Abstract
Background
Inadequately controlled symptoms incur a substantial burden on patients with neuroendocrine tumors and carcinoid syndrome (CS). The effectiveness of telotristat ethyl (TE) with a somatostatin analog for uncontrolled CS diarrhea has been demonstrated in clinical trials and observational studies. TELEPRO-II was a prospective observational study evaluating TE’s effectiveness in clinical practice over the first 3 months of treatment.
Methods
Patients initiating TE in 2018 participated in an optional nurse support program reporting CS symptoms during interviews at baseline and 1, 2, and 3 months after TE initiation. Eligible patients received TE for ≥3 months and reported symptom burden at baseline and ≥1 follow-up visit within the first 3 months. Daily bowel movement (BM) frequency and flushing episodes were reported as events/episodes per day. Stool consistency, nausea severity, urgency severity, and abdominal pain were reported on a severity scale (1–10). Symptom changes were evaluated using paired-sample t-tests and Wilcoxon signed-rank tests. Analysis of symptoms based on achievement of <30% or ≥30% reduction in daily BM frequency was conducted using a cumulative distribution function.
Results
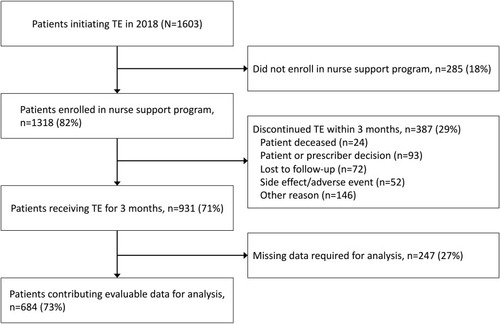
A total of 684/1603 (43%) patients were eligible for analysis. At baseline, patients reported a mean of 6.3 BM/day, nausea severity of 8.4/10 and stool urgency of 8.2/10. Significant improvements in all CS symptoms were observed after 3 months of TE. Mean daily BMs were reduced 64% after 3 months of TE (mean reduction [SD], –3.99 [3.8]; P<0.0001). Most patients (74%, n=503) reported ≥30% reduction in daily BM frequency; these patients also reported improvements in other symptoms (76–87%). Patients with <30% reduction in daily BMs also reported improvements in nausea severity (62%, n=24), daily flushing episodes (66%, n=98), abdominal pain (50%, n=60), urgency severity (38%, n=64), and stool consistency (24%, n=44).
Conclusion
Patients treated with TE in a real-world setting experienced significant, clinically meaningful improvements in CS symptoms.
Plain Language Summary
Over the first 3 months of treatment, patients with neuroendocrine tumors and carcinoid syndrome diarrhea who received telotristat ethyl experienced significant and clinically meaningful improvements in carcinoid syndrome symptoms.
Introduction
Carcinoid syndrome (CS) symptoms incur a substantial burden on the health and quality of life of patients with secretory neuroendocrine tumors and CS.Citation1–Citation4 One-fifth or more of patients with well-differentiated neuroendocrine tumors are likely to have carcinoid syndrome symptoms,Citation1,Citation5 many of whom may also develop carcinoid heart disease.Citation6 CS diarrhea and flushing are the most common and disruptive symptoms of CS, with CS diarrhea having a substantial negative impact on quality of life.Citation2 Patients with CS diarrhea are hospitalized more often and incur higher overall healthcare costs than those without CSD,Citation7,Citation8 and experience impairment in work productivity and indirect costs,Citation9 reinforcing the need for more effective management of CS.
The efficacy, safety and tolerability of the tryptophan hydroxylase inhibitor telotristat ethyl (TE; Xermelo®, TerSera Therapeutics, Lake Forest, IL, USA) to treat inadequately controlled CS diarrhea in combination with a somatostatin analog for patients with CS diarrhea were demonstrated in the randomized TELESTAR and TELECAST clinical trials,Citation10,Citation11 which supported the US FDA approval of TE in 2017. TE treatment, together with a long-acting somatostatin analog, is currently also recommended for inadequately controlled CS diarrhea by the National Comprehensive Cancer Network (NCCN).Citation12 To further evaluate the real-world use and effectiveness of TE in US clinical practice, a patient registry for patients receiving telotristat ethyl (RELAX) was established in 2017 (NCT03223428). Early analyses from the RELAX registry suggested that the majority of patients treated with TE experienced improved control of diarrhea and non-diarrhea symptoms of CS, as well as improvements in health- and wellness-related outcomes such as weight and activities of daily living.Citation13,Citation14 A second study, the prospective observational TELEPRO-I study, was also in initiated in 2017 to evaluate TE’s effectiveness in clinical practice in the first 3 months of treatment, when it was first approved in the US in 2017. This study provided further evidence of improvements in CS diarrhea and other CS symptoms associated with TE treatment; improvements in this study were observed independently of the frequency of baseline daily bowel movements.Citation15,Citation16
TELEPRO-II was a follow up study to TELEPRO-I, and evaluated TE effectiveness in a patient cohort initiating TE in the second year of its availability in the US. The study was conducted using similar methodology to TELEPRO-I, with the goal of further evaluating patient experience with TE in a large study population and to validate the TELEPRO-I findings. We present the following article in accordance with the STROBE reporting checklist.
Materials and Methods
TELEPRO-II used a similar design as TELEPRO-I, reported previously.Citation15 TELEPRO-II collected information from patients initiating TE in 2018 who opted in to participate in a nurse support program. Patient responses were collected during monthly telephone interviews before initiating TE (baseline) and at 1, 2, and 3 months after initiating TE. Patients could discontinue participation in the nurse support program at any time. The analytic sample reported here included patients participating in the nurse support program who remained on TE for at least 3 months (determined by delivery of TE by the specialty pharmacy) and reported CS symptom burden at baseline and at least one post-baseline timepoint. Patients who did not report bowel movement frequency at baseline (or reported zero BM/day) and at least one post-baseline follow-up visit were excluded.
Patients were asked to provide basic demographic information and clinical information related to their CS symptoms, including gender, age, type of health insurance, years since diagnosis of CS, somatostatin analog treatment history, and burden of CS symptoms. In reporting CS symptom burden, the frequency of daily bowel movements and flushing episodes were reported as the number of events or episodes per day, consistent with measures in clinical trials.Citation10,Citation11 Stool consistency was reported on a scale of 1 (“very hard”) to 10 (“watery”). Nausea, urgency, and abdominal pain were reported on a severity scale of 0 (“no/not at all”) to 10 (“worst imaginable/very urgent”). Changes in CS symptoms were evaluated overall and in subgroups of patients who did or did not experience a ≥30% reduction in daily bowel movement frequency.
Statistical Analysis
Descriptive statistics were used to analyze baseline demographics and disease burden. Post-baseline changes in CS symptoms were evaluated using paired-sample t-tests and Wilcoxon signed-rank tests. Improvement in symptoms was determined by lower reported bowel movement frequency and lower CS symptom ratings in post-baseline follow-up visits. Each patient served as their own control. Analysis of patients experiencing 30% improvement in daily bowel movement frequency was conducted using a cumulative distribution function. The ≥30% cutoff was based on the threshold for clinical meaningfulness reported by TELESTAR participants and approved by the US Food and Drug Administration as an acceptable threshold for clinical effect.Citation17,Citation18 Missing data were computed using the last observation carried forward methodology based on the latest post-baseline reported value for each outcome measure. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC).
Human Subjects Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki in a de-identified dataset with no patient identifiers, and determined to be exempt from Institutional Review Board oversight (Advarra®, Columbia, MD).
Results
Out of 1603 eligible patients initiating TE before December 31, 2018, 1318 (82%) opted in to the nurse support program, of whom 387 (29%) discontinued TE within the first 3 months. Of the 931 patients who received TE for 3 months, 247 (27%) did not have both a baseline and at least one post-baseline value for reported bowel movement frequency, and were excluded from this analysis. A total of 684 (43%) patients receiving TE for at least 3 months participated in the nurse support program and had both a baseline and at least one post-baseline measure of bowel movement frequency (). Approximately half of included patients were women (54%) and ≥65 years old (49%); patients tended to have commercial (43%) or Medicare (37%) insurance coverage (). The mean time from neuroendocrine tumor diagnosis to initiation of TE was 6.8 (SD, 8.5) years. Baseline characteristics were similar between patients enrolled in the nurse support program, those who were also eligible for analysis, and those who discontinued TE within the first 3 months. Patients reported a substantial burden of CS diarrhea (mean 6.3 bowel movements per day) and other CS symptoms, particularly nausea (mean, 8.4/10) and urgency (mean, 8.2/10).
Table 1 Baseline Demographic and Clinical Characteristics
CS Symptom Changes from Baseline
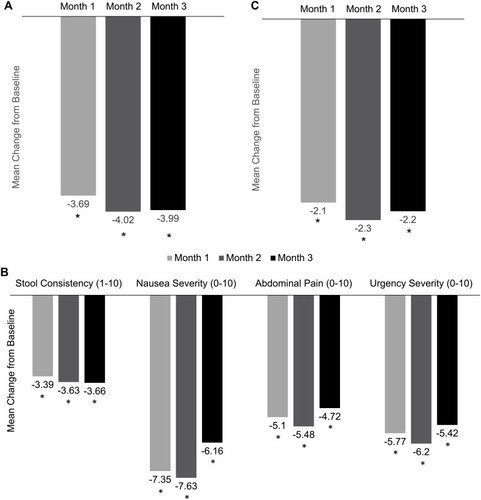
Patients receiving TE for at least 3 months who had baseline and ≥1 follow-up value reported significant improvements in CS diarrhea and all CS symptoms. Included patients had follow-up symptom ratings through 3 months. Mean (SD) daily bowel movements were reduced 64% after 3 months of TE treatment, from 6.3 (3.3) at baseline to 2.3 (2.8) per day (mean reduction, –3.99 [3.8]; P<0.0001; ). Other CS symptoms with the highest baseline burden, nausea severity and urgency severity, respectively, were reduced 73% (8.4/10 [2.9] to 2.2/10 [4.1]; mean reduction, –6.16 [4.4]; P<0.0001) and 66% (8.2/10 [2.3] to 2.9/10 [3.9]; mean reduction, –5.42 [4.1]; P<0.0001; ). Patients also reported significant 3-month reductions in stool consistency and abdominal pain, 55% and 73%, respectively. Daily flushing episodes were reduced 73% from 3.0 (3.2) to 0.9 (2.0) per day (mean reduction, –2.23 [3.3]; P<0.0001; ).
Figure 2 CS symptom mean changes from baseline. (A) Daily bowel movement frequency. (B) Stool consistency, nausea, abdominal pain, and urgency. (C) Daily flushing episodes. *P<0.0001.
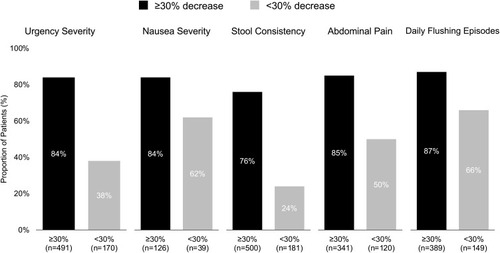
The majority of patients reported ≥30% reduction in daily bowel movement frequency after 3 months of TE treatment (503/684, 74%). Nearly all of these patients also reported improvements in other CS symptoms (range, 76–87%; ). Of those who did not report ≥30% improvement in bowel movement frequency, most patients reported improvement (lower symptom severity scores) in nausea severity (62%, n=24) and daily flushing episodes (66%, n=98). This subgroup also reported improvements in abdominal pain (50%, n=60), urgency severity (38%, n=64), and stool consistency (24%, n=44).
Discussion
This real-world study of patient-reported experience demonstrated significant improvements in CS symptoms in the first 3 months of TE initiation when added to a somatostatin analog, and further supports findings from the TELEPRO study. The baseline burden of CS symptoms was high in this population despite having received SSAs, confirming the need for treatments that improve symptoms of CS. Many patients who did not achieve ≥30% reduction in daily bowel movement frequency still experienced reductions in CS symptoms.
Our findings are consistent with the known effects of reducing peripheral serotonin production on CS symptoms and with previous observational studies of TE treatment in clinical practice.Citation14–Citation16 Elevated serotonin is associated with proliferative effects on fibroblasts in both the small bowel mesentery and cardiac valvesCitation19–Citation21 and with increased 1-year mortality for patients with CS.Citation22 Serotonin has further been associated with increased secretion, motility, and inflammation in the gastrointestinal tract, contributing to CS diarrhea. TE is postulated to improve these symptoms by reducing tumoral production of serotonin.Citation23
Our findings are consistent with and confirm the findings from TELEPRO-I.Citation15 TELEPRO-II included a larger sample of TE patients (684 vs 369 in TELEPRO-I) with generally similar proportions opting in to the nurse support program (82% and 88%, respectively) and providing evaluable data (73% and 62%, respectively). Both studies showed a high burden of pre-TE bowel movement frequency (mean of 6.3 per day in both studies), stool consistency (means of 6.5 and 6.6/10 in TELEPRO-II and TELEPRO-I, respectively), and daily flushing episodes (means of 3.1 and 3.0, respectively). Improvements in bowel movement frequency appeared generally greater in TELEPRO-II (3.99 fewer per day at Month 3 vs 2.1 in TELEPRO-I), as did improvements in stool consistency (3.7- and 1.4-point mean reductions, respectively) and daily flushing episodes (2.2- and 1.3-point mean reductions, respectively). Scoring was changed from 100- to 10-point scales for urgency, abdominal pain and nausea severity for TELEPRO-II, but patients reported improved symptoms across all timepoints in both studies.
Our study is the largest real-world analysis of patient-reported improvements after TE treatment reported to date, providing data on a large patient cohort with CS treated with TE over 3 months. Of note, this was a single-arm observational study where patients served as their own controls, and findings should be interpreted in this context. Additionally, nurses administering the telephone interviews were unblinded to patient treatment status. Patients declining to participate in the specialty pharmacy nurse support program (18%) and overall attrition of participants over time may have contributed to potential selection bias that could not be evaluated, though baseline characteristics were similar across the TE treatment and discontinuation groups.
Conclusions
In summary, our study provides further evidence of significant, clinically meaningful improvements in CS symptoms among a large cohort of patients receiving TE in real-world clinical practice. Further studies, with longer follow-up, are warranted to explore the impact of reduced peripheral serotonin on longer-term complications of CS including mesenteric fibrosis and cardiac valvular disease.
Authors’ Responsibility
The authors confirm they have not previously published or submitted this manuscript elsewhere; they took a significant part in the work and approved the final version of the manuscripts; they have complied with ethical standards; they agree AME Publishing Company to get a license to publish the accepted article when the manuscript is accepted; and they have obtained all necessary permissions to publish any figures or tables in the manuscript, and assure that the authors will pay for any necessary charges.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki in a de-identified dataset with no patient identifiers, and determined to be exempt from Institutional Review Board oversight (Advarra®, Columbia, MD).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
MHK and HFK received research support from Lexicon Pharmaceuticals, Inc. and TerSera Therapeutics. KM was employed by DataWave Solutions which received research funding from Lexicon Pharmaceuticals, Inc. and TerSera Therapeutics. VNJ was employed by Lexicon Pharmaceuticals, Inc. and TerSera Therapeutics. The authors report no other conflicts of interest in this work.
Acknowledgments
This work was supported by TerSera Therapeutics. The authors wish to thank Neha Kapur and Salma Sayeed for their review of the manuscript. Medical writing support was provided by Jeff Frimpter, MPH, funded by TerSera Therapeutics.
References
- HalperinDM, ShenC, DasariA, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18(4):525–534.28238592
- AnthonyL, ErvinC, LapuertaP, et al. Understanding the patient experience with carcinoid syndrome: exit interviews from a randomized, placebo-controlled study of telotristat ethyl. Clin Ther. 2017;39(11):2158–2168. doi:10.1016/j.clinthera.2017.09.01329074312
- ShenC, ChuY, HalperinDM, et al. Carcinoid syndrome and costs of care during the first year after diagnosis of neuroendocrine tumors among elderly patients. Oncologist. 2017;22(12):1451–1462. doi:10.1634/theoncologist.2017-014928642335
- SinghS, GranbergD, WolinE, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Glob Oncol. 2016;3(1):43–53. doi:10.1200/JGO.2015.00298028717741
- DasariA, ShenC, HalperinD, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi:10.1001/jamaoncol.2017.058928448665
- Grozinsky-GlasbergS, GrossmanAB, GrossDJ. Carcinoid heart disease: from pathophysiology to treatment – “something in the way it moves”. Neuroendocrinol. 2015;101(4):263–273. doi:10.1159/000381930
- DasariA, JoishVN, Perez-OlleR, et al. Direct costs of carcinoid syndrome diarrhea among adults in the United States. World J Gastroenterol. 2019;25(47):6857–6865. doi:10.3748/wjg.v25.i47.685731885426
- BroderMS, ChangE, RomanusD, CherepanovD, NearyMP. Healthcare and economic impact of diarrhea in patients with carcinoid syndrome. World J Gastroenterol. 2016;22(6):2118–2125. doi:10.3748/wjg.v22.i6.211826877616
- DasariA, JoishVN, Perez-OlleR, et al. Work productivity burden and indirect costs associated with carcinoid syndrome diarrhea. Expert Rev Pharmacoecon Outcomes Res. 2020;20:507–511.31448649
- KulkeMH, HörschD, CaplinME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35(1):14–23. doi:10.1200/JCO.2016.69.278027918724
- PavelME, HörschD, CaplinM, et al. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015;100(4):1511–1519. doi:10.1210/jc.2014-224725636046
- National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology – v2. 2020: neuroendocrine and adrenal tumors. Available from:http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed 728, 2020.
- DardenC, JoishVN, PriceMA, et al. Patient-reported activity impairment, work productivity loss, and carcinoid syndrome outcomes: interim analyses of the XERMELO patient registry. Presented at the North American Neuroendocrine Tumor Society virtual symposium; 102–3, 2020. Paper no. 113.
- PriceMA, JoishVN, SchwartzS, et al. XERMELO patient registry: improvements in clinical outcomes, patient satisfaction, and weight with telotristat ethyl in the real-world. In: Annual Symposium of the North American Neuroendocrine Tumor Society; Boston, Massachusetts; 103–5, 2019. Paper no. ID77.
- StrosbergJ, JoishVN, GiacaloneS, et al. TELEPRO: patient-reported carcinoid syndrome symptom improvement following initiation of telotristat ethyl in the real world. Oncologist. 2019;24(11):1446–1452. doi:10.1634/theoncologist.2018-092131189618
- BensonAB, StrosbergJ, JoishVN, et al. Clinical benefits of telotristat ethyl in patients with neuroendocrine tumors and low bowel movement frequency: an observational patient-reported outcomes study. Pancreas. 2020;49(3):408–412. doi:10.1097/MPA.000000000000149632132510
- HudgensS, GableJ, KulkeM, et al. Evaluation of meaningful change in bowel movement frequency for patients with carcinoid syndrome. Presented at the annual meeting of the Gastrointestinal Cancers Symposium; January 19–21; 2017; San Francisco, CA.
- Food and Drug Administration. Center for drug evaluation and research. Summary Review. Application Number 208794Orig1s000. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208794Orig1s000SumR.pdf. Accessed 1019, 2018.
- SiddiquiEJ, ShabbirMA, MikhailidisDP, MumtazFH, ThompsonCS. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 2006;97:634–639. doi:10.1111/j.1464-410X.2006.06056.x16469039
- IshizukaJ, BeauchampRD, TownsendCM, GreeleyGH, ThompsonJC. Receptor-mediated autocrine growth-stimulatory effect of 5-hydroxytryptamine on cultured human pancreatic carcinoid cells. J Cell Physiol. 1992;150(1):1–7. doi:10.1002/jcp.10415001021309821
- DrozdovI, KiddM, GustafssonBI, et al. Auto-regulatory effects of serotonin on proliferation and signaling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer. 2009;115(21):4934–4945. doi:10.1002/cncr.2453319634160
- JoishVN, ShahS, TierceJC, et al. Serotonin levels and 1-year mortality in patients with neuroendocrine tumors: a systematic review and meta-analysis. Future Oncol. 2019;15(12):1397–1406. doi:10.2217/fon-2018-096030734573
- Lexicon Pharmaceuticals, Inc. Xermelo® (telotristat ethyl) prescribing information; 2017. Available from: https://www.xermelo.com/Media/Default/pdfs/Product_Info_telotristat_ethyl.pdf. Accessed 36, 2020.