ChenJZ, et al. Long term outcomes and prognostic factors of n0 stage nasopharyngeal carcinoma: a single institutional experience with 610 patients. Asian Pac J Cancer Prev. 2012;13(5):2101–2107. doi:10.7314/APJCP.2012.13.5.210122901177
- PengH, ChenL, GuoR, et al. Clinical treatment considerations in the intensity-modulated radiotherapy era for patients with N0-category nasopharyngeal carcinoma and enlarged neck lymph nodes. Chin J Cancer. 2017;36(1):32. doi:10.1186/s40880-017-0199-228340596
- WuLR, YuHL, JiangN, et al. Prognostic value of chemotherapy in addition to concurrent chemoradiotherapy in T3-4N0-1 nasopharyngeal carcinoma: a propensity score matching study. Oncotarget. 2017;8(44):76807–76815. doi:10.18632/oncotarget.2001429100350
- SunY, TangLL, ChenL, et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer. 2012;12:1–8. doi:10.1186/1471-2407-12-6822212211
- SuSF, HanF, ZhaoC, et al. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer. 2011;30(8):565–573. doi:10.5732/cjc.010.1054721801605
- ZhangF, ZhangY, LiWF, et al. Efficacy of concurrent chemotherapy for intermediate risk NPC in the intensity-modulated radiotherapy era: a propensity-matched analysis. Sci Rep. 2015;5:17378. doi:10.1038/srep1737826611462
- ChanATC, TeoPML, LeungTWT, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1995;33(3):569–577. doi:10.1016/0360-3016(95)00218-N7558945
- ChuaDTT, ShamJST, ChoyD, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998;83(11):2270–2283. doi:10.1002/(SICI)1097-0142(19981201)83:11<2270::AID-CNCR6>3.0.CO;2-T9840526
- TannockIF. Combined modality treatment with radiotherapy and chemotherapy. Radiother Oncol. 1989;16(2):83–101. doi:10.1016/0167-8140(89)90025-X2687956
- CaoC, LuoJ, GaoL, et al. Concurrent chemotherapy for T4 classification nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. PLoS One. 2015;10(3):e0119101. doi:10.1371/journal.pone.011910125747589
- LuoY, GaoY, YangG, LangJ. Clinical outcome and prognostic factors of intensity-modulated radiotherapy for T4 stage nasopharyngeal carcinoma. Biomed Res Int. 2016;2016. doi:10.1155/2016/4398498
- ZhangS, HuangX, ZhouL, LinS. Efficacy of concurrent chemoradiotherapy combined with nimotuzumab for low-risk T4 stage nasopharyngeal carcinoma: a pilot study. Medicine. 2018;97(38):e12503. doi:10.1097/MD.000000000001250330235761