Abstract
Background
Lenvatinib treatment of 24 mg/day for radioiodine-refractory differentiated thyroid carcinoma (RRDTC) patients was almost intolerable, with high rates of dose reduction, interruption and discontinuation. Balancing treatment safety with disease risks remains challenging, and the appropriate dosage remains unclear in Asia.
Patients and Methods
A total of 65 RRDTC patients treated with lenvatinib were retrospectively collected from Oct. 2015 to Jun. 2020 from two medical centers of South Taiwan. The drug tolerability, treatment efficacy and clinical outcomes were analyzed.
Results
Different doses of lenvatinib were initiated but ultimately maintained with a median dose of 10 mg/day within the first 3 months. The disease control rate reached 89.2%, including 24.6% partial response and 64.6% stable disease. Disease progression occurred in 10.8% of patients and increased to 40.0% at the end. Eventually, the treatment dose achieved a median progression-free survival (PFS) of 26.1 months (95% CI: 17.1-NA) with overall survival (OS) not reached yet (24.1~NA). Overall, the 48-month PFS rate was 35.6% (95% CI: 18.5–68.4) and 48-month OS was 54.3% (95% CI: 41.2–71.7). The dose was tolerable with a dose reduction rate of 44.6%, dose interruption rate of 40.0% and fewer high-graded adverse events. The drug discontinuation rate was only 3.1%. However, RRDTC patients with bone metastasis or maximal dose exposure to RAI (≥600 mCi) may have less efficacy to the low maintenance dose treatment.
Conclusion
Assessing treatment intensity, safety and efficacy, low-dose lenvatinib treatment was well tolerated by RRDTC patients and displayed acceptable drug efficacy and outcomes.
Plain Language Summary
The low maintenance dose of lenvatinib (10 mg/day) was well tolerated for patients of radioiodine-refractory differentiated thyroid carcinoma with relatively less dose reduction, interruption, drug discontinuation and minor grades of adverse events.
The low maintenance dose lenvatinib treatment could achieve disease control rate of 89.2% and median progression-free survival of 26.1 months.
Patients with radioiodine-refractory differentiated thyroid carcinoma with bone metastasis or maximal dose exposure to RAI (≥600 mCi) may have less efficacy to the low maintenance dose treatment.
The initial drug response to lenvatinib at first 3 months could predict the outcome well.
Introduction
The incidence of thyroid cancer continues to rise worldwide. Generally, most differentiated thyroid carcinoma (DTC) is near-curable after surgery combined with radioiodine (RAI) therapy. However, disease persistence and recurrence are reported up to 30%.Citation1,Citation2 And 10~15% of these patients may progress to advanced stage with local invasion and/or distant metastasis. Concurrently, 25~50% of these patients are refractory to RAI therapy; then, the management becomes very challenging. Overall, patients with RAI-refractory DTC (RRDTC) are estimated 4–5 cases/million/year. Afterward, the 10-year survival drops to 10% with a life expectancy by 3–5 years.Citation3–Citation6
Lenvatinib, an approved multi-kinase inhibitor (TKI), significantly prolongs median progressive-free survival (PFS, 18.3 vs 3.6 months of placebo) for RRDTC patients.Citation7–Citation10 The Phase II study of lenvatinib treatment determined a maximal tolerated dose of 25 mg/day and initiated a dose of 24 mg/day.Citation11 However, the incidence of adverse events (AEs) of any grade and higher grade were 97.3% and 75.9%, respectively, which definitely restricted maintenance of the standard dose. The dose reduction or interruption was reported to be 59.0 ~73.6% and 31.0 ~ 86.8% in clinical reality.Citation8,Citation12 The Japanese studies ever reported significant higher liver toxicities and higher (~90%) discontinuous rate for a standard dosage of 24 mg/day lenvatinib.Citation7,Citation9,Citation13,Citation14 Therefore, balancing treatment intensity, safety, and the risk posed by the disease remains challenging. Especially, it is still unclear how to manage the tolerably effective dose for Asian population.
Thus, a retrospective observational study from two medical centers in South Taiwan was conducted, where all RRDTC patients were treated with a low dose of lenvatinib. We assessed this real-world experience for drug efficacy and outcome by disease control rate (DCR), median PFS, and overall survival (OS). Our results demonstrated that the low dose (~10 mg/day) of lenvatinib treatment was well tolerated for RRDTC patients to achieve acceptable outcome with relatively less dose reduction, interruption, drug discontinuation and minor grades of AE.
Patients and Methods
This study was conducted after approval by the Institutional Review Boards of both medical centers (KMUHIRB-E (I)-20190014 and CGMHIRB No. 201801270B0). Since this study was a retrospective observational study without any intervention, the clinical dataset was permitted for review without informed consent from patients of advanced RRDTC patients treated with lenvatinib at Kaohsiung Medical University and Kaohsiung Chang-Gung Memorial Hospital from Oct.2015 to Jun. 2020. All of the medical record reviews were carried out confidentially to comply with the Declaration of Helsinki. The study included 65 DTC patients who all met the RRDTC criteria with cumulated doses of radioiodine therapy ≥600 mCi, presence of any RAI refractory new lesion or RAI-avid lesion with progressive enlargement.
The medical record was independently reviewed by two authors, including the patient’s Eastern Cooperative Oncology Group (ECOG) performance status, pathology, AEs, lesion or disease progression by imaging evidences, and drug efficacy. For consistent reporting between hospitals, all the image studies for tumor assessment from radioiodine whole body scan, computerized tomography, bone scan, magnetic resonance image or positron emission tomography were reviewed again and standardized according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by one professional nuclear medicine radiologist. The drug response was assessed by image evidences at the first 3 months of lenvatinib treatment and then kept with an interval of 3 ~ 6 months. The AEs were recorded following the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Treatment timeline of the study population was illustrated using R4.0.2 (R Core Team, 2020). All patients were initially tracked from the starting date of lenvatinib treatment, and the time point of PD, death or censor were marked according to the date of PD documented, death date or end date of follow-up, respectively.
Statistical Analysis
The statistical analyses were performed with Stata version 14.0. (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The continuous variables were indicated as frequency (percentage), mean (standard deviation) or median (interquartile range, IOR). The differences of categorical variables among groups were estimated by Fisher’s exact test. For continuous variables, the differences among groups were estimated using Kruskal–Wallis test, and the Mann–Whitney U-test was used in pairwise comparison for each group. Median PFS and OS of each group were reported with 95% confidence intervals (CIs), and the survival curve of all patients and group stratification were illustrated by Kaplan–Meier estimator. The survival differences between groups were estimated by the Log rank test. P values less than 0.05 were considered statistically significant.
Results
Patients enrolled in this study were followed from 0.4 to 48 months. The median follow-up period was 17.1 months, and the treatment timeline of all patients is shown in Supplementary Figure 1. Most of the recruited patient (85.4%) presented with ECOG performance score 0–1 before initiation of lenvatinib treatment. Lung was primarily documented to be the most common metastatic lesions, followed by neck and bone. Advanced loco-regional invasion to adjacent critical organs, including carotid artery, jugular vein, trachea or esophagus, occurred in 30.8% of the patients. Some patients (21.5%) were previously exposed to other TKI therapy before lenvatinib ().
Table 1 Clinicopathological Characteristics Before Initiation of Lenvatinib
The lenvatinib treatment was differently initiated with doses ranged 4 to 24 mg/d, which was determined by individual physician (Supplementary Figure 1). The median dose was 10 mg/day with an interquartile range (IOR) of 10~14 mg/day within first 2~4-week. The dose titration was almost achieved a tolerable maintenance dose within the first month. The median maintenance dose was eventually around 10 (IOR, 8~14) mg/day until the 3rd month of treatment, with AE-related dose reduction and interruption in 44.6% and 40.0%, respectively (). However, lenvatinib was discontinued in 2 patients (3.1%) because of rapid disease progression and they all died soon after.
Table 2 Overall Efficacy and Tolerability of Low Maintenance Dose of Lenvatinib
At the initial 3 months, the DCR reached 89.2%, including 24.6% of partial response (PR) and 64.6% of stable disease (SD). Progressive disease (PD) occurred in 10.8% and reached 40.0% of patients at the end of follow (). Ultimately, the median PFS was 26.1 months (17.1~not available (NA)) and the median OS has not reached (). Overall, the 48-month PFS was 35.6% (95% CI: 18.5–68.4) and 48-month OS was 54.3% (95% CI: 41.2–71.7). Within period, 21 (32.3%) patients died and most of them were caused by terminal RRDTC ().
The reported AEs mainly occurred in the first 3 months, which were primarily presented in low grade and rare with high grade. The AE associated dose reduction or interruption of lenvatinib was always resumed within days to maintain at tolerable levels until the end of follow-up. However, hypertension, asthenia and proteinuria were seen in over half of the patients (Supplementary Table 1).
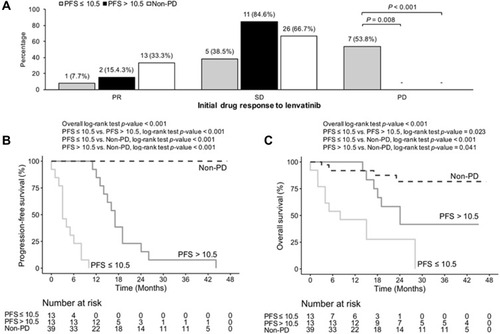
In our study, the treatment response was widely distributed with a median PFS of 10.5 months in PD group. To further analyze the difference in treatment efficacy and outcome, we divided the PD patients into group 1: PFS ≤10.5 months (n=13), and group 2: PFS >10.5 months (n=13) as well as group 3: non-PD patients (n=39). The ECOG status among 3 groups had no significant change before treatment. It was almost unchanged by low-dose lenvatinib treatment among the three groups (Supplementary Table 2). Patients ever exposed to maximal dose of RAI therapy (≥600 mCi) and those with lesion involved metastasis to bone and other sites (brain, liver and skin, etc.) occurred more in the PD than non-PD groups (). The initial dose and dose reduction rate were significantly higher in group 2 than the other groups. But the maintenance dose and dose interruption rate had no difference among the three groups (). However, the initial drug response displayed a significant discrimination at the first 3 months (). The group 1 patients displayed a significantly higher (53.8%) PD and less PR (7.7%) rates than the other groups. The initial drug response of SD was relatively less (38.5%) in group 1 patients than the other groups (84.6% of group 2 and 66.7% of group 3). Initial drug response to the low-dose lenvatinib treatment may predict future drug efficacy and outcome. Consequently, the group 1 patients had significantly worse PFS (3.0 months) and median OS (8.0 months) with greater mortalities (69.2%) than the other groups (). The 48-month PFS among the 3 groups was 0, 0 and 100%, respectively. And the 48-month OS among 3 groups was 0, 41.7% (95% CI: 21.3–81.4) and 81.7% (95% CI: 67.5–98.9), respectively ( ). Frequency of AEs was similar among groups in whole treatment course, but proteinuria (greater than grade 2) appeared more in group 2 than group 1 patients. Sub-analysis of the proteinuria, including previously existing proteinuria, co-morbidities, incidental proteinuria or worsening of proteinuria after lenvatinib treatment, did not reveal any difference among the three groups.
Table 3 Comparison of the Clinicopathological Characteristics Among Patients with PFS ≤10.5 Months, PFS >10.5 Months and Non-PD Patients
Table 4 Comparison of the Drug Efficacy, Tolerability, Adverse Events and Outcome Among Patients with PFS ≤10.5 Months, PFS >10.5 Months and Non-PD Patients
Discussion
TKI is used as a salvage therapy for advanced RRDTC patients. It is nearly impossible to achieve complete responses or tumor eradication. In fact, the median OS could not be significantly prolonged by TKI therapy in both the DECISION and SELECT trials.Citation8,Citation15 Therefore, it seems more practical to target prolongation of the median PFS, sustain a durable drug response and maintain an acceptable QoL by TKI therapy. Accordingly, optimization of TKI treatment should consider how to balance the efficacy, tolerability, risk posed by therapy and the impact on patient’s QoL.Citation16,Citation17 Several factors have been reported to correlate with the response and outcome of lenvatinib treatment for RRDTC patients, such as initial dose, patient’s age, patient’s body weight, AEs or dose interruption. The SELECT Phase III trial initiating 24 mg/day of lenvatinib for advanced RRDTC patients significantly improved PFS to achieve a 64.8% response rate and 87.7% DCR but the discontinuation rate of lenvatinib was 14.2%.Citation8 A retrospective review in France, mainly initiated lenvatinib 24 mg/day, demonstrated a median PFS of 10 months and 82% DCR (PR, 31% and SD, 51%) with 14.7% of drug discontinuation.Citation12 A retrospective report of 56 DTC patients with distant metastases showed a significantly extended SD period with the sorafenib or lenvatinib to reach 28.5% of response rate and the 75% of DCR. However, the median time of treatment failure and failure rate was 3.8 months and 86.4% for sorafenib, and 5.9 months and 28.6% for Lenvatinib.Citation18 A study compared initial 24 mg/day vs lower doses of 20, 14 and 10 mg/day of lenvatinib for 30 Japanese DTC patients. The median PFS duration was 696 days (95% CI: 318~not available) with response rate of 43% in the full-dose group, but the dose reduction was required in 93% of patients. The group with initial lower doses displayed a lower response rate (33%) and dose reduction rate 67%.Citation19 Therefore, the proper dose of lenvatinib for RRDTC patients to achieve significant outcomes remains an important issue. Balancing the drug efficacy, AEs and quality of residual life (QoL) is always a great challenge for clinician. This real-world experience clearly demonstrated that low dose of lenvatinib treatment was well tolerated by our patients with less dose reduction, interruption, drug discontinuation and also achieved an acceptable outcome and QoL.
Generally, the recommended initial dose of lenvatinib is 24 mg/day with a dose down-escalated to 20, 14 and finally 10 mg/day gradually by AEs. However, the AEs rapidly resulted in dose discontinuation (82.4%) and reduction (67%) to initiate with 24 mg/d in SELECT trial.Citation9,Citation17 Based on a study evaluating tumor sizes treated with lenvatinib, drug-tolerance-related treatment failure was highly concerned to initiate smaller doses of lenvatinib in an up-escalating titration schedule.Citation20 The impact of dose interruption was further analyzed in SELECT trial. All 261 patients experienced a dose interruption, but those with shorter dose interruptions (duration <10% of total treatment course, median 19 days) had significantly longer PFS (not reached yet) versus 12.8 months (95% CI: 9.3~16.5) in those with longer interruptions of lenvatinib (duration ≥10% of total treatment course, median 61 days).Citation21 There was a distinct risk of accelerated tumor growth and sudden death following TKI drug withdrawal.Citation18, Citation22–Citation24 The flare-up phenomenon was rationally correlated to in vivo evidence from mice suggesting that tumor angiogenesis is halted by TKI therapy but rapid and full revascularization develops in 7 days from drug discontinuation.Citation25,Citation26 Thus, the option to continue TKI therapy as long as possible has been emphasized to avoid lethal tumor regrowth after drug discontinuation, especially in patients who progress slowly during treatment.Citation18,Citation26,Citation27 Therefore, it is very important for timely management of AE to minimize the dose interruption and maximize the drug efficacy. Until now, no evidence documents the clinical efficacy of doses less than 10 mg/day. Our study to initiate and maintain with low dose (~10 mg/day) of lenvatinib treatment displayed compatible drug efficacy with fewer high graded AE and less drug interruption.
So far, no biomarkers have been able to predict the response to lenvatinib even with many reports exploring potential predictive markers for drug response before treatment.Citation18 The lesion-based evaluation in a Korean study, with the mean dose of 16.0 mg/day for 5 months, found that patients with rapid PD and shorter initial tumor doubling time could be predicted as better treatment responder but was still unable to obtain a longer PFS. Metastases to lung and brain occurred more frequently in lenvatinib treatment responders, but metastases to lymph nodes and bone were more prevalent in non-responders.Citation28 Bone metastasis was regarded as a better independent negative prognostic factor for PFS than lung metastasis.Citation29 The SELECT trial found that lenvatinib responder could achieve a longer median PFS of 33.1 months than 7.9 months in non-responders. The median duration of response (DOR) for all lenvatinib responder was 30.0 months, but appeared shorter in patients with greater tumor burden, liver metastases or brain metastases. Previous study also suggests the initial response can sustain a prolonged response to treatment.Citation30 Sub-analysis of the SELECT trial demonstrated tumor size response was also a predictive or prognostic factor. Tumor shrinkage by lenvatinib treatment was initially rapid (24.7% within 2 months) and then gradually slowing down (1.3% per month). The initial decrement of tumor size was a marginally significant positive predictor for PFS (P = 0.06).Citation20 The maximal benefit was also reported in patients with a better ECOG performance status.Citation6 Our clinical experience obtained a similar finding. The initial drug response to lenvatinib at the first 3 months was highly correlated with the future prognosis well. A higher DCR achieved by initial lenvatinib treatment could significantly refer to a longer PFS and OS. In the PD groups, RRDTC patient with maximal dose exposure of RAI (≥600 mCi), or metastatic lesions to bone or other sites may be poor response indicators for low dose of lenvatinib treatment. Therefore, the drug response of the first 3 months could help physician to adjust the future treatment strategy.
In the SELECT trial, PD was reported in 6.9~8.4% of RRDTC patients even maintained with relative high dose of Lenvatinib.Citation8,Citation31 From this cohort, PD occurred in 10.8% of patients after initial treatment and then increased to 40.0% of patient at the end of follow-up. Also, PD occurred initially in 53.8% of group 1 patients, who had the shortest PFS and OS (3.0 months). The relative low-dose treatment may be a plausible factor in the rapid progression to death in our group 1 patients. However, intrinsic drug resistance to lenvatinib could also have had a critical role in the rapid PD, since most cancer patients may eventually develop an escape phenomenon or resistance to TKI therapy.Citation18,Citation26 Up to date, there is still very little exploring the potential mechanisms of lenvatinib resistance. However, the pathways may involve activation of the cancer stem cells, which could become resistance to anti-angiogenic drugs through stimulation of alternative signaling pathways or upregulation of tumor cell receptors to promote tumor growth. To overcome or delay resistance to lenvatinib, combination with multiple drugs to achieve synergistic inhibition of different angiogenic or proliferative pathways may be a future strategy.Citation18,Citation32
Limitations of this study warrants mention, specifically the retrospective nature, conducted in a Taiwanese population only, without comparative dosage or a randomized design. Hence, our findings require validation by a prospective randomized trial to recruiting a larger and more general patient population.
In conclusion, low-dose lenvatinib treatment was well tolerated by RRDTC patients and displayed acceptable drug efficacy and outcomes with 89.2% DCR and a median PFS of 26.1 months. The relatively low dose of lenvatinib treatment may be more suitably applied in Asia.
Figure 1 Kaplan–Meier curve of median progression-free survival (PFS) and overall survival (OS) in patients with advanced radioiodine-refractory differentiated thyroid carcinoma treated by low maintenance dose lenvatinib (n=65). (A) The 48-month of PFS rate was 35.6% (95% CI: 18.5–68.4) and median PFS was 10.5 months in group of progressive disease. (B) The 48-month of OS rate was 54.3% (95% CI: 41.2–71.7) in all patients.
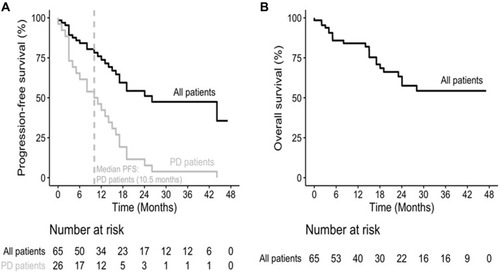
Figure 2 Comparison of the initial drug response (A), median progression-free survival (PFS) (B), and overall survival (OS) (C) in PD patients with PFS ≤10.5 months (n=13), PFS >10.5 months (n=13), and the patients with non-progressive disease (non-PD, n=39). The 48-month PFS rate in the 3 groups were 0%, 0%, and 100%, respectively. The 48-month OS rate in the 3 groups were 0%, 41.7% (95% CI: 21.3–81.4), and 81.7% (95% CI:67.5–98.9), respectively.
Abbreviations
RRDTC, radioiodine-refractory differentiated thyroid carcinoma; PFS, progression-free survival; OS, overall survival; DCR, Disease control rate; PFS, Progression-free survival; OS, Overall survival; DTC, differentiated thyroid carcinoma; RAI, radioiodine; TKI, multi-kinase inhibitor; AEs, adverse events; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; IOR, interquartile range; CIs, confidence intervals; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available; QoL, quality of residual life; DOR, duration of response.
Ethics Approval and Informed Consent
This case series study was conducted and approved by the Institutional Review Boards of Medical centers of Kaohsiung Medical University (KMUHIRB-E (I)-20190014) and Kaohsiung Chang-Gung Memorial Hospital (CGMHIRB No. 201801270B0).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declared that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
The authors are grateful to Sin-Hua Moi, PhD, Institute of Biotechnology and Chemical Engineering, I-Shou University, Kaohsiung, Taiwan, for her valuable assistance with statistical analysis.
References
- CabanillasME, McFaddenDG, DuranteC. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi:10.1016/S0140-6736(16)30172-627240885
- TamaiT, HayatoS, HojoS, et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J Clin Pharmacol. 2017;57(9):1138–1147. doi:10.1002/jcph.91728561918
- HaugenBR, ShermanSI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev. 2013;34(3):439–455. doi:10.1210/er.2012-103823575762
- SchmidbauerB, MenhartK, HellwigD, GrosseJ. Differentiated thyroid cancer—treatment: state of the art. Int J Mol Sci. 2017;18(6):1292. doi:10.3390/ijms18061292
- PaciniF. Which patient with thyroid cancer deserves systemic therapy and when?Best Pract Res Clin Endocrinol Metab. 2017;31(3):291–294. doi:10.1016/j.beem.2017.08.00128911725
- BerdelouA, LamartinaL, KlainM, LeboulleuxS, SchlumbergerM. Treatment of refractory thyroid cancer. Endocr Relat Cancer. 2018;25(4):R209–R223. doi:10.1530/ERC-17-054229371330
- CabanillasME, HabraMA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47–55. doi:10.1016/j.ctrv.2015.11.00326678514
- SchlumbergerM, TaharaM, WirthLJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi:10.1056/NEJMoa140647025671254
- CostaR, CarneiroBA, ChandraS, et al. Spotlight on lenvatinib in the treatment of thyroid cancer: patient selection and perspectives. Drug Des Devel Ther. 2016;10:873–884. doi:10.2147/DDDT.S93459
- NairA, LemerySJ, YangJ, et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin Cancer Res. 2015;21(23):5205–5208. doi:10.1158/1078-0432.CCR-15-137726324740
- FerrariSM, RuffilliI, CentanniM, et al. Lenvatinib in the therapy of aggressive thyroid cancer: state of the art and new perspectives with patents recently applied. Recent Pat Anticancer Drug Discov. 2018;13(2):201–208. doi:10.2174/157489281366618022011072929468981
- BerdelouA, BorgetI, GodbertY, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 2018;28(1):72–78. doi:10.1089/thy.2017.020529048237
- KiyotaN, SchlumbergerM, MuroK, et al. Subgroup analysis of Japanese patients in a Phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015;106(12):1714–1721. doi:10.1111/cas.1282626426092
- NagahamaM, OzekiT, SuzukiA, et al. Association of lenvatinib trough plasma concentrations with lenvatinib-induced toxicities in Japanese patients with thyroid cancer. Med Oncol. 2019;36(5):39. doi:10.1007/s12032-019-1263-330919115
- BroseMS, NuttingCM, JarzabB, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. doi:10.1016/S0140-6736(14)60421-924768112
- YuST, GeJN, LuoJY, WeiZG, SunBH, LeiST. Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:1525–1532. doi:10.2147/cmar.s19149930863162
- CapdevilaJ, NewboldK, LicitraL, et al. Optimisation of treatment with lenvatinib in radioactive iodine-refractory differentiated thyroid cancer. Cancer Treat Rev. 2018;69:164–176. doi:10.1016/j.ctrv.2018.06.01930032061
- IwasakiH, YamazakiH, TakasakiH, et al. Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosine kinase inhibitors. Oncol Lett. 2019;17(6):5292–5300. doi:10.3892/ol.2019.1018031186746
- YamazakiH, IwasakiH, TakasakiH, et al. Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine. 2019;98(10):e14774. doi:10.1097/md.000000000001477430855484
- RobinsonB, SchlumbergerM, WirthLJ, et al. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101(11):4103–4109. doi:10.1210/jc.2015-398927548104
- TaharaM, BroseMS, WirthLJ, et al. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer. 2019;106:61–68. doi:10.1016/j.ejca.2018.10.00230471649
- YunKJ, KimW, KimEH, et al. Accelerated disease progression after discontinuation of sorafenib in a patient with metastatic papillary thyroid cancer. Endocrinol Metab. 2014;29(3):388–393. doi:10.3803/EnM.2014.29.3.388
- UchidaT, YamaguchiH, NagamineK, et al. Rapid pleural effusion after discontinuation of lenvatinib in a patient with pleural metastasis from thyroid cancer. Endocrinol Diabetes Metab Case Rep. Epub 2019 Mar 18. doi:10.1530/EDM-18-0158
- MarkmanM. Lenvatinib real-life experience. Oncology. 2019;97(4):189–191. doi:10.1159/00050164131390640
- MancusoMR, DavisR, NorbergSM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. doi:10.1172/JCI2461217016557
- MatroneA, ValerioL, PieruzziL, et al. Protein kinase inhibitors for the treatment of advanced and progressive radiorefractory thyroid tumors: from the clinical trials to the real life. Best Pract Res Clin Endocrinol Metab. 2017;31(3):319–334. doi:10.1016/j.beem.2017.06.00128911728
- ViolaD, ValerioL, MolinaroE, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23(4):R185–R205. doi:10.1530/ERC-15-055527207700
- LeeEK, KimS-M, KimBH, et al. Lesion-based evaluation predicts treatment response to lenvatinib for radioactive iodine-refractory differentiated thyroid cancer: a Korean multicenter retrospective study. Thyroid. 2019;29(12):1811–1819. doi:10.1089/thy.2019.002231482759
- SuzukiC, KiyotaN, ImamuraY, et al. Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head Neck. 2019;41(9):3023–3032. doi:10.1002/hed.2578431013380
- GianoukakisAG, DutcusCE, BattyN, GuoM, BaigM. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer. 2018;25(6):699–704. doi:10.1530/erc-18-004929752332
- KiyotaN, RobinsonB, ShahM, et al. Defining radioiodine-refractory differentiated thyroid cancer: efficacy and safety of lenvatinib by radioiodine-refractory criteria in the SELECT TRIAL. Thyroid. 2017;27(9):1135–1141. doi:10.1089/thy.2016.054928665259
- TuttleRM, BroseMS, GrandeE, KimSW, TaharaM, SabraMM. Novel concepts for initiating multitargeted kinase inhibitors in radioactive iodine refractory differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31(3):295–305. doi:10.1016/j.beem.2017.04.01428911726
