Abstract
Whole-gland low-dose rate (LDR) brachytherapy has been a well-established modality of treating low-risk prostate cancer. Treatment in a focal manner has the advantages of reduced toxicity to surrounding organs. Focal treatment using LDR brachytherapy has been relatively unexplored, but it may offer advantages over other modalities that have established experiences with a focal approach. This is particularly true as prostate cancer is being detected at an earlier and more localized stage with the advent of better detection methods and newer imaging modalities.
Introduction
Prostate cancer is the most common malignancy in men, with an estimated incidence in the United States of 241,740 cases and 28,170 deaths in 2012 (Surveillance, Epidemiology and End Results [SEER] stats).Citation1 Low-dose rate (LDR) brachytherapy has been an effective form of definitive therapy for low-risk localized prostate cancer with excellent long-term outcomes.Citation2–Citation5 Traditionally, LDR brachytherapy has been delivered to the whole gland of the prostate. While the outcomes have proven successful, it is not without adverse effects that could include urinary, rectal, and sexual toxicities that may affect a patient’s quality of life.Citation6 Treating a portion of the gland or a focal region of well-identified disease within the prostate gland may offer an option of reducing the toxic effects of treatment while maintaining similar treatment outcomes when compared with whole-gland therapy. Advantages in quality of life could be exhibited in the form of reduced urinary incontinence, rectal symptoms, and improved erectile and prostatic gland function.
Approaches to partial-prostate gland treatment include hemigland therapy, the addition of a focal boost to whole-gland therapy, and finally treatment of a focal region alone. These distinctions are important, as the term focal therapy at times has been used interchangeably in the past to represent one of these partial approaches.
The experience with focal LDR brachytherapy in prostate cancer to date is limited. There are ongoing trials evaluating hemigland and focal approaches in selected patients,Citation7,Citation8 and there has been experience with delivering LDR brachytherapy to the peripheral zone alone.Citation9 Much of the experience with a focal approach in itself has been seen with ablative techniques that include high-intensity focused ultrasound (HIFU) and cryotherapy. Other, less-studied partial approaches to deliver radiotherapy include hemigland high-dose rate (HDR) brachytherapyCitation10 and a focal boost to a course of whole-gland radiation. In the latter experiences, the focal boost has been delivered with differential dose external beam radiation, differential HDR brachytherapy, and LDR brachytherapy.Citation11–Citation14
Selecting appropriate patients for focal therapy will be a vital component of exploring this therapeutic modality as an option. Prostate cancer is being detected at an earlier and more localized stage of disease with the widespread use of prostate-specific antigen (PSA) detection as well as improvement in biopsy and imaging techniques.Citation15 Such advances offer improvements in selecting patients with focal low-disease burden while excluding those with multifocal disease to preferentially select patients for focal treatment.Citation16,Citation17
Here, we review various partial and focal therapy experiences to date, evaluate the advancements in disease detection and their role in the future of selecting patients for focal LDR brachytherapy, and assess aspects of LDR brachytherapy delivery in the context of focal treatment.
Focal therapy experience
Much of the experience with focal therapy to date has been with ablative techniques that include HIFU and cryotherapy. In properly selected groups, these techniques have shown good results in terms of outcomes and toxicities. For example, in one focal HIFU experience, a 2-year biochemical disease-free survival rate of 83.3% was reported.Citation18 It was observed that the focally treated group had decreased the indwelling catheter period, frequency of urinary stricture, and urinary tract infection in comparison to whole-gland treatment. In another series described as hemiprostate HIFU ablation, the 10-year overall survival was reported at 83% and cancer-specific survival at 100%. All patients maintained urinary continence.Citation19 In the focal cryotherapy experience, one report indicated a biochemical disease-free survival rate of 88% over a median follow-up time of 28 months, with maintenance of continence and potency at 71%. In another experience, the biochemical disease-free survival rate was 95% over a mean follow-up time of 3.6 years, with maintenance of continence and sexual potency rates of 85%.Citation20–Citation22 These experiences and other focal HIFU and cryotherapy experiences are summarized in .
Table 1 Summary of partial and focal therapy modalities reported
The dosimetric feasibility to plan hemigland HDR brachytherapy has been evaluated. In this study, hemigland treatment plans were compared to whole-gland treatment plans. Hemigland dosimetric assessment revealed effective target coverage, and doses to the rectum, bladder, and urethra were reduced significantly. The degree of reduction in the dose to 2cc to these organs was from 64.1%–53.1% for the rectum, 67.5%–55.9% for the bladder, and 95.2%–69.3% to the urethra.Citation10 The addition of a focal radiation boost by stereotactic radiation or HDR brachytherapy to a course of whole-gland external beam radiation has also been studied.Citation11–Citation13 In one experience, patients received a conventionally fractionated external radiation dose to 64–64.4 Gy followed by a hypofractionated course of 5–7 Gy in two fractions delivered stereotactically.Citation11 In another experience, a hypofractionated course of external radiation was delivered stereotactically to a dose of 38 Gy in four daily fractions, followed by an integrated boost to 11 Gy per fraction to the dominant lesion if visible on magnetic resonance imaging (MRI).Citation12 With HDR brachytherapy as a partial boost, one experience treated patients with external beam radiation doses of 64–64.4 Gy, followed by either a bilateral or unilateral HDR brachytherapy boost. Unilateral HDR boost doses ranged from 12–16 Gy in two fractions.Citation13 LDR brachytherapy to the whole gland with a concomitant brachytherapy boost to identified lesions has been evaluated as well. In these experiences, the feasibility of using magnetic-resonance spectroscopy imaging (MRSI) to identify tumor deposits and deliver additional dose to these regions with I125 seeds was demonstrated. Improved estimated tumor control probability was shown while maintaining acceptable dose constraints to the urethra.Citation14 While the outcomes of focal boost added to whole-gland treatments cannot easily be compared to hemigland or focal experiences, acceptable and, in some cases, reduced toxicity were demonstrated and provided a basis for a movement toward a focal delivery of radiation.
LDR brachytherapy for prostate cancer has been used for a number of decades with the majority of recent experiences emerging from the 1980s with the introduction of transrectal ultrasound to plan and guide the delivery of treatment. LDR brachytherapy has traditionally been delivered to the whole gland with excellent long-term outcomes. Fifteen-year biochemical relapse-free survival has been reported at 85.9% for low-risk patientsCitation3 and 10-year disease-specific survival at 96%.Citation4 Toxicities primarily involve urinary, rectal, and sexual function. Urinary symptoms exhibited as mild obstructive or irritative symptoms may be seen in 50% of patients in the immediate postimplant period, with 90% returning to their baseline urinary function by 12 months, and <3% requiring surgical management to relieve obstructive urinary symptoms long term.Citation23–Citation26 Rectal irritation may be seen in up to 30% of patients within the frst 2 years which resolves, while rectal bleeding may be seen in up to 7% of patients, and rectal fistulas in <1% of patients.Citation27,Citation28 Erectile function is maintained in 50%–80% of patients with variations dependent on pretreatment erectile function, age at the time of treatment, and pharmacologic assistance.Citation25,Citation29 While the use of LDR brachytherapy for whole-gland treatment is very well established, there is very limited data with its use in focal-only treatment. There is one experience in which LDR brachytherapy was delivered to the peripheral zone alone. The PSA failure-free survival for low-risk patients at 5 and 8 years was 95.6% and 90.0%, respectively.Citation9 The successful experience and established dose response with whole-gland LDR brachytherapy,Citation5,Citation30 along with acceptable toxicities, may have reduced the motivation toward a focal approach. However, with the advent of better disease detection and the potential for further minimizing toxicity, greater experience with focal LDR brachytherapy should be considered. As such, its use in a focal manner is currently explored with protocols looking at hemiglandCitation8 and focal LDR brachytherapy.Citation7
Selection of appropriate patients
Selecting appropriate patients for focal therapy would be of utmost importance, specifically patients with low-disease burden that is confined to a portion of the prostate gland. Advances in ultrasound and MRI techniques, as well as tissue sampling, have enhanced the ability to select these patients. Advances in ultrasound techniques include B mode ultrasound, color Doppler imaging, and contrast-enhanced ultrasound. These techniques can assist in localizing tumors based on vascular variance and tumor tissue differences.Citation31–Citation34 Ultrasound imaging, based on spectrum analysis of radiofrequency signals, has shown good biopsy correlation.Citation35,Citation36 In addition, there is some evidence that elastography and histoscanning may add diagnostic value as well.Citation37,Citation38
The use of MRI is gaining in popularity for the diagnosis and planning of prostate therapy. Localization of tumors within the prostate gland has improved with the use of multiparametric MRI, which adds functional sequences that include diffusion weighting, dynamic contrast enhancement, and MRI spectroscopy. It has been reported that dynamic contrast-enhanced imaging has a sensitivity of 86%–90% and a specificity of 88%–94% in identifying a tumor sized at 0.5 mL.Citation39–Citation41 As well, identification of tumors down to 0.2 mL in size has been reported, particularly in the intracapsular transition or peripheral zone.Citation39–Citation41 MRI spectroscopy has shown a sensitivity of 91.9% and specificity of 98.3%.Citation42 When correlated with Gleason score, MRI has been shown to have good sensitivity and specificity in identifying tumors with more aggressive disease.Citation43,Citation44 One important consideration is that MRI results may be obscured by biopsy changes, and it is recommended that MRI evaluation should be performed either prior to biopsy or no earlier than 6 weeks following biopsy.Citation45
With respect to tissue diagnosis, optimizing tissue sampling would also be imperative in identifying populations appropriate for focal therapy. One aspect to consider is the inhomogeneity in tissue sampling and interpretation standards. This includes the variability in the number of cores taken, biopsy length, and location sampled by the clinician as well as tissue processing and interpretation by the pathologist.Citation46,Citation47 This may lead to undergrading in as many as 30% of the cases and understaging in as many as 25% of the cases.Citation48 As a result, guidelines for the standardization and optimization of tissue sampling would be an important component of candidacy for focal therapy.
The common standard for prostate biopsy involves six to twelve transrectal biopsy cores. It has been shown that this may not be adequate for identifying patients for focal therapy, as it may result in missing the disease in 31% of cases.Citation49,Citation50 MRI-guided biopsy could be considered, as it has been shown to have greater detection rate when compared to transrectal ultrasound-guided biopsy and has a detection rate of 59% after an initial negative biopsy.Citation51 A better method of pathologically mapping the prostate is by transperineal three-dimensional mapping biopsy with a 5 mm template.Citation52 This has been shown to identify bilateral disease in 39% of cases that were found to be negative on transrectal ultrasound-guided biopsy.Citation48
Review of biopsy findings suggests that the expected number of cases with unifocal disease may be in the range of 13%–35%.Citation53,Citation54 Of those with low-risk unifocal disease, only 1% displayed extracapsular extension.Citation55 In cases where multifocal disease was observed, it was found that 97% of index lesions displayed the same Gleason score as the overall cancer.Citation56
While advances in imaging techniques as well as tissue sampling will help identify patients appropriate for focal therapy, other factors may need to be considered. These additional factors can help select patients who are likely to have minimal toxicity from brachytherapy and may have low-risk, low-volume disease that could be appropriate for focal therapy. A multidisciplinary international consensus group has proposed a number of factors to consider, which include life expectancy, PSA burden, the use of multiparametric MRI, the use of bilateral template-guided prostate-mapping biopsy, unilateral disease, Gleason score of 6–7, and tumor stage (≤T2b) ().Citation57 In comparison, in the Phase II hemigland brachytherapy protocol, the inclusion criteria include life expectancy, pathology, performance status, stage (T1c–T2a), PSA value, MRI evidence of unilateral disease, and prostate size ().Citation8 As well, the exclusion criteria for this protocol include inability to tolerate anesthesia, extracapsular extension involvement, International Prostate Symptom Score (IPSS),Citation58 ability to undergo MRI, and prior radiation ().
Table 2 Multidisciplinary international consensus group proposal selection
Table 3 Phase II hemigland protocol inclusion criteria
Table 4 Phase II hemigland protocol exclusion criteria
Technique
LDR brachytherapy is delivered using permanently implanted radioactive seeds that allow the delivery of very high doses of radiation to the target while limiting toxicities to organs at risk. Focal LDR brachytherapy would entail the insertion of such radioactive seeds into regions with well-identified lesions within the prostate while sparing the remainder of the gland and further reducing toxicity. Brachytherapy may offer certain advantages over other modalities, which include the geographic flexibility of shaping the treatment region by positioning the radioactive seeds as well as clear radiologic delineation of the region treated once implanted. As with whole-gland LDR brachytherapy, there may be differing opinions on certain aspects of delivering focal LDR brachytherapy. Methodological considerations include preplanning versus intraoperative planning, stranded versus loose seeds, and the choice of isotope. The focal brachytherapy consensus panel recommended a preplanned approach, although they did suggest that intraoperative planning software to monitor the procedure would be desirable.Citation57 In contrast, the Phase II hemigland brachytherapy protocol uses an intraoperative planning approach and utilizes information/coordinates from the biopsy and the dominant tumor on the preoperative endorectal or pelvic coil MRI. In that protocol, the MRI information is fused to the intraoperative ultrasound images.Citation8 With respect to stranded versus loose seeds, the consensus panel felt there may be a reduced potential for seed migration with stranded seeds, particularly at the periphery. However, some investigators have found that stranded seeds may migrate as well,Citation59 having potentially detrimental consequences in the setting of focal therapy. Loose seeds may offer the advantage of greater flexibility in geographic positioning of individual seeds, which may be of superior importance in focal treatment. This may be particularly true in cases of small lesions or lesions in close proximity to organs at risk. Isotopes used in LDR brachytherapy include I125, Pd103, and Cs131. Differences between the isotopes include relatively shorter half-lives with Pd103 and Cs131 when compared to I125, and higher energies with I125 and Cs131, when compared to Pd103. The guidelines for use of these isotopes should follow the general recommendations for whole-gland brachytherapy. For example, use of isotopes with shorter half-lives, compared to edema half-life, could potentially lead to lower delivered dose than intended. Higher energy isotopes may be more forgiving in the context of geographic miss, due to a less acute dose fall off. However, care should be taken when placing seeds of higher energy in the proximity of sensitive structures, such as the urethra. The choice of methodology may best be chosen based on the institution’s standard approach and familiarity with delivering whole-gland brachytherapy.
With respect to planning volume definitions and dosimetric parameters, the Phase II hemigland brachytherapy protocol defines the gross target volume as the visible disease on preoperative MRI that can be fused to the intraoperative ultrasound images. The clinical target volume represents a 3 mm margin beyond the gross target volume and the planning target volume would represent the involved hemilobe of the prostate gland. Normal tissue constraints include urethra (volume, in percent) V100 <130% of the prescription dose and a rectal V100 <100% of the prescription dose. In general, the computed tomography (CT) unit should be employed for the focal brachytherapy procedure, to confirm accurate placement of the seeds. Specific to the Phase II protocol, if it were determined based on the scan that there was a deficient dose region; this could be corrected with the placement of additional seeds after regeneration of the treatment plan and prior to the reversal of anesthesia. An example of a hemigland implant, with contours and isodose lines is shown in . In comparison, a representative image of a focal implant, with contours and isodose lines, is shown in .
Figure 1 Hemigland brachytherapy implant.
Note: Computed tomography (contours of the prostate in red, target in yellow, rectum in blue. 100%, 150%, and 200% isodose lines in green, yellow, and red). The green triangles and circles indicate seed positions.
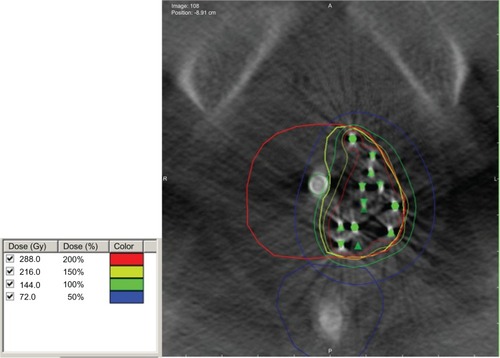
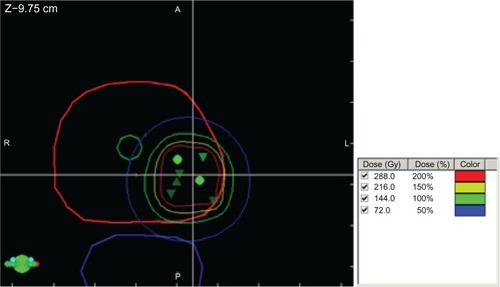
Figure 2 Representative image of a focal brachytherapy implant.
Notes: Contours of the prostate in red, urethra in green, and rectum in blue. The 100%, 150%, and 200% isodose lines are in green, yellow, and red. The green triangles and circles indicate seed positions.
Similar to whole-gland brachytherapy, routine postimplantation CT scan should be obtained for quality-assurance assessment of the adequacy of the implanted region. For whole-gland brachytherapy, the American Brachytherapy Society (ABS) recommends a postimplantation CT scan to be performed within 60 days of the implant. The optimum timing of the CT to minimize edema-derived dosimetry error is radionuclide specific: 16 days ± 4 days for Pd103 and Cs131; and 30 days ± 7 days for I125. A number of dosimetric parameters for the prostate, rectum, and urethra to assess implant quality have also been defined.Citation60 These recommendations can be used as a guide to assess the adequacy of a partially implanted prostate. While the prostatic D90 (dose, in Gy and percent, received by 90% of the volume) for whole-gland brachytherapy has been used, due to the reduced target volume in partial-gland brachytherapy, its significance in relation to outcome is in question. However, the V100 and V150 for a partial-gland target, whether hemigland or a focal target, should be assessed. A V100 of at least 95% is desired while the V150 is radionuclide specific, but generally should be equal or less than 50%–60%. For the urethra, the V150 (in volume), V5 (in percent), V30 (in percent), and the V100 for the rectum have been defined.Citation61 Dosimetric goals for the urethra and rectum in whole-gland brachytherapy include a V5 <150% and V30 <125% for the urethra and a V100 of <1 cm3 on day 1 and <1.3 cm3 on day 30 dosimetry for the rectum.Citation61 These goals can be considered acceptable constraints for partial-prostate brachytherapy dosimetry.
Other critical structures important for erectile dysfunction have been evaluated, including the internal pudendal artery, penile bulb, and neurovascular bundles; however, this is up for debate and is not recommended to be reported routinely.Citation62–Citation64
Post-treatment care
Patients should be evaluated on a regular basis post-treatment in a fashion similar to that performed for whole-gland therapy. In addition, the assessment of quality of life would be of particular importance, as a goal of focal therapy would be to reduce potential toxicities. Assessments would focus on erectile, urinary, and rectal function, as well as overall general health. A number of quality-of-life scales have been developed and used that could be applied to this population. The Memorial Sloan-Kettering Cancer Center Prostate-Health Related Quality of Life QuestionnaireCitation65 includes erectile function assessments derived from the Erectile Function Domain of the International Index of Erectile Function Questionnaire,Citation66 as well as urinary and rectal function, and overall general health assessments. Other assessment scales include the IPSS, Expanded Prostate Cancer Index Composite, Short Form-36, International Index of Erectile Function 15, European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire C30, EORTC quality of life questionnaire Pr25, pain score, and urinary diaries.Citation67–Citation70
One challenge of patients treated with focal brachytherapy is finding the optimal method to monitor for disease recurrence. While some focal experiences to date have used the traditional method of PSA as a measure of outcome, the traditional definitions of PSA failure would not fully apply, due to the presence of normal prostatic tissue that was not treated. One proposal for better prediction of clinical failure following focal therapy is PSA velocity greater than 0.75 ng/mL per year, in addition to nadir +2.Citation9 Significant changes in PSA could be used as an indicator to warrant further tests to rule out recurrence, but it is clear that PSA evaluations alone would not be an effective method of assessing response or monitoring for recurrence. Other biomarkers to follow prostate cancer are being studied and could find utility in following patients with partial prostate treatment. These include transmembrane protease serine 2-ETS (erythroblast transformation-specific) related gene fusion,Citation71 the phosphatase and tensin homolog gene, prostate cancer gene 3, and urinary engrailed-2.Citation72
An advantage with partial prostate treatment is that digital rectal examination could be helpful in detecting new abnormalities in the untreated region, which could warrant further investigations, such as a biopsy. However, similar to whole-gland treatment, a digital rectal examination in the treated area may be difficult to interpret due to soft tissue changes, such as fibrosis, post-treatment.
As previously discussed, multiparametric MRI, with functional sequences that include diffusion weighting, dynamic contrast enhancement, and MRI spectroscopy, is a major advancement in imaging that will play a vital role in identifying patients that will be appropriate for partial prostate brachytherapy. Postirradiation, there are morphologic changes that include inflammation, atrophy, and fibrosis that can pose challenges with traditional MRI evaluation. The addition of multiparametric MRI would be helpful to identify recurrent lesions in the treated area or new lesions in the untreated area. Its value has been demonstrated in detecting recurrent disease after HIFU ablationCitation73,Citation74 or whole-gland external beam radiotherapy,Citation75,Citation76 and has been described in the setting of recurrence after focal therapy with HIFU, cryotherapy, external radiation, and brachytherapy.Citation77
Interval biopsy, in conjunction with multiparametric MRI, should be considered as a follow-up tool. By sampling all areas of the prostate, potential recurrent lesions in the treated area or new lesions in the untreated areas can be identified. This is the only method to confirm the presence of disease. The follow-up regimen, outlined by the ongoing Phase II hemigland brachytherapy protocol, includes clinic evaluations at 3, 6, 12, 18, and 24 months after implantation, an MRI at 6 months and 24 months, and a transrectal or transperineal biopsy at 12 months and 24 months after the brachytherapy implant.
Failure after initial partial-prostate brachytherapy may occur either as a recurrence in the treated area, as a new lesion in the untreated areas, or both. In the setting of recurrence after whole-gland radiation, there are a number of recognized salvage treatment options, which include HIFU, cryotherapy, external radiation, radical prostatectomy, or brachytherapy.Citation78–Citation80 However, it should be noted that each modality, as a salvage, has its own toxicity profiles to consider, and there is no consensus as to the optimal option after whole-gland radiation.Citation81 Because partial-gland brachytherapy is in its infancy, the optimal management of salvage for recurrent disease or retreatment of patients with new disease is even less clear, due to the limited experience. However, the salvage experiences for whole-gland therapy could be extrapolated as options. In addition, it could be hypothesized that initial partial-gland brachytherapy allows for the possibility of less toxicity when compared to salvage experiences after whole-gland radiation as well as the possibility to offer further partial-gland therapy to recurrent or new disease.
The limiting factor for retreatment with partial-gland brachytherapy would involve the normal tissue constraints, with the goals of maintaining the combined dosimetric parameters of the urethra, bladder, and rectum below the currently accepted constraints used for whole-gland brachytherapy. Therefore, salvage of a recurrent region or treatment of a new region with further partial-gland brachytherapy could potentially be done so long as the combined normal tissue constraints are met. However, while the presence of the brachytherapy seeds allows for the radiological delineation of the previously treated region, it may be a challenge to accurately combine the dosimetric plans, particularly in areas to be retreated or with new lesions that are in proximity to the previously treated regions. It is clear that further development of planning systems for this purpose and greater experience is needed to explore partial-gland brachytherapy as an option for retreatment or salvage.
Conclusion
The use of whole-gland LDR brachytherapy for localized prostate cancer has been well established. In comparison, the use of focal LDR brachytherapy is still in its early stages. Other modalities have shown promise in delivering focal treatment with comparable outcomes and reduced toxicity compared with whole-gland therapy. Extending these experiences to LDR brachytherapy is promising because of the well-established and successful experience with LDR brachytherapy for whole-gland treatment, and the added advantages of flexibility and the ease of transitioning a technique at an institution that may already have an established whole-gland brachytherapy program. The reduction in toxicity can be of significant importance particularly in a well-selected population. These include reduced rectal, urinary, and sexual toxicities. While it has not been established, it could also be hypothesized that an additional advantage of focal therapy could be the preservation of gland function and possibly the maintenance of fertility. A focal approach may also allow for an intermediary management option between active surveillance and initiating whole-gland therapy.Citation82 With the advent of recent developments in ultrasound and MRI as well as advances and standardization of tissue sampling and interpretation, the appropriate population with unifocal, low-risk disease may be identified as optimal candidates to consider for focal therapy. Reduction in toxicity may lead to improved quality of life without the reduction in outcome. Post-treatment monitoring will have its challenges since the PSA alone would be an inadequate measure for disease recurrence. Digital rectal examination, multiparametric MRI, and interval biopsy will be important components of detecting disease recurrence in the treated area or new disease in the untreated areas. The optimal management for disease recurrence after partial-gland brachytherapy is unclear and the options may need to be considered on a case by case basis. While there is limited data for the use of partial-gland LDR brachytherapy, there are ongoing studies evaluating hemigland and focal approaches, and further analysis and studies are required to establish the validation and feasibility of offering focal LDR brachytherapy as an option to selected patients.
Disclosure
Yoshiya Yamada is a consultant for Varian Medical Systems, Palo Alto, CA, USA, and a member of the Speakers Bureau for the Institute for Medical Education. The authors report no other conflicts of interest in this work.
References
- National Cancer Institute [homepage on the Internet]Previous version: SEER cancer statistics review, 1975–2009 (Vintage 2009 Populations)US National Institutes of Health2012 [updated August 20, 2012]. Available from http://seer.cancer.gov/csr/1975_2009_pops09/index.htmlAccessed June 23, 2013
- HeidenreichABellmuntJBollaMEAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised diseaseEur Urol2011591617121056534
- SylvesterJEGrimmPDWongJGalbreathRWMerrickGBlaskoJCFifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experienceInt J Radiat Oncol Biol Phys201181237638120864269
- StockRGCesarettiJAStoneNNDisease-specific survival following the brachytherapy management of prostate cancerInt J Radiat Oncol Biol Phys200664381081616309852
- ZelefskyMJKubanDALevyLBMulti-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantationInt J Radiat Oncol Biol Phys200767232733317084558
- SandaMGDunnRLMichalskiJQuality of life and satisfaction with outcome among prostate-cancer survivorsN Engl J Med2008358121250126118354103
- CossetJMWakilGPierratNCathelineauXMarchandVVallancienGPoster 710. Focal brachytherapy for prostate cancer: a pilot studyRadiotherapy and Oncology201199S283
- Memorial Sloan-Kettering Cancer CenterPhase II study assessing the potential for reduced toxicity using focal brachytherapy early stage, low volume in prostate cancerClinicalTrials.gov [website on the Internet]New York, NYMemorial Sloan-Kettering Cancer Center2011 [updated May 13, 2013]. Available from: http://clinicaltrials.gov/ct2/show/NCT01354951?term=focal+prostate&rank=5. NLM identifier: NCT01354951Accessed June 23, 2013
- NguyenPLChenMHZhangYUpdated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapyJ Urol201218841151115622901567
- KamravaMChungMPKayodeOFocal high-dose-rate brachytherapy: a dosimetric comparison of hemigland vs conventional whole-gland treatmentBrachytherapy Epub2013211
- MiralbellRMollàMRouzaudMHypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: a sequential dose escalation pilot studyInt J Radiat Oncol Biol Phys2010781505719910135
- AluwiniSvan RooijPHoogemanMKirkelsWKolkman-DeurlooIKBangmaCStereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: early resultsRadiat Oncol201388423570391
- SchickUPopowskiYNouetPHigh-dose-rate brachytherapy boost to the dominant intra-prostatic tumor region: hemi-irradiation of prostate cancerProstate201171121309131621308714
- ZaiderMZelefskyMJLeeEKTreatment planning for prostate implants using magnetic-resonance spectroscopy imagingInt J Radiat Oncol Biol Phys20004741085109610863082
- CooperbergMRLubeckDPMengMVMehtaSSCarrollPRThe changing face of low-risk prostate cancer: trends in clinical presentation and primary managementJ Clin Oncol200422112141214915169800
- BottSRAhmedHUHindleyRGAbdul-RahmanAFreemanAEmbertonMThe index lesion and focal therapy: an analysis of the pathological characteristics of prostate cancerBJU Int2010106111607161120553262
- KaravitakisMWinklerMAbelPLivniNBeckleyIAhmedHUHistological characteristics of the index lesion in whole-mount radical prostatectomy specimens: implications for focal therapyProstate Cancer Prostatic Dis2011141465220498680
- MutoSYoshiiTSaitoKKamiyamaYIdeHHorieSFocal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancerJpn J Clin Oncol200838319219918281309
- El FegounABBarretEPrapotnichDFocal therapy with high-intensity focused ultrasound for prostate cancer in the elderly. A feasibility study with 10 years follow-upInt Braz J Urol2011372213219 discussion 220–22221557838
- BahnDde Castro AbreuALGillISFocal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 yearsEur Urol2012621556322445223
- LambertEHBolteKMassonPKatzAEFocal cryosurgery: encouraging health outcomes for unifocal prostate cancerUrology20076961117112017572198
- OnikGVaughanDLotenfoeRDineenMBradyJ“Male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-upUrol Oncol200826550050518774463
- KeyesMMillerSMoravanVPredictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patientsInt J Radiat Oncol Biol Phys20097341023103219111402
- CrookJFleshnerNRobertsCPondGLong-term urinary sequelae following 125iodine prostate brachytherapyJ Urol2008179114114517997424
- StoneNNStockRGLong-term urinary, sexual, and rectal morbidity in patients treated with iodine-125 prostate brachytherapy followed up for a minimum of 5 yearsUrology200769233834217320674
- KeyesMMillerSMoravanVUrinary symptom fare in 712 125I prostate brachytherapy patients: long-term follow-upInt J Radiat Oncol Biol Phys200975364965519211199
- KeyesMSpadingerILiuMRectal toxicity and rectal dosimetry in low-dose-rate (125)I permanent prostate implants: a long-term study in 1006 patientsBrachytherapy201211319920821763213
- TranAWallnerKMerrickGRectal fistulas after prostate brachytherapyInt J Radiat Oncol Biol Phys200563115015416111583
- CrookJBorgJEvansA10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patientsInt J Radiat Oncol Biol Phys20118051323132920675072
- PollackAZagarsGKStarkschallGProstate cancer radiation dose response: results of the MD Anderson phase III randomized trialInt J Radiat Oncol Biol Phys20025351097110512128107
- HalpernEJFrauscherFStrupSENazarianLNO’KanePGomellaLGProstate: high-frequency Doppler US imaging for cancer detectionRadiology20022251717712354987
- MitterbergerMHorningerWPelzerAA prospective randomized trial comparing contrast-enhanced targeted versus systematic ultrasound guided biopsies: impact on prostate cancer detectionProstate200767141537154217705242
- NewmanJSBreeRLRubinJMProstate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy siteRadiology1995195186907534429
- RoyCBuyXLangHSaussineCJacqminDContrast enhanced color Doppler endorectal sonography of prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedureJ Urol20031701697212796647
- FeleppaEJFairWRLiuTThree-dimensional ultrasound analyses of the prostateMol Urol20004313313911062367
- FeleppaEJFairWRTsaiHProgress in two-dimensional and three-dimensional ultrasonic tissue-type imaging of the prostate based on spectrum analysis and nonlinear classifiersMol Urol19993330331010851337
- BraeckmanJAutierPSovianyCThe accuracy of transrectal ultrasonography supplemented with computer-aided ultrasonography for detecting small prostate cancersBJU Int2008102111560156518710457
- HoytKCastanedaBZhangMTissue elasticity properties as biomarkers for prostate cancerCancer Biomark200844–521322518957712
- NishidaSKinoshitaHMishimaTKurokawaHSakaidaNMatsudaTProstate cancer detection by prebiopsy 3.0-Tesla magnetic resonance imagingInt J Urol201118965365821790792
- PuechPPotironELemaitreLDynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimensUrology20097451094109919773038
- VillersAPuechPMoutonDLeroyXBallereauCLemaitreLDynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findingsJ Urol20061766 Pt 12432243717085122
- YamamuraJSalomonGBuchertRMR imaging of prostate cancer: diffusion weighted imaging and (3D) hydrogen 1 (H) MR spectroscopy in comparison with histologyRadiol Res Pract2011201161685222091382
- ArumainayagamNAhmedHUMooreCMFreemanASohaibAKirkhamAA negative multi-parametric MRI can rule out up to 97% of clinically significant prostate cancerEur Urol Suppl20111026667
- KobusTHambrockTHulsbergen-van de KaaCAIn vivo assessment of prostate cancer aggressiveness using magnetic resonance spectroscopic imaging at 3 T with an endorectal coilEur Urol20116051074108021419565
- DickinsonLAhmedHUAllenCMagnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meetingEur Urol201159447749421195536
- BostwickDGMeiersIProstate biopsy and optimization of cancer yieldEur Urol200649341541716442209
- BostwickDGQianJDrewnowskaKProstate needle biopsy quality in reduction by dutasteride of prostate cancer events study: worldwide comparison of improvement with investigator training and centralized laboratory processingUrology20107561406141019942263
- OnikGMiessauMBostwickDGThree-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer managementJ Clin Oncol200927264321432619652073
- FalzaranoSMZhouMHernandezAVMoussaASJonesJSMagi-GalluzziCCan saturation biopsy predict prostate cancer localization in radical prostatectomy specimens: a correlative study and implications for focal therapyUrology201076368268720206973
- SinnottMFalzaranoSMHernandezAVDiscrepancy in prostate cancer localization between biopsy and prostatectomy specimens in patients with unilateral positive biopsy: implications for focal therapyProstate201272111179118622161896
- HambrockTSomfordDMHoeksCMagnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigenJ Urol2010183252052720006859
- AhmedHUHuYCarterTCharacterizing clinically significant prostate cancer using template prostate mapping biopsyJ Urol2011186245846421679984
- HorningerWBergerAPRogatschHCharacteristics of prostate cancers detected at low PSA levelsProstate200458323223714743461
- MastersonTAChengLMehanRMKochMOTumor focality does not predict biochemical recurrence after radical prostatectomy in men with clinically localized prostate cancerJ Urol2011186250651021679993
- OhoriMEasthamJAKohHKuroiwaKSlawinKMWheelerTMIs focal therapy reasonable in patients with early stage prostate cancer (CaP) – an analysis of radical prostatectomy (RP) specimensJ Urol2006Suppl 175507
- AroraRKochMOEbleJNUlbrightTMLiLChengLHeterogeneity of Gleason grade in multifocal adenocarcinoma of the prostateCancer2004100112362236615160339
- LangleySAhmedHUAl-QaisiehBReport of a consensus meeting on focal low dose rate brachytherapy for prostate cancerBJU Int2012109Suppl 171622239224
- Urology Specialists, PCInternational Prostate Symptom Score (I-PSS)Middlebury, CT, USAUrology Specialists, PC2013 Available from: http://www.urospec.com/uro/Forms/ipss.pdfAccessed June 23, 2013
- SaibishkumarEPBorgJYeungICummins-HolderCLandonACrookJSequential comparison of seed loss and prostate dosimetry of stranded seeds with loose seeds in 125I permanent implant for low-risk prostate cancerInt J Radiat Oncol Biol Phys2009731616818823713
- DavisBJHorwitzEMLeeWRAmerican Brachytherapy SocietyAmerican Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapyBrachytherapy201211161922265434
- SalembierCLavagniniPNickersPGEC ESTRO PROBATE GroupTumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapyRadiother Oncol200783131017321620
- BuyyounouskiMKHorwitzEMUzzoRGThe radiation doses to erectile tissues defined with magnetic resonance imaging after intensity-modulated radiation therapy or iodine-125 brachytherapyInt J Radiat Oncol Biol Phys20045951383139115275723
- GillanCKirilovaALandonAYeungIPondGCrookJRadiation dose to the internal pudendal arteries from permanent-seed prostate brachytherapy as determined by time-of-flight MR angiographyInt J Radiat Oncol Biol Phys200665368869316626892
- MerrickGSButlerWMWallnerKEThe importance of radiation doses to the penile bulb vs crura in the development of postbrachytherapy erectile dysfunctionInt J Radiat Oncol Biol Phys20025441055106212419431
- BefortCAZelefskyMJScardinoPTBorrayoEGieslerRBKattanMWA measure of health-related quality of life among patients with localized prostate cancer: results from ongoing scale developmentClin Prostate Cancer20054210010816197610
- RosenRCRileyAWagnerGOsterlohIHKirkpatrickJMishraAThe international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunctionUrology19974968228309187685
- BarryMJFowlerFJJrO’LearyMPThe American Urological Association symptom index for benign prostatic hyperplasiaJ Urol19921485154915571279218
- WeiJTDunnRLLitwinMSDevelopment and Validation of the Expanded Prostate Cancer Index Composite (EPIC) for Comprehensive Assessment of Health-Related Quality of Life in Men with Prostate CancerUrology200056689990511113727
- GroenvoldMKleeMCSprangersMAValidation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreementJ Clin Epidemiol19975044414509179103
- van AndelGBottomleyAFossåSDAn international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancerEur J Cancer200844162418242418774706
- RajputABMillerMADe LucaAFrequency of the TMPRSS2: ERG gene fusion is increased in moderate to poorly differentiated prostate cancersJ Clin Pathol200760111238124317259299
- MorganRBoxallABhattAEngrailed-2 (EN2): a tumor specific urinary biomarker for the early diagnosis of prostate cancerClin Cancer Res20111751090109821364037
- KimCKParkBKLeeHMKimSSKimEMRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancerAJR Am J Roentgenol200819051180118618430829
- RouvièreOGirouinNGlasLProstate cancer transrectal HIFU ablation: detection of local recurrences using T2-weighted and dynamic contrast-enhanced MRIEur Radiol2010201485519690866
- ArumainayagamNKumaarSAhmedHUAccuracy of multi-parametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapyBJU Int2010106799199720230392
- HaiderMAChungPSweetJDynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapyInt J Radiat Oncol Biol Phys200870242543017881141
- De VisscherePJDe MeerleerGOFüttererJJVilleirsGMRole of MRI in follow-up after focal therapy for prostate carcinomaAJR Am J Roentgenol201019461427143320489080
- AllenGWHowardARJarrardDFRitterMAManagement of prostate cancer recurrences after radiation therapy-brachytherapy as a salvage optionCancer200711071405141617685384
- NguyenPLD’AmicoAVLeeAKSuhWWPatient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literatureCancer200711071417142817694553
- ChalasaniVMartinezCHLimDChinJSalvage HIFU for recurrent prostate cancer after radiotherapyProstate Cancer Prostatic Dis200912212412918852702
- BoukaramCHannoun-LeviJMManagement of prostate cancer recurrence after definitive radiation therapyCancer Treat Rev420103629110020100637
- TsivianMAbernMRPolascikTJEvolution of the concept of focal therapy for prostate cancerOncology (Williston Park)2013271646823461042
- AhmedHUHindleyRGDickinsonLFocal therapy for localised unifocal and multifocal prostate cancer: a prospective development studyLancet Oncol201213662263222512844
- AhmedHUFreemanAKirkhamAFocal therapy for localized prostate cancer: a phase I/II trialJ Urol201118541246125421334018
- Eastern Cooperative Oncology Group [homepage on the Internet]ECOG performance status2011 [updated July 27, 2006]. Available from: http://www.ecog.org/general/perf_stat.htmlAccessed June 23, 2013