?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The aim of this study was to evaluate the cost-effectiveness of gefitinib plus chemotherapy (GCP) versus gefitinib alone for advanced non-small-cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations in China.
Methods
A decision-analytic Markov model was conducted to simulate the disease process of advanced NSCLC patients with EGFR mutations. Three distinct health states: progression-free survival (PFS), progressive disease (PD) and death were included. Clinical data were derived from the NEJ009 study. The cost was evaluated from the perspective of the Chinese society. Quality-adjusted life-years (QALYs) and incremental cost–effectiveness ratios (ICER) were calculated over a 10-year lifetime horizon. One-way sensitivity analysis and probabilistic sensitivity analysis were also performed to explore the uncertainty of parameters in the study.
Results
The base case analysis demonstrated that gefitinib plus chemotherapy gained 2.44 QALYs at an average cost of $59,571.34, while the effectiveness and cost of gefitinib group were 1.82 QALYs and $52,492.75, respectively. The ICER for gefitinib plus chemotherapy was $11,499.98 per QALY gained. The ICER was lower than the accepted willingness-to-pay (WTP) threshold, which was three times gross domestic product (GDP) per capita of China ($31,498.70 per QALY). Variation of parameters did not reverse the cost-effectiveness of gefitinib plus chemotherapy through univariable and probabilistic sensitivity analyses.
Conclusion
Our results showed that gefitinib plus chemotherapy is a cost-effective treatment option compared with gefitinib for advanced NSCLC patients with EGFR mutations in China.
Introduction
According to the global cancer statistics in 2020, there were 2.207 million new cases of lung cancer and 1.79 million associated deaths worldwide, ranking first among all cancers in mortality.Citation1,Citation2 In China, lung cancer is a malignant tumor with the highest incidence and mortality. It was estimated that 816,000 new lung cancer cases and 715,000 deaths occurred in China in 2020, accounting for 23.8% of all the cancer deaths.Citation3 The costs of diagnosis and treatment of lung cancer bring huge economic burden to both the country and society. NSCLC was the most common histological subtype, which accounted for approximately about 85% to 90% of all lung cancers.Citation2,Citation4,Citation5 The symptoms of NSCLC patients in the early stage are not typical, and most patients are advanced when they are newly diagnosed, so they can only receive palliative treatment. Approximately 35% to 40% of NSCLC patients are caused by epidermal growth factor receptor (EGFR) mutations in China,Citation6 and National Comprehensive Cancer Network (NCCN) guidelines recommend EGFR-TKIs for the first-line treatment of EGFR-mutated metastatic NSCLC.Citation7
Although EGFR-TKIs have significantly improved the PFS and quality of life (QoL) of advanced NSCLC patients with EGFR mutations, most patients cannot escape the fate of drug resistance. About 30% of the patients may lose the opportunity of follow-up treatment due to the rapid disease progression.Citation8 Compared with traditional chemotherapy, first-generation EGFR-TKIs did not bring significant extension of overall survival (OS) either in first-line use or sequential maintenance after chemotherapy. In order to overcome drug resistance and improve OS, the bottleneck of efficacy of single-drug therapy can be broken through the combination of EGFR-TKIs with chemotherapy via strategic adjustment. However, in the era without driver gene screening, 4 Phase III randomized controlled studies (INTACT1, INTACT2, TRIBUTE and TALENT) showed that combined with EGFR-TKIs (gefitinib or erlotinib) could not improve OS in patients with advanced NSCLC on the basis of first-line chemotherapy.Citation9–Citation12 The main reason for the negative results was that the EGFR mutation status in the treated population was not identified.
NEJ009 study is the first phase III clinical study comparing gefitinib alone with gefitinib plus two platinum-containing drugs (pemetrexed and carboplatin) in first-line treatment of advanced NSCLC patients with EGFR mutations,Citation13 and the results have attracted wide attention since they were announced at the 2018 American Society of Clinical Oncology (ASCO). The study met its primary endpoint, with median OS significantly longer in the combination group than in the monotherapy group. In addition, the PFS of the combined treatment group reached 20.9 months, even surpassing the data of 18.9 months for third-generation EGFR-TKI osimertinib for the first-line treatment of NSCLC in the FLAURA study,Citation14 which broke a new record for first-line treatment of EGFR mutant patients.
Although the NEJ009 study demonstrated a significant PFS and OS benefit, the economics of both treatments are unknown to the patients and physicians. The purpose of this study was to evaluate the cost-effectiveness of gefitinib plus chemotherapy compared with gefitinib alone in the treatment of advanced NSCLC patients with EGFR mutations from Chinese societal perspective.
Methods
NEJ009 Study
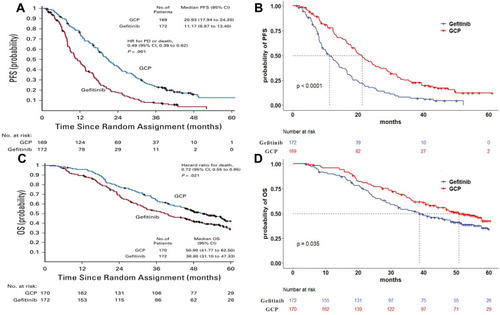
The clinical data was based on the results of the NEJ009 study, an open-label, randomized phase III trial comparing gefitinib alone with gefitinib plus chemotherapy for NSCLC patients with EGFR mutations.Citation13 A total of 345 eligible patients with newly diagnosed metastatic NSCLC with EGFR mutations were randomly assigned to gefitinib (gefitinib 250 mg orally per day) or GCP regimen (gefitinib 250 mg orally per day combined with carboplatin area under the curve 5 and pemetrexed 500 mg/m2 in a 3-week cycle for up to six cycles, followed by concurrent gefitinib and pemetrexed maintenance) until disease progression or the development of unacceptable toxic effects or death. The GCP group demonstrated a better median PFS than the gefitinib group (20.93 vs 11.17 months, HR 0.49, 95% CI 0.39 to 0.62, p<0.001), and median OS in the GCP group was also significantly longer than in the gefitinib group (50.9 vs 38.8 months, HR 0.722, 95% CI 0.55 to 0.95, p=0.021). The most frequently reported serious adverse events (SAEs, the rate of grade ≥3) in the GCP group were neutropenia, anemia, and thrombocytopenia compared with liver toxicity in the gefitinib group.
Markov Model
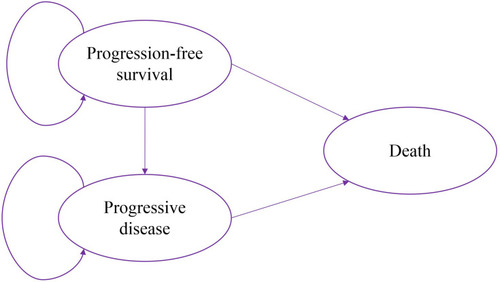
A Markov model was constructed using TreeAge Pro software (TreeAge Pro 2019, Williamstown, MA, USA) to estimate the cost and QALYs of GCP and gefitinib. The Markov model had three mutually exclusive health states including PFS, PD and death. It was assumed that all patients entered the model in the PFS state and could move to the other state or remain in the same state, and patients could only stay in the PD state or move to death after transferring to the PD state. The model diagram is shown in . A cycle length of 1 month was set to capture relevant changes in the health states, with a half-cycle correction applied to adjust for the timing of events. According to the survival curve, time of follow-up and treatment in the NEJ009 study, a total of 120 cycles of simulation, which was the equivalent of 10 years in the Markov model was adopted. A 3% annual discount rate was used for costs and effectiveness.Citation15
Survival Estimates and Utilities
Transition probabilities for the different health states were estimated from Kaplan–Meier survival curves obtained from the NEJ009 study. The Kaplan–Meier curves of PFS and OS for the two groups were read by GetData Graph Digitizer software (Version 2.26) to get the survival data. The Weibull distribution was fitted to the data for PFS and OS curves using R statistical software (version 4.0.5). The calculated scale parameter (λ) and shape parameter (γ) are presented in . The survival curve simulation results are shown in . Formula S(t)=exp(-λtγ) was used to calculate the survival probability at time t and we used formula P(t)=1-exp[λ(t-1)γ-λtγ] to estimate the transition probability at a given cycle t.Citation16,Citation17 The transition probability from PFS to death state is derived from the natural death rate of Chinese population in 2020 (0.707%).Citation18 Health utility values were obtained from a recently published study.Citation19,Citation20 The utility values of the PFS state, PD state and death were 0.804, 0.321 and 0, respectively.
Table 1 Weibull Parameters of Model Estimated for Progression-Free and Overall Survival Curves
Figure 2 (A) Kaplan–Meier curve of the progression-free survival from the NEJ009 study. (B) Simulate progression-free survival curve for the GCP group and the gefitinib group. (C) Kaplan–Meier curve of overall survival from the NEJ009 study. (D) Simulate overall survival curve for the GCP group and the gefitinib group.
Cost Estimates and Outcomes
Costs were estimated from the perspective of Chinese society. The cost of this study only covered direct medical costs, which included drug costs of gefitinib and chemotherapies, follow-up costs, supportive care costs, SAEs treatment costs, and terminal care costs. To calculate the drug costs of chemotherapy per cycle, a base-case patient with a body surface area of 1.72 m2 was assumed. The costs of follow-up included hospitalization expenses, the costs of outpatient-based physician visits, laboratory examination fees (inpatient and/or outpatient), and costs of computed tomography and magnetic resonance imaging. Once the disease progressed, patients were assumed to receive salvage chemotherapy.Citation21 SAEs’ management strategies were based on clinical practice and expert opinions, and SAEs related to costs were collected from the NEJ009 study as shown in . The costs of drugs and examinations were based on the 2020 fee standards of local hospitals in China. All costs were presented in US dollars, with an exchange rate of $1 =Ұ6.9 (2020). Details of the cost information are provided in .
Table 2 The Incidence and Expenditures of SAEs
Table 3 Costs, Utilities, and Discount Rates in the Model
Incremental cost–effectiveness ratio (ICER) was calculated to evaluate the outcomes. The treatment is considered affordable and economical when the ICER value is less than the willingness-to-pay (WTP) threshold. The formula of ICER is as follows:
The World Health Organization recommended that the increased cost was extremely cost-effectiveness when the ICER was less than GDP per capita (1 GDP), but could still count as cost-effectiveness if the ICER did not exceed three times GDP per capita (3 GDP).Citation15 Thus, we used $10,499.57 (1 GDP of China in 2020) per QALY and $31,498.70 (3 GDP of China in 2020) per QALY gained as the WTP threshold in different situations.Citation18
Sensitivity Analysis
One-way sensitivity and probabilistic sensitivity analyses were performed to evaluate the effect of the model uncertainty on the cost-effectiveness of different treatment options. A one-way sensitivity analysis kept other parameters unchanged, and altered individual model parameters in the range of variation, and then verified the effect of individual model parameters on the results. The key parameters in the model were changed with a range of ±20% of their baseline value to examine their impact on the results. Results of the one-way sensitivity analysis were represented by a tornado diagram. The probabilistic sensitivity analysis was performed to assess the effects of uncertainty in all model parameters simultaneously using a second-order Monte Carlo simulation for 1000 times to obtain an acceptable cost-effectiveness curve with different hypothetical WTP thresholds. The beta distribution was applied to the utilities, and the triangle distribution was applied to the others.
Results
Base-Case Analysis
The results of a base-case analysis with a 10-year time horizon, as well as economic and health outcomes estimated by the model, are shown in . The total costs of the GCP group and gefitinib group were $59,571.34 and $52,492.75, respectively. The overall QALYs in the GCP group were higher than those in the gefitinib group (2.44 QALYs vs 1.82 QALYs). The GCP group generated a gain of 0.62 QALYs over gefitinib group, resulting in an ICER of $11,499.98/QALY gained, which was lower than the commonly accepted threshold for cost-effectiveness (3 GDP, $31,498.70 per QALY in China).
Table 4 The Cost and Outcome Results of the Cost-Effectiveness Analysis
Sensitivity Analysis
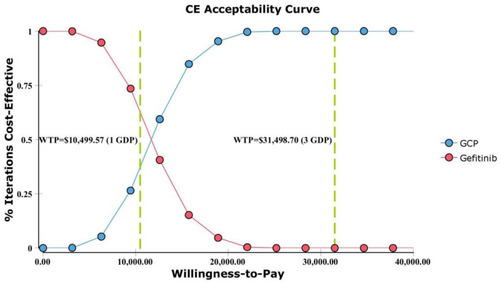
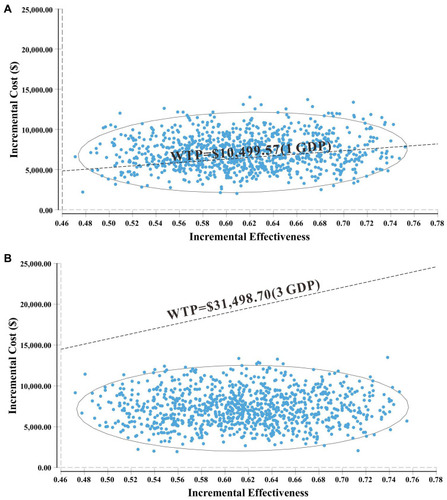
One-way deterministic sensitivity analysis of key variables revealed that the duration of PFS for GCP group, the duration of PFS for the gefitinib group, the utility of PFS, the cost of Gefitinib per 250 mg and cost of salvage therapy per cycle were the top five influential parameters in the model (). The duration of PFS for GCP group had the greatest influence on the results of the model. However, when the duration of PFS for GCP varied from 16.74 to 25.12, the ICER ranged from $19,875.78 per QALY to $5,918.34 per QALY, which was still lower than WTP (3 GDP). Furthermore, the top five influential parameters could gain ICER lower than 1 GDP within the range of variation. Other variables, such as body surface area (m2), the utility of PD, and discount rate had a moderate or mild impact on the ICER results. The probabilistic sensitivity analysis showed that the probability of GCP being cost-effective reached to 100% when 3 GDP was set as the WTP threshold (), and 38.75% being extremely cost-effective when 1 GDP was WTP threshold. Correspondingly, the cost-effectiveness acceptability curve showed the probabilistic sensitivity analysis results of different WTP thresholds (). If WTP threshold was $11,500/QALY, GCP treatment would have a 50% probability of being cost-effective.
Figure 3 Tornado diagram of one-way sensitivity analysis. It summarized the results of one-way sensitivity analysis, which listed influential parameters in descending order according to their effect on the ICER over the variation of each parameter value.
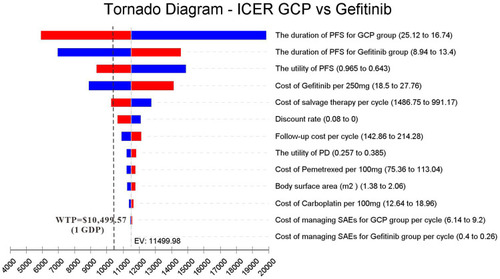
Figure 4 A probabilistic scatter plot of the ICER between the GCP and gefitinib group. Each dot represents the ICER for 1 simulation. An ellipse means 95% confidence interval. Dots that are located below the ICER threshold represent cost-effective simulations. (A) A probabilistic scatter plot of under WTP=$10,499.57 (1 GDP). (B) A probabilistic scatter plot of under WTP=$31,498.70 (3 GDP).
Discussion
In recent years, first-generation EGFR-TKIs such as gefitinib and erlotinib have been widely used in clinical practice and proved to be able to significantly improve patient survival.Citation22,Citation23 However, resistance mutations are inevitable due to the long-term use of these targeted drugs. Studies have found that the combination of gefitinib or erlotinib with chemotherapy drugs in advanced NSCLC patients with EGFR mutations can produce synergistic anti-proliferation and pro-apoptotic effects, which can effectively inhibit the occurrence of targeted drug resistance.Citation24–Citation26 Besides, several studies of targeted drugs in combination with chemotherapy have shown significant survival benefits. It has become a new direction of targeted therapy to explore the combined application mode of targeted drugs with chemotherapy to achieve the maximum survival benefit. However, the cost-effectiveness of these regimens in advanced NSCLC patients with EGFR mutations remains unknown. In this study, we investigated the cost-effectiveness of gefitinib alone versus gefitinib plus chemotherapy for advanced NSCLC patients with EGFR mutations based on NEJ009 study.
According to our analysis results, the addition of carboplatin and pemetrexed to gefitinib generated an ICER of $11,499.98/QALY, which was lower than the commonly accepted WTP threshold of $31,498.70/QALY (3 GDP), indicating that the GCP was cost-effective as the first-line treatment for advanced NSCLC patients with EGFR mutations compared with gefitinib alone in China. The acceptability curve also supported this finding, which demonstrated that GCP was the preferred option at this WTP threshold (3 GDP). It is worth noting that GCP had a 38.75% probability to be extremely cost-effective at 1 GDP, which strongly suggested that GCP was not only more effective but also the added cost was well worth. The one-way sensitivity analysis revealed that the duration of PFS for GCP group had the greatest influence on the ICER. Generally, the cycle costs of chemotherapy in the model were influenced by drug costs and duration of PFS, and the longer the PFS, the lower the chemotherapy cost per cycle. The top five influential parameters were the main tradeoffs when generalizing the results of clinical trials to real-world outcomes, because they could gain ICER lower than 1 GDP in China.
To the best of our knowledge, there are few studies reporting the cost-effectiveness of EGFR-TKIs alone versus EGFR-TKIs plus chemotherapy for first-line treatment of NSCLC. Some cost-effective studies between EGFR-TKIs, including osimertinib, gefitinib, afatinib, and erlotinib have been performed by other researchers.Citation27,Citation28 In Japan, use of gefitinib and EGFR testing could be considered as a cost-effective first-line therapy with an ICER of $32,500/QALY, and Kimura et al demonstrated that gefitinib was more cost-effective in comparison with afatinib and erlotinib regimens, although afatinib and erlotinib regimens were well tolerated and could achieve sufficient effects.Citation27,Citation28 Cai et al showed gefitinib or erlotinib first-line and chemotherapy second-line strategies were the most cost-effective first-line treatments for EGFR mutations in patients with NSCLC in China.Citation29 Different conditions, such as the model structure, time horizon, countries and regions, the measurement of costs and health utilities, may lead to inconsistent conclusions in similar clinical reports. Due to the superior efficacy and economy of gefitinib in EGFR-TKIs, it is meaningful and necessary to study the cost-effectiveness of gefitinib combined with chemotherapy.
Notably, the second generation of EGFR-TKIs could not overcome the drug resistance of the first-generation, and simultaneously showed greater adverse reactions, resulting in its unsatisfactory clinical application.Citation30,Citation31 In order to overcome drug resistance and improve survival time, NEJ009 was the first phase III clinical trial to evaluate the clinical efficacy of EGFR-TKI first-line platinum-containing two-drug combination chemotherapy in patients with EGFR-mutant advanced NSCLC. Although the third-generation EGFR-TKI osimertinib has received marketing authorization for its significant survival benefit in EGFR-mutated NSCLC, the price of osimertinib is 7.5-times of gefitinib and 5-times of afatinib in China. The cost disadvantage caused by such a huge price difference might not be compensated by its clinical output. From the economic point of view, the first-generation EGFR-TKIs were still a more economical treatment option for EGFR-mutated NSCLC in China.Citation32
The study had some limitations that are worth discussing. First, basic information was retrospectively collected from a phase III trial, and we used the Weibull distribution to extrapolate the results beyond the follow-up duration of the RCTs, which was not patient-level data in clinical practice. Second, the value of utilities of health states were derived from previously published studies, which might not reflect the health state of patients in China. Third, drug discounts and patient assistance programs were not considered in this study, making the costs slightly higher than those in the real-world in the long term. Fourth, due to the short hospitalization time of chemotherapy patients in each cycle, or even outpatient chemotherapy, the length of hospitalization had little effect on the results, so it was not included in the calculation. Finally, since it was difficult to accurately estimate the impact of SAEs on utility values, in order to calculate the cost-effectiveness for convenience, the negative effects of SAEs on utility were excluded in our calculation, which may also decrease the accuracy of our analysis.
Conclusion
This is the first study to investigate the cost-effectiveness of gefitinib plus chemotherapy for advanced NSCLC patients with EGFR mutations in China. Gefitinib plus chemotherapy is cost-effective compared with gefitinib alone from Chinese societal perspective. In addition to the efficacy and safety obtained from the clinical trial, our study could also provide evidences to evaluate the economy of gefitinib plus chemotherapy for the treatment of NSCLC from a pharmacoeconomic perspective. The results of our study are potentially significant for the decision-making of the patients, the government as well as the healthcare financial institutions.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi:10.3322/caac.2165433433946
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.2166033538338
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.2133826808342
- Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39:22. doi:10.1186/s40880-019-0368-6
- Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861. doi:10.1056/NEJMra170341328854088
- Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29:i3–i9. doi:10.1093/annonc/mdx70229462253
- National Comprehensive Cancer Network. Non-small cell lung cancer version 5; 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 15, 2021.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi:10.1056/NEJMoa090953020573926
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi:10.1200/JCO.2005.02.84016043829
- Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi:10.1200/JCO.2004.07.21514990633
- Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–784. doi:10.1200/JCO.2004.08.00114990632
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi:10.1200/JCO.2005.05.147417442998
- Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi:10.1200/JCO.19.0148831682542
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi:10.1056/NEJMoa171313729151359
- Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9:235–251. doi:10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O10790702
- Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32:101–108. doi:10.1007/s40273-013-0123-924338265
- Liu M, Zhang L, Huang Q, Li N, Zheng B, Cai H. Cost-effectiveness analysis of ceritinib and alectinib versus crizotinib in the treatment of anaplastic lymphoma kinase-positive advanced non-small cell lung cancer. Cancer Manag Res. 2019;11:9195–9202. doi:10.2147/CMAR.S22344131749634
- National Bureau of Statistics. National data of National Bureau of Statistics in 2020. Available from: https://data.stats.gov.cn/tablequery.htm?code=AD02. Accessed October 28, 2021.
- Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–e203. doi:10.1111/ajco.1247726990789
- Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi:10.1186/1477-7525-6-8418939982
- You R, Liu J, Wu DB, et al. Cost-effectiveness analysis of EGFR mutation testing and afatinib versus gemcitabine-cisplatin as first-line therapy for advanced non-small-cell lung cancer in China. Cancer Manag Res. 2019;11:10239–10248. doi:10.2147/CMAR.S21972231824194
- Kim Y, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Efficacy and safety of afatinib for EGFR-mutant non-small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat. 2019;51:502–509. doi:10.4143/crt.2018.11729898592
- Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116:568–574. doi:10.1038/bjc.2016.45628103612
- Rossi A, La Salvia A, Di Maio M. Chemotherapy and intercalated gefitinib or erlotinib in the treatment of advanced non-small-cell lung cancer. Expert Rev Respir Med. 2017;11:171–180. doi:10.1080/17476348.2017.129052628152323
- Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38:124–136. doi:10.1200/JCO.19.0115431411950
- Zhao Q, Sun K, Lei X, Cai L. A meta-analysis of the therapeutic effect of gefitinib combined with chemotherapy and chemotherapy alone in treating non-small cell lung cancer. Medicine. 2020;99:e21490. doi:10.1097/MD.000000000002149032756179
- Narita Y, Matsushima Y, Shiroiwa T, et al. Cost-effectiveness analysis of EGFR mutation testing and gefitinib as first-line therapy for non-small cell lung cancer. Lung Cancer. 2015;90:71–77. doi:10.1016/j.lungcan.2015.07.00626259876
- Kimura M, Yasue F, Usami E, et al. Cost-effectiveness and safety of the molecular targeted drugs Afatinib, gefitinib and erlotinib as first-line treatments for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Mol Clin Oncol. 2018;9:201–206.30101022
- Cai H, Zhang L, Li N, et al. Cost-effectiveness of osimertinib as first-line treatment and sequential therapy for EGFR mutation-positive non-small cell lung cancer in China. Clin Ther. 2019;41:280–290. doi:10.1016/j.clinthera.2018.12.00730639208
- Svaton M, Bratova M, Fischer O, et al. Real-life effectiveness of afatinib versus gefitinib in patients with non-small-cell lung cancer: a Czech multicentre study. Anticancer Res. 2021;41:2059–2065. doi:10.21873/anticanres.1497533813414
- Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-lung 7 trial. Ann Oncol. 2017;28:270–277. doi:10.1093/annonc/mdw61128426106
- Li WQ, Li LY, Chai J, Cui JW. Cost-effectiveness analysis of first-line treatments for advanced epidermal growth factor receptor-mutant non-small cell lung cancer patients. Cancer Med. 2021;10:1964–1974. doi:10.1002/cam4.373333626238