Abstract
Purpose
High‐dose methotrexate (HD‐MTX)-based chemotherapy regimen is the first-line treatment of primary central nervous system lymphoma (PCNSL). At present, doses of MTX in the range of 3.5–8 g/m2 are frequently used. However, the optimal dose of methotrexate for PCNSL remains controversial. The purpose of this real-world study was to compare the efficacy and toxicity of HD-MTX in patients with untreated PCNSL.
Methods
Immunocompetent adults with newly diagnosed PCNSL between January 2015 and December 2018 were investigated and followed up to June 2019. All patients’ initial treatments were based on HD‐MTX chemotherapy regimens.
Results
A total of 73 patients were reviewed. For patients who received HD-MTX at 8 g/m2 vs.3.5 g/m2, the complete response (CR) rates were 68.29% vs 43.75% (p = 0.03), and the median PFS times were 17.7 months vs 9.05 months (HR=0.455, 95% CI 0.239–0.865, p=0.016). There was no significant difference in OS between the two groups. Serious adverse effects were uncommon and clinically manageable.
Conclusion
There is a correlation of treatment response and clinical outcomes between the dosage of MTX in initial induction therapy in newly diagnosed PCNSL. MTX dose of 8 g/m2 provided a higher CR rate and PFS benefits with acceptable adverse effects.
Introduction
Primary central nervous system lymphoma (PCNSL) is an uncommon extranodal non-Hodgkin’s lymphoma (NHL) that is defined as lymphoma involving the brain, leptomeninges, cerebrospinal fluid, eyes, or spinal cord without evidence of systemic disease at the time of diagnosis. PCNSL accounts for only 2–4% of intracranial tumors and 4–6% of extranodal lymphomas in Western countries.Citation1–Citation3 Unfortunately, PCNSL has been classified as a highly aggressive lymphoma with poor clinical outcomes.Citation4
In the past three decades, many therapeutic regimens for PCNSL have been studied and recommended for use in patients, but with evidence-based clinical case series, small sample prospective clinical trials, and clinical experience. The role of surgery in PCNSL is typically limited to diagnostic biopsy, as aggressive surgery for PCNSL has been discouraged due to a high risk of significant postoperative neurologic deficits and other adverse outcomes.Citation5,Citation6 Because of neurotoxicity risk after radiotherapy, whole-brain radiation therapy (WBRT) is also not the treatment of choice, especially in elderly patients.Citation4,Citation7–Citation9 Given the limited roles of surgery and radiotherapy, chemotherapy is the treatment of choice, particularly as first-line therapy.
High-dose methotrexate (HD-MTX) based regimens remain the first option for newly diagnosed PCNSL because of high response rates as demonstrated by numerous studies. However, there is little consensus on optimal dosing for induction and consolidation therapy. MTX therapy at doses greater than 3 g/m2 has been shown to penetrate the blood-brain barrier and enter the cerebrospinal fluid (CSF) at therapeutic levels.Citation10 Most studies employed doses between 3 and 8 g/m2, but the optimal dose for initial treatment has yet to be identified.Citation11–Citation13 Efforts have been made to determine the optimal dose of HD-MTX for patients with PCNSL, including strategies to calculate appropriate individualized doses using different algorithms.Citation14,Citation15 Due to the rarity of PCNSL, prospective randomized trials to compare various treatment options are not feasible. Therefore, this retrospective cohort analysis in a real-world fashion was conducted to investigate the efficacy of HD-MTX in various dosages and to determine an effective standard MTX dose for PCNSL. We reviewed 73 cases of pathologically confirmed PCNSL in our hospital to evaluate the relationship between treatment and outcome.
Patients and Methods
Patients
Immunocompetent patients with newly diagnosed primary CNS lymphoma were reviewed between January 2015 and December 2018. The inclusion criteria for this study were as follows: (a) pathological diagnosis of diffuse large B-cell lymphoma (DLBCL); (b) no systemic involvement other than CNS; (c) availability of complete information on the patient’s treatment and outcomes; (d) HIV-negative and non-immunosuppression-related PCNSL; (e) no previous treatment except for steroid therapy. Patients were excluded if they were HIV-positive, showed evidence of systemic lymphoma on imaging studies, had any associated immunodeficiency or received radiotherapy alone as initial treatment. The study protocol was approved by the Ethics Committee of Huashan Hospital, and all patients provided written informed consent.
The clinical features of all patients were collected from the medical records, including demographics, performance status, time of diagnosis, surgical resection or biopsy, number and site of lesions, immunochemical staining, HIV status, serum lactate dehydrogenase (LDH) levels, CSF protein level, CSF cell counts, CSF pressure and hepatorenal function, etc. We also recorded the initial treatment dose of HD-MTX, any toxicity associated with HD-MTX, the number of cycles of HD-MTX-based therapy, and the treatment response. The location and number of lesions pre and post-therapy were evaluated by contrast-enhanced magnetic resonance imaging (MRI) for all patients. Deep brain involvement (corpus callosum, basal ganglia, periventricular region, thalamus, brainstem, and/or cerebellum) was determined based on the International Extranodal Lymphoma Study Group (IELSG).Citation16
Treatment Regimens and Response Assessment
Between January 2015 and December 2018, treatment options may be changed over time in our institution. In recent years, the treatment options were that fit patients aged ≤ 65 years old were treated with an HD-MTX (8 g/m2) on day 1, while those aged > 65 years received a reduced MTX dose at 5 g/m2. Those aged ≥ 70 years or unfit received only HD-MTX at 3.5 g/m2 on day 1. Dexamethasone was administered at 15 mg/d on days 1–3. The methotrexate dose may be adjusted based on the patients’ renal function. All patients received an HD-MTX-based chemotherapy regimen. Each HD-MTX treatment was administered as a 3-h infusion. Prehydration and alkalinization were initiated at least 72 h before MTX administration. Diuresis was kept at 3000 mL/24 h. Standard leucovorin rescue was initiated 24 h after the start of MTX infusion at a dose of 15 mg/m2 every 6 h for a total of eight times. If delayed elimination occurred, the leucovorin dose or rate of intravenous fluid hydration and alkalinization was increased. The patients received additional chemotherapy cycles every 3 weeks for at least 8 cycles. The data for initial treatments were obtained, and the categories were MTX as a single drug, MTX combined with idarubicin (IDA), MTX combined with rituximab (R), and a combination of these drugs. When the disease progressed, treatment was adjusted to include whole-brain radiotherapy or second-line treatment. The primary endpoints of the study were progression-free survival (PFS) and complete remission (CR) rate, and the secondary endpoints were overall survival (OS) and safety.
Treatment response was evaluated using contrast-enhanced magnetic resonance imaging (MRI) of the brain at baseline and before each cycle of chemotherapy. CR was defined as complete disappearance of all lesions; partial response (PR) was defined as the tumor size reduction of ≥50%; progressive disease (PD) was defined as an increase in tumor size by ≥25% for all lesions or the occurrence of new lesions; and stable disease (SD) was defined as a condition that could not be classified as CR, PR, or PD. An overall response (OR) was considered if either CR or PR was observed. PFS was calculated from the date of diagnosis to the date of disease progression, the first relapse, death from any cause, or last follow-up. OS was assessed from the date of diagnosis until death or the last follow-up. Treatment toxicities were assessed separately for each chemotherapy course and graded using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. After the completion of treatment, patients were clinically evaluated every 3 months for the first 2 years, every 6 months for 3 years, and then annually. The date of the last follow-up was June 30, 2019.
Statistical Analysis
The patients’ baseline characteristics were summarized using descriptive statistics, and descriptive analyses were conducted for all variables. The Chi-square, Fisher’s exact test, Mann–Whitney, and Kruskal–Wallis tests were used for statistical analyses. Survival curves were plotted by the Kaplan–Meier method and analyzed by the log-rank test. Multivariate Cox proportional hazards regression analysis was performed for multivariate analysis. All tests were two-sided, and p<0.05 was taken as statistically significant. All statistical analyses were performed using Stata version 15.1.
Results
Patient Characteristics
In this study, 73 patients with newly diagnosed PCNSL were reviewed in this study (). These patients included 49 males and 24 females, with a median age of 53 years (range, 24 to 81 years). Six patients (8.22%) had an elevated LDH level at diagnosis, and 46 (63.01%) had deep brain involvement. A diagnostic biopsy procedure was performed for all patients, with 47 patients (64.38%) undergoing a stereotactic biopsy and 26 (35.62%) undergoing gross total resection of their mass. No cases of PCNSL were diagnosed on the basis of CSF analysis.
Table 1 Clinical Characteristics of the Included PCNSL Patients
Treatment Responses
We compared the effects of patients receiving methotrexate at 8g/m2 and 3.5g/m2. Forty-one patients received HD-MTX at 8 g/m2, and 32 received an MTX dose of 3.5 g/m2. Thirty patients received treatment with HD-MTX monotherapy (). Among the 43 patients who received combination therapy, 17 received HD-MTX combined with idarubicin, 24 received HD-MTX plus rituximab, and 2 received HD-MTX combined with rituximab and idarubicin treatment.
Table 2 Comparison of Clinicopathological Features Between Patients Who Received MTX 3.5 g/m2 vs 8 g/m2
The median follow-up duration was 28.8 months (range, 6.3–51.9 months). By the end of observation, 26 (35.62%) patients died. The CR rates after 3 courses of chemotherapy were 43.75% for the patients who received HD-MTX at 3.5 g/m2 and 68.29% for the patients who received HD-MTX at 8 g/m2 (p= 0.03). The corresponding OR rates were 65.63% and 73.1%, respectively (). The median PFS was 9.05 months in the HD-MTX 3.5 g/m2 group compared with 17.7 months in the HD-MTX 8 g/m2 group (p=0.03; ). The median OS in the HD-MTX 3.5 g/m2 group was 42.5 months, and it has not yet been reached in the MTX dose 8 g/m2 group (p=0.83; ). From the multivariate analysis, HD-MTX at 8 g/m2 and single lesion were significant independent predictors of longer PFS (p=0.016, HR=0.455 [95% CI, 0.239–0.865]; p=0.031, HR=1.908 [95% CI, 1.060–3.432] respectively; ). These two cohorts showed similar distributions of gender, performance status, deep brain structure involvement, number of lesions, use of MTX monotherapy, and use of multi-agent therapy, and a difference in patients’ age was noted. Patients who received HD-MTX (8 g/m2) were younger than those who received the lower dosages.
Table 3 HD-MTX Doses and Treatment Responses
Table 4 Univariate and Multivariate Analyses of Factors Affecting PFS of PCNSL Patients
Figure 1 Kaplan-Meier PFS, OS curve stratified by MTX doses. Progression-free (A) and overall (B) survival of patients with newly diagnosed PCNSL treated with MTX 3.5 g/m2 vs MTX 8 g/m2. Progression-free (C) and overall (D) survival of patients younger than 65 years treated with HD-MTX 3.5 g/m2 vs MTX 8 g/m2.
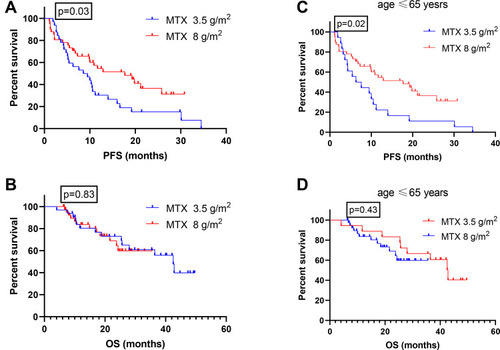
Furthermore, we compared patients younger than 65 years who received methotrexate doses at 8g/m2 and 3.5g/m2. The median PFS was 7.0 months in the HD-MTX at 3.5 g/m2 group compared with 17.7 months in the HD-MTX at 8 g/m2 group (p=0.02; ). The median OS in the HD-MTX 3.5 g/m2 group was 42.8 months, and it has not yet been reached in the MTX dose 8 g/m2 group (p=0.43; ).
Among the 30 patients who received methotrexate monotherapy, there were 7 patients (36.84%) with intracranial lesions ≥3 in the HD-MTX 8g/m2 group, and only 1 patient (9.09%) in the HD-MTX 3.5g/m2 group. A relatively small number of patients and different distributions of the two groups, no comparison of the effects of different doses for patients with single-agent chemotherapy.
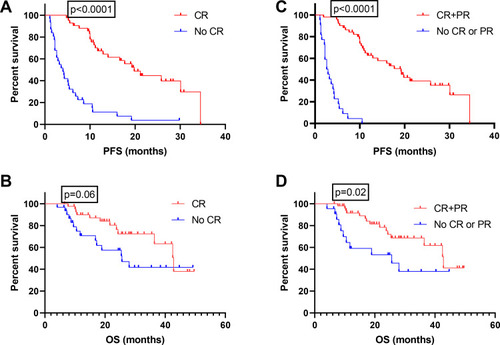
We then assessed the median OS and PFS in all patients who achieved a CR or PR after 3 cycles of chemotherapy compared with those who did not achieve a CR or PR. In patients who did achieve a CR, the median PFS was 19.8 months compared with only 4 months in patients who did not achieve CR (p<0.0001; ), median OS was 42.8 months vs 25.6 months respectively (p =0.06; ). The median PFS for patients with an OR was 19.2 months compared with only 2.75 months for those who did not achieve a CR or PR (p<0.0001; ), median OS was 42.5 months vs 25.6 months respectively (p =0.02; ). These results indicate that essentially only the patients who achieved a CR could gain a longer PFS.
Figure 2 Survival of patients with CR vs others. Progression-free (A) and overall (B) survival of patients who did achieve a CR vs those who did not; progression-free (C) and overall (D) survival of patients who did achieve an OR vs those who did not.
Toxicity
In the HD-MTX 8 g/m2 group, 5 patients experienced grade 3 hepatotoxicities or grade 1–2 nephrotoxicities. Hepatotoxicities were more common in patients with chronic liver diseases, including viral hepatitis, alcoholic liver toxicity, and non-alcoholic fatty liver disease. Grade 3‐4 hematological toxicities (anemia, neutropenia, and thrombocytopenia) were not frequent with any MTX dose. Overall, there were no significant differences in treatment toxicities between the patients who received HD-MTX at 8 g/m2 vs 3.5 g/m2 (). All treatment-related toxicities were manageable without severe events, and there were no treatment-associated deaths in either group.
Table 5 Main Adverse Effects Between the Two Groups
Discussion
PCNSL is a rare extra-nodal subtype of non-Hodgkin’s lymphoma, and most cases are of DLBCL histology with an aggressive presentation.Citation17 Its incidence has been steadily increasing during the last two decades.Citation18,Citation19 The treatment of PCNSL has evolved over the years from radiation alone to multi-agent chemotherapy.Citation20,Citation21 With concerns over the long-term neurotoxicity of radiation,Citation22 oncologists have moved away from WBRT as consolidation in first-line therapy.Citation23,Citation24
Given the rarity of PCNSL and the paucity of Phase III randomized clinical trials for potential treatments, no consensus exists on the optimal frontline regimens, chemotherapeutic agents in addition to HD-MTX, or consolidation therapy with WBRT versus high-dose chemotherapy and autologous stem cell transplant (ASCT). HD-MTX has been proven to reach a therapeutic concentration in the brain and is now considered the first-line treatment of PCNSL,Citation13 and doses of MTX in the range of 3–8 g/m2 are frequently used.Citation11,Citation21,Citation25 The current National Comprehensive Cancer Network (NCCN) guidelines recommend doses of MTX of 3.5 g/m2 or higher for PCNSL.Citation17 However, the optimal dose of MTX for PCNSL remains uncertain. A previous prospective analysis of 357 patients suggested that MTX ≥3 g/m2 improved survival in PCNSL patients.Citation26 A study that recruited 25 patients showed that the cases receiving MTX dose at 8 g/m2 and got CR and OR rates of 52 and 74%, respectively, median PFS and OS times of 12.8 and 22.8 months, and modest toxicity.Citation27 However, there are also controversial conclusions in clinical investigations, especially in different populations.Citation28,Citation29
In this study, we compared the dosage for patients who received HD-MTX at 8 g/m2 vs.3.5 g/m2, the CR rates were 68.29% vs 43.75% (p= 0.03), and the median PFS times were 17.7 months vs 9.05 months (p=0.03). Our results indicate that a higher cumulative dose of HD-MTX can improve the CR rate and PFS. The 2 cohorts showed a similar distribution of clinical characteristics, except for patients’ age. Younger patients were more frequently observed in the higher MTX dose group. However, age itself is one of the most important prognostic factors in patients with PCNSL. We thus further evaluated the dose of MTX (8 g/m2 vs 3.5 g/m2) in patients younger than 65 years. Median PFS was 17.7 months vs 7.0 months (p=0.02). Notably, better therapeutic effects were still achieved in the higher dose group. A recent study showed that higher dose intensity of MTX was a major contributor to favorable outcomes for PCNSL patients.Citation30 Our result was in line with previous work identifying MTX dose as a factor associated with survival.
Our results did not show a benefit in OS for patients receiving HD-MTX (8 g/m2) chemotherapy. However, it was thought to be relatively less reliable for assessing the therapeutic effect of different MTX doses than the response rate and PFS, because these patients had received different salvage therapeutic schemes, such as cytarabine, lenalidomide, temozolomide, and WBRT after disease progression.
Our results highlighted that most PCNSL patients were able to tolerate initial HD-MTX treatments. Adverse events associated with HD-MTX at a dose of 8 g/m2 were modest and tolerable in the present study, although some cases of grade 3–4 hematological toxicities, hepatotoxicity, and febrile neutropenia were reported. All toxicities were manageable, and no treatment-related deaths occurred. Perhaps, for patients who are younger and have no impairment of organ function, a HD-MTX dose of 8 g/m2 may be an effective and safe choice for the first‐line treatment of PCNSL.
Potential limitations in this study should be acknowledged. Selection bias is inevitable in retrospective cohort designs. Compared with previous years, patients have received higher doses of MTX (8 g/m2) in recent years, and the total number of chemotherapy cycles have increased from 8 to 12. Thus, future randomized, well-controlled, multi-center, prospective studies are needed to confirm our findings.
Conclusions
In conclusion, this study compared the outcomes among patients with newly diagnosed PCNSLs treated with different doses of HD-MTX suggested that higher MTX doses potentially improve the overall response and PFS. Given findings of favorable efficacy and toxicity ratio presented in this study, an HD-MTX dose of 8 g/m2 is recommended as the first-line treatment for PCNSL patients younger than 65 years old. Further evidence from a prospective randomized trial is needed to confirm this recommendation.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors declare that they have no conflicts of interest regarding this work or the publication of this article.
References
- Qian L, Tomuleasa C, Florian IA, et al. Advances in the treatment of newly diagnosed primary central nervous system lymphomas. Blood Res. 2017;52(3):159–166.29043230
- Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49.23095881
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446.30060998
- Seidel S, Schlegel U. Have treatment protocols for primary CNS lymphoma advanced in the past 10 years. Expert Rev Anticancer Ther. 2019;19(10):909–915.31594423
- Jahr G, Da Broi M, Holte H, et al. The role of surgery in intracranial PCNSL. Neurosurg Rev. 2018;41(4):1037–1044.29383600
- Hirono S, Iwadate Y, Higuchi Y, et al. Stereotactic radiosurgery in combination with up-front high-dose methotrexate as a first-line treatment for newly diagnosed primary central nervous system lymphoma. J Neurooncol. 2015;123(2):237–244.25911295
- van der Meulen M, Dirven L, Habets EJJ, et al. Cognitive functioning and health-related quality of life in patients with newly diagnosed primary CNS lymphoma: a systematic review. Lancet Oncol. 2018;19(8):e407–e418.30102235
- Herrlinger U, Schäfer N, Fimmers R, et al. Early whole brain radiotherapy in primary CNS lymphoma: negative impact on quality of life in the randomized G-PCNSL-SG1 trial. J Cancer Res Clin Oncol. 2017;143(9):1815–1821.28434043
- Correa DD, Braun E, Kryza-Lacombe M, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neuro-oncol. 2019;144(3):553–562.
- Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293(4):161–166.806016
- Holdhoff M, Mrugala MM, Grommes C, et al. Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma. J Natl Compr Canc Netw. 2020;18(11):1571–1578.33152700
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) Phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227.27132696
- Fox CP, Phillips EH, Smith J, et al. Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br J Haematol. 2019;184(3):348–363.30467845
- Joerger M, Ferreri AJ, Krahenbuhl S, et al. Dosing algorithm to target a predefined AUC in patients with primary central nervous system lymphoma receiving high dose methotrexate. Br J Clin Pharmacol. 2012;73(2):240–247.21838788
- Joerger M, Huitema AD, Krahenbuhl S, et al. Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: a pharmacokinetic-pharmacodynamic analysis from the IELSG no 20 trial. Br J Cancer. 2010;102(4):673–677.20125159
- Ferreri AJM, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272.12525518
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers (Version 1.2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed June 4, 2021.
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424.27018254
- Farrall AL, Smith JR. Changing incidence and survival of primary central nervous system lymphoma in Australia: a 33-year national population-based study. Cancers. 2021;13(3):403.33499081
- Schaff LR, Grommes C. Updates on primary central nervous system lymphoma. Curr Oncol Rep. 2018;20(2):11.29492682
- Grommes C, Rubenstein JL, DeAngelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296–305.30418592
- Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84–92.23685932
- Schlegel U, Korfel A. Is whole-brain radiotherapy still a standard treatment for primary central nervous system lymphomas? Curr Opin Neurol. 2018;31(6):733–739.30300241
- Song J, Samant R, Jay M, et al. Whole brain radiotherapy improves survival outcomes in primary CNS lymphoma patients ineligible for systemic therapy. Support Care Cancer. 2020;28(11):5363–5369.32140974
- Choi YS. Recent advances in the management of primary central nervous system lymphoma. Blood Res. 2020;55(S1):S58–S62.32719178
- Reni M, Ferreri AJ, Guha-Thakurta N, et al. Clinical relevance of consolidation radiotherapy and other main therapeutic issues in primary central nervous system lymphomas treated with upfront high-dose methotrexate. Int J Radiat Oncol Biol Phys. 2001;51(2):419–425.11567816
- Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21(6):1044–1049.12637469
- Herrlinger U, Kuker W, Uhl M, et al. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann Neurol. 2005;57(6):843–847.15929034
- Herrlinger U, Schabet M, Brugger W, et al. German cancer society neuro-oncology working group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51(2):247–252.11835382
- Martinez-Calle N, Poynton E, Alchawaf A, et al. Outcomes of older patients with primary central nervous system lymphoma treated in routine clinical practice in the UK: methotrexate dose intensity correlates with response and survival. Br J Haematol. 2020;190(3):394–404.32232989
