Abstract
Purpose
To determine clinical predictors of recurrence and metastasis in patients with pathological complete response (pCR) after neoadjuvant chemotherapy (NCT).
Methods
Patients treated with NCT who achieved pCR (n=285) were classified into three groups according to pre-NCT clinical stage (cStage): group I (IIa–IIb), group II (IIIa), and group III (IIIb–IIIc). Survival was analysed using the Kaplan–Meier method. The relationships between clinicopathological factors and recurrence were determined using Cox regression analysis.
Results
The median follow-up was 31 months. The 3-year disease-free survival and overall survival rates in groups I, II, and III were 92.7%, 87.8%, and 66.7% (P < 0.01) and 98.6%, 98.3%, and 90.6% (P=0.370), respectively. Lymph node status and tumour size were independent risk factors for recurrence and metastasis after NCT. In the human epidermal growth factor receptor 2-positive subgroup, advanced cStage and lymph node metastasis were associated with recurrence (P < 0.01). In the hormone receptor-positive subgroup, disease-free survival rates differed for cStages I–II compared to cStage III (P=0.049) and clinical node status 0–2 compared to clinical node status 3 (P=0.037).
Conclusion
Pre-NCT cStage predicted the prognosis of pCR for different breast cancer subtypes. Patients with advanced cStage, lymph node metastasis, and large tumour sizes had a higher risk of recurrence or metastasis.
Introduction
Neoadjuvant chemotherapy has gradually become popular in breast cancer, and achieving pathological complete response (pCR) (ypT0/is, ypN0) in high-risk breast cancer has been associated with a relatively good prognosis.Citation1–Citation3 The effects of neoadjuvant chemotherapy may be used as an external proxy of in vivo drug sensitivity. Prognostic information can be more accurately provided to patients via the objective evaluation of postoperative pathological findings, which constitutes an important advancement towards individualised medicine.Citation4,Citation5 Meta-analyses have indicated that pCR has a better prognostic value than that of other pathological findings.Citation3,Citation6 Therefore, achieving pCR via treatment has become a surrogate endpoint for a large proportion of neoadjuvant chemotherapy clinical study designs and serves as a neoadjuvant treatment goal for high-risk types of breast cancer, such as human epidermal growth factor receptor 2-positive (HER2+) and triple-negative breast cancer. Nevertheless, evidence suggests that achievement of pCR after treatment does not preclude the risk of recurrence or metastasis.Citation7 Therefore, clarifying the clinicopathological factors associated with these risks in patients with pCR is crucial for the postoperative treatment of neoadjuvant patients and screening of populations enrolled in neoadjuvant clinical studies.Citation8
Currently, various criteria for pCR evaluation after neoadjuvant chemotherapy exist, including the American Joint Committee on Cancer’s post-neoadjuvant staging system, the net reclassification improvement, Miller and Payne system, Residual Cancer Burden, and Neo-Bioscore. These methods provide an approximate indication of differential patient prognoses based on residual pathology after neoadjuvant chemotherapy, thereby facilitating the provision of distinct treatment methods for patients during adjuvant therapy.Citation9–Citation11 However, the majority of these methods have not elucidated the key factors influencing the prognosis of patients with pCR.Citation7,Citation12,Citation13 Although relevant factors that affect prognosis, such as hormone receptor (HR) expression, HER2 status, and clinical stage (cStage), are listed in the Neo-Bioscore, this evaluation system is too generalized. Notably, based on current treatment approaches, distinct treatment modalities are required for different subtypes of breast cancer, and prognoses are inconsistent. Therefore, the stratification of prognoses for all breast cancer types according to uniform standards is a major issue that contributes to inaccuracy.Citation6,Citation14,Citation15 In addition, several studies have explored prognostic factors that affect patients with pCR. However, data have been unreliable owing to outdated, retrospective nature of the studies, inconsistent definitions or treatments for pCR, and lack of classification and refinement.Citation16–Citation18
To address these gaps in the literature, we performed a retrospective analysis of patients with different types of breast cancer in the context of corresponding standard neoadjuvant chemotherapy. We aimed to determine the prognosis of patients with different subtypes of breast cancer who achieved pCR and elucidate the relevant clinicopathological factors influencing their prognosis.
Materials and Methods
Patient Population
This study retrospectively analysed patients who received neoadjuvant chemotherapy and achieved pCR at Harbin Medical University Cancer Hospital between June 2012 and May 2019. Patients who were initially diagnosed with metastatic breast cancer, underwent neoadjuvant chemotherapy without surgery, presented with inflammatory breast cancer, had incomplete follow-up data, and/or underwent surgery or chemotherapy at other institutions were excluded. The initial cStage of all patients was reviewed based on the American Joint Committee on Cancer staging system (seventh edition). Patients were classified into three groups according to cStage: group I (IIa–IIb), group II (IIIa), and group III (IIIb–IIIc). Electronic medical records were reviewed to obtain information on age, tumour characteristics, initial cStage, type of neoadjuvant chemotherapy received, recurrence, and survival. All procedures performed in this study complied with the ethical standards of the institution and/or the National Research Council, as well as the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. The retrospective study design was approved by the Ethics Committee of Harbin Medical University Cancer Hospital. Written informed consent was obtained from each patient.
Pathological Assessment
Professional pathologists reviewed pathological specimens from diagnostic core biopsies from each patient to determine the HR and HER2 status, which was recorded by specialised breast doctors. HR status was assessed by immunohistochemistry. Specimens were classified as HR+ when more than 1% of cancer cells stained positive. HER2 positivity was defined according to the American Society of Clinical Oncology/American Society of Pathologists guidelines.Citation19 If the immunohistochemical score of the tumour was 3+, or the immunohistochemical score was 2+ and the fluorescence in situ hybridisation test was positive, the specimen was classified as HER2+. Based on HR and HER2 status, intrinsic subtypes were classified as HER2+, HR+ (HR+/HER2–), or triple-negative breast cancer. pCR was defined as the absence of any invasive disease in the breast and no micro-/macro-metastases in the ipsilateral axillary lymph nodes. All patients received at least four cycles of anthracycline- or taxane-based neoadjuvant chemotherapy. Trastuzumab was recommended for patients who were HER2+. After completing neoadjuvant chemotherapy, patients underwent radical breast surgery, axillary lymph node dissection, or sentinel lymph node dissection. The decision to undergo breast-conserving surgery was made via consensus between the patient and surgeon. For all patients with metastatic cancer in the axillary region at the diagnostic core biopsy, axillary lymph node dissection was used after the completion of neoadjuvant chemotherapy. If there was no lymph node involvement, lymph node dissection or sentinel lymph node biopsy was performed according to the patient’s wishes. If the patient had undergone breast-conserving surgery or had locally advanced disease at the time of consultation, postoperative radiotherapy was performed. Fifty Gy in 25 fractions was prescribed for these patients; radiotherapy regimens included whole breast, chest wall, and regional nodal radiation. Patients with HR+ breast cancer were offered adjuvant endocrine therapy for 5 years.
Statistical Analyses
The primary endpoint was disease-free survival (DFS), defined as the time from disease diagnosis to the first documentation of cancer recurrence or last follow-up. The secondary endpoint was overall survival (OS), defined as the time from disease diagnosis to death from any cause or last follow-up. Survival curves were plotted using the Kaplan–Meier method and compared using the Log rank test. The χ2 test or Fisher’s exact test was used to determine the factors that could predict recurrence and evaluate the impact of cStage on recurrence in each subgroup.
Univariate and multivariate analyses combined with Cox proportional hazards regression models were used to identify high-risk factors associated with survival. Known determinants in the logistic regression model were adjusted, including age, cStage, primary tumour size, lymph node status, HER2 status, HR status, administration of chemotherapy regimens, and Ki-67 labelling index. All tests were two-tailed. P < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
From 2012 to 2019, a total of 2359 patients received neoadjuvant therapy. 285 patients achieved pCR and were included in the survival analysis. In total, 147 (51.6%), 77 (27.0%), and 61 (21.4%) patients were classified into cStage groups I, II, and III, respectively. All 285 patients underwent surgery. Detailed patient characteristics are summarised in . Among cStages based on clinical tumour (cT) size, cT2 accounted for the largest proportion (68.4%), while clinical lymph node metastasis constituted the highest proportion (71.6%) of the overall population. Among tumour subtypes, HER2+, triple-negative, and HR+ breast cancer accounted for 58.9%, 26.3%, and 15.8% of the population, respectively. The immunohistochemical results of one patient were unidentifiable.
Table 1 Patient Characteristics of the Final Cohort
The Regimens of Treatment and Features in Prognosis of Different Subtypes
Regimens for the majority of patients treated with neoadjuvant chemotherapy included anthracyclines and taxane (85.9%). In addition, 168 (58.9%) patients completed the standard treatment cycle preoperatively, while the remaining patients did not complete the standard treatment cycle before surgery and did not undergo postoperative adjuvant chemotherapy. The majority of patients in the HER2+ subgroup (80.9%) received preoperative chemotherapy with anthracyclines and taxane (Supplementary Table S1), and the recurrence rate was 16.9%, which was numerically higher than that in patients who received taxane or anthracyclines alone, although the difference was not statistically significant (P = 0.584). A numerical difference in the recurrence rate was also observed between patients treated with platinum and not statistically significant (4.2% vs 17.4%, respectively; P = 0.132). Fifty-eight patients (34.5%) were treated with combined targeted therapy during neoadjuvant chemotherapy, whereas the majority of patients (n=110, 65.5%) did not receive targeted therapy. The risk of recurrence was significantly higher in patients without targeted therapy compared to those who received targeted therapy (20.0% vs 6.9%, respectively; P = 0.026). We further classified the HER2+ group into HER2+/HR+ and HER2+/HR– subgroups and evaluated the correlation between clinical factors of targeted therapy and recurrence. In the HER2+/HR+ subgroup, the risk of recurrence was significantly higher in patients who did not receive targeted therapy than in those who received targeted therapy (21.4% vs 0.0%, respectively; P = 0.048) (Supplementary Table S3). This difference was not observed in the HER2+/HR− subgroup. One hundred and four patients (61.9%) completed standard treatment cycles before surgery. The risk of recurrence was higher in patients who did not complete the standard treatment cycle than in those who completed the standard treatment cycle, although it was not statistically significant (17.2% vs 14.4%, respectively; P = 0.458). In the triple-negative subgroup, the majority of patients (91.4%) received a combination of anthracyclines and taxane before surgery (Supplementary Table S2). More than half of the patients (51.7%) completed the standard treatment cycle of neoadjuvant therapy; the remaining patients did not complete the standard treatment cycle owing to pCR. The risk of recurrence between the group that did not complete the standard treatment cycle and the group that completed the standard treatment cycle was 13.2% vs 18.9%, respectively (P = 0.544). Three patients (4%) received platinum in addition to the neoadjuvant treatment regimen. The characteristics of the HR+ group are summarised in Supplementary Table S4.
Recurrence Events
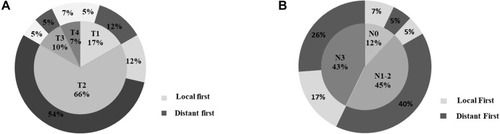
The median time to recurrence was 31 (range, 2–99) months. The mean follow-up time was 39.7 months. In total, 42 end-point events related to recurrence or death occurred in patients with recurrence, of whom 12 (28.6%) presented with local recurrence and 30 (71.4%) developed distant metastases. Of these, 10 (23.8%) presented with liver metastases, 7 (16.7%) with brain metastases, and 4 (9.5%) with lung metastases ().
Table 2 Details of the First Site of Cancer Recurrence for the Final Cohort and Three Cancer Subtypes
Among patients with different tumour subtypes, 26 recurrence-related events were observed in the HER2+ subgroup. Eight (30.1%) patients presented with local recurrence and 18 (69.9%) with distant metastases. Most patients presented with brain (n=5, 19.2%) and liver metastases (n=5, 19.2%). Lung and bone metastases were identified in three patients (11.5%). In the triple-negative subgroup, 12 recurrence-related events were observed. Three patients presented with local recurrence and nine with distant metastases, while most patients presented with liver metastases (n=4, 30.0%). In the HR+ subgroup, four recurrence-related events were observed. One patient (25.0%) presented with local recurrence, and three (75.0%) with distant metastases.
Local recurrence and distant metastasis events were distinguished based on primary tumour size and lymph node status (). T2 and N3 accounted for the largest proportion of local recurrence events. For distant metastatic events, T2 and N1–2 accounted for the largest proportion. T4 recurrence events were local recurrence events without distant metastasis.
Survival Outcomes
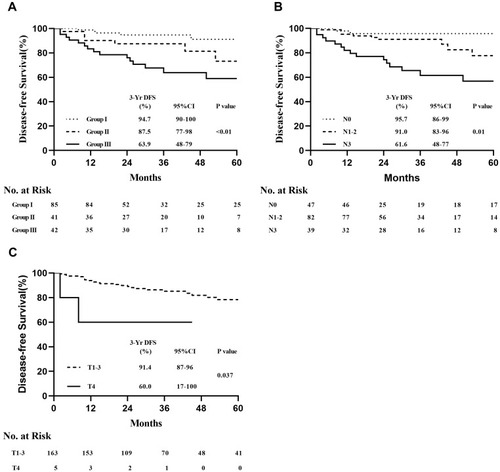
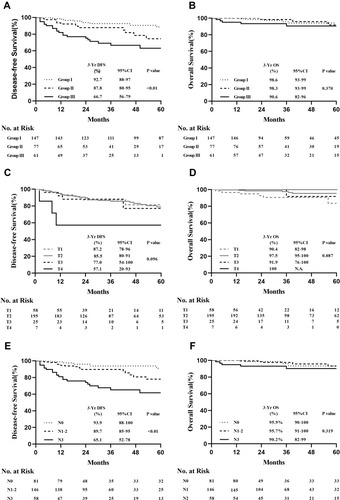
The 3-year DFS rates were 92.7%, 87.8%, and 66.7% in groups I, II, and III, respectively, with significant differences observed among groups (Log rank test, P < 0.01) (). The 3-year OS rates were 98.6%, 98.3%, and 90.6% in groups I, II, and III, respectively, with no significant differences observed among groups (Log rank test, P = 0.370) (). Clinical tumour size was not significantly correlated with DFS (Log rank test, P = 0.096) or OS (Log rank test, P = 0.087) (). Lymph node status was significantly correlated with DFS (Log rank test, P < 0.01) (), but not OS (Log rank test, P = 0.319) ().
Figure 2 (A) DFS and (B) OS among the three clinical stages in the final cohort. (C) DFS and (D) OS among patients stratified by clinical tumour size in the final cohort. (E) DFS and (F) OS among patients stratified by lymph node status in the final cohort.
Cox regression analysis of factors predicting recurrence revealed that cStage III (hazard ratio: 3.2, 95% confidence interval [CI]: 1.6–6.3; P < 0.01) was associated with a higher risk of recurrence than cStage I–II (). The analysis of primary clinical tumour size- and lymph node involvement-related factors revealed that the risk of recurrence was significantly higher in patients with cT4 than in those with cT1–3 (hazard ratio: 4.4, 95% CI: 1.1–15.6; P = 0.03). The risk of recurrence was significantly higher in cN3 patients than in cN0–2 patients (hazard ratio: 13.3, 95% CI: 2.6–16.6; P < 0.01).
Table 3 Cox Proportional Hazard Model of Predictors of Cancer Recurrence in Patients Who Achieved pCR
In the correlation analysis, age at diagnosis (<50 vs ≥50 years), HER2 status (HER2– vs HER+), HR status (HR– vs HR+), chemotherapy regimen (1 vs 3 weeks), Ki-67 level (<20% vs ≥20%), axillary surgical approach (sentinel lymph node biopsy versus axillary lymph node dissection), and treatment cycle (completion vs non-completion) were not significantly associated with recurrence. In the multivariate analyses, lymph node status was the only independent factor predicting recurrence in patients with pCR (hazard ratio: 2.2, 95% CI: 1.1–4.4; P = 0.03).
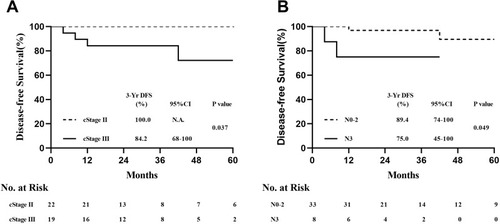
Based on the correlation analysis results for recurrence factors, we analysed different tumour types. In the HER2+ subgroup, significant differences were observed in DFS rates (Log rank test, P < 0.01) () and lymph node status (Log rank test, P < 0.01) (), but not tumour size (Log rank test, P = 0.191) (Supplementary Figure S1A), among the three cStage groups. Separate analyses revealed that DFS was significantly different between patients with cT4 and those with cT1–3 (Log rank test, P = 0.037) (). In the HR+ subgroups, cStage, lymph node status, and tumour size were not significantly correlated with DFS (Supplementary Figure S1B–D). However, the Log rank test P-values in the Kaplan–Meier analyses were significant for DFS for cStage I–II vs cStage III (Log rank test, P = 0.049) () and cN0–2 vs cN3 (Log rank test, P = 0.037) (). No significant correlations with DFS were observed in the triple-negative subgroup, irrespective of cStage, clinical tumour size, or lymph node status (Supplementary Figure S1E–G).
Discussion
Increased research on the efficacy of neoadjuvant chemotherapy has contributed to an increase in its clinical implementation. Meta-analyses have indicated that the efficacy of neoadjuvant chemotherapy is comparable to that of adjuvant therapy for the same treatment regimen; however, the prognosis of patients with pCR is superior to that of patients without pCR.Citation3,Citation20 Moreover, studies of high-risk populations have revealed that the prognosis of pCR patients may be suboptimal, and the implications of pCR have been questioned.Citation17,Citation21 In this study, we examined the prognosis of patients with different breast cancer subtypes who achieved pCR and identified the clinicopathological factors relevant to their prognosis. We identified lymph node status as an independent risk factor for recurrence in patients with breast cancer who received neoadjuvant chemotherapy and achieved pCR. There was a trend in T4, although it was not statistically significant. We analysed the correlation of recurrence and metastasis in patients with pCR but did not identify any clear patterns. Recurrence events in the T4 population were all in situ; DFS exhibited the poorest performance while OS exhibited the best performance in this population. Based on these findings, caution should be exercised when formulating clinical decisions after neoadjuvant chemotherapy for patients with locally advanced or inoperable breast cancer. Clinical decisions should not be made solely based on references to previous criteria for neoadjuvant chemotherapy for operable breast cancer. Treatment options should instead be tailored according to advances in genetic mapping, as well as tumour and patient characteristics, following the achievement of pCR after neoadjuvant chemotherapy.
Our findings may differ from the results of previous retrospective studies because we included patients receiving neoadjuvant chemotherapy with cStage II–III. As cStage I patients have a better prognosis, the patients in this study presented with a higher risk of recurrence and metastasis. In addition, our patient groups differed from those in previous studies. The use of tumour size and lymph node status to determine the included population may lead to considerable differences in the residual risk of recurrence. Based on the findings of Wei et al,Citation21 we balanced the risk of recurrence in each group and classified patients as cStage IIa–IIb, IIIa, or IIIb–IIIc according to disease stage. Therefore, our results may more closely reflect the conditions observed in clinical practice. Notably, this is the first study to identify and analyse different immunohistochemical types based on cStage-, tumour size-, and lymph node-related prognoses to enable identification of prognostic risk and formulation of optimal clinical decisions.
We observed that a higher cStage, extensive lymph node involvement, and T4 were significantly associated with poor prognosis in the HER2+ population. For this group of patients, multimodal therapy (eg intensive drugs, including trastuzumab emtansine and tyrosine kinase inhibitors) is recommended, even if pCR is achieved. In the HR+ population, cStage III and N3 were key factors associated with poor prognosis. Compared to HER2+, which is a more aggressive tumour type, HR+ tumours grow more slowly. No HR+ patients with T4 primary tumours were identified in our study. HR+ patients have a lower probability of four or more lymph node metastases compared to HER2+ patients.Citation22 Therefore, for HR+ patients, tumour size may not reflect disease duration, and a more accurate evaluation of axillary lymph nodes is required for disease diagnosis. In patients with severe lymph node involvement, early intensive treatment is required for cases with a high risk of recurrence, and, if necessary, continuous intensive endocrine therapy (eg cyclin-dependent kinase 4/6 inhibitors) should be considered. We were unable to differentiate the prognosis of patients with triple-negative breast cancer using the appropriate stratification owing to the small number of patients and single-treatment regimen used. Thus, the combination of traditional anthracyclines and taxane was commonly used, with the addition of platinum or taxane to neoadjuvant chemotherapy regimens accounting for only a small proportion.
A large proportion of HER2+ patients achieved pCR, despite not receiving targeted therapy during neoadjuvant chemotherapy. At follow-up, we observed that the risk of recurrence was higher in patients without targeted therapy than in those who received targeted therapy. Even though these patients achieved pCR without the support of targeted therapy, their prognosis was still affected by the absence of targeted therapy. However, for this population, the duration of targeted therapy is open to discussion since the application of short courses of targeted adjuvant therapy is a contradictory topic.Citation23–Citation25 Although this study was a single-centre, retrospective analysis with a small sample, from the results, other studies had corroborated these findings, including a retrospective analysis by von Minckwitz et al,Citation6 which demonstrated that patients with pCR after anti-HER2 treatment had better clinical outcomes compared to those who did not receive targeted therapy during neoadjuvant chemotherapy, especially with regard to OS (hazard ratio: 14.11, P = 0.009). For HR+ patients, as demonstrated in our study, potential synergy between signals may be key to improving the prognosis of HR+ and HER2+ patients. Further, long-term targeting and maintenance endocrine therapy are critical factors for improving prognosis, as supported by Exnet research.Citation26 Collectively, these results suggest that targeted therapy for HER2+ breast cancer is crucial for achieving and maintaining good long-term survival. However, this needs to be confirmed by rigorous clinical studies in the future, given the discrepancies in the literature.Citation27 Generally, no difference in long-term prognosis was observed, regardless of whether standard treatment cycles were completed or continued for three weeks during neoadjuvant pCR (including the replenishment of previously missing treatments after neoadjuvant chemotherapy). Notably, the effects of chemotherapy during the neoadjuvant period may affect long-term prognosis. Achievement of pCR may be employed to identify the optimal treatment plan for patients. Indeed, this issue has been investigated in several retrospective studies and clinical research, which may ultimately help to guide clinical treatment strategies.Citation28,Citation29
In our study, the recurrence of liver metastases accounted for a large proportion of patients. In a prospective study that included liver ultrasound and hepatic function tests, no significant differences in mortality were observed between intensive monitoring and minimal examination during follow-up.Citation30 However, the optimal follow-up approach for patients with breast cancer who achieve pCR after treatment requires clarification. Notably, patients with brain metastases comprised a large proportion of patients with HER2+ breast cancer. In the KATHERINE study,Citation11 a similar incidence of brain metastases was observed in the trastuzumab and T-DM1 groups. Of note, there is significant scope for improvement in the monitoring and prevention of recurrence. In the future, effective tyrosine kinase inhibitor drugs and monoclonal antibodies that can cross the blood-brain barrier should be included in neoadjuvant chemotherapy regimens to prevent brain metastases.Citation31 Similarly, the National Comprehensive Cancer Network guidelines do not recommend magnetic resonance imaging of the head during follow-up of patients with breast cancer. Based on these issues, the present results clearly emphasise the need for systemic examination of patients with breast cancer who have achieved pCR after neoadjuvant chemotherapy. In this regard, it is necessary to develop clear methods and standards for the early detection of distant metastases in patients who have achieved pCR to improve prognosis and survival.
Our study had several limitations. The study was retrospective in nature, and was conducted in a single institution, thus limiting the generalisability of the results. In addition, the follow-up time was insufficient to identify the most relevant endpoints. Furthermore, tumour size was not uniformly distributed. One explanation for this is that the patients included in our study were diagnosed at an early stage and that Asian women typically have smaller breasts than European and American women. Further research should incorporate larger sample sizes and uniformly distributed tumour sizes.
In conclusion, our findings indicate that patients with breast cancer who achieve pCR after neoadjuvant chemotherapy have a good prognosis. However, patients with a late clinical stage before neoadjuvant chemotherapy remain at risk of recurrence after treatment. Therefore, close follow-up is warranted to improve survival rates and prevent recurrence and metastasis in this population.
Data Sharing Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
All procedures performed in this study complied with the ethical standards of the institution and/or the National Research Council, as well as the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. The retrospective study design was approved by the Ethics Committee of Harbin Medical University Cancer Hospital.
Consent to Participate
Written informed consent was obtained from each patient.
Consent to Publication
The authors affirm that all research participants provided written consent for the publication of their data in this study.
Disclosure
All authors declare that they have no conflicts of interest.
References
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi:10.1200/JCO.1998.16.8.26729704717
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. doi:10.1200/JCO.2001.19.22.422411709566
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-824529560
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–647. doi:10.1016/S1470-2045(14)70080-424657003
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi:10.1016/S1470-2045(11)70336-922153890
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi:10.1200/JCO.2011.38.859522508812
- Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–1060. doi:10.1200/JCO.2015.63.101028135148
- Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017;15(10):1216–1223. doi:10.6004/jnccn.2017.015828982747
- Cho WK, Park W, Choi DH, et al. Is tumor bed boost necessary in patients who achieved ypCR following neoadjuvant chemotherapy and breast conserving therapy? (KROG 12-05 and 16-16). Breast. 2019;45:43–47. doi:10.1016/j.breast.2019.02.01030844692
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi:10.1056/NEJMoa161264528564564
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi:10.1056/NEJMoa181401730516102
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327. doi:10.1016/S0960-9776(03)00106-114659147
- Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2017;165(1):181–191. doi:10.1007/s10549-017-4303-828577078
- Bergquist JR, Murphy BL, Storlie CB, Habermann EB, Boughey JC. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2017;24(12):3510–3517. doi:10.1245/s10434-017-6010-428828583
- Mittendorf EA, Vila J, Tucker SL, et al. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–936. doi:10.1001/jamaoncol.2015.647826986538
- Ju NR, Jeffe DB, Keune J, Aft R. Patient and tumor characteristics associated with breast cancer recurrence after complete pathological response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;137(1):195–201. doi:10.1007/s10549-012-2312-123149464
- Gonzalez-Angulo AM, McGuire SE, Buchholz TA, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005;23(28):7098–7104. doi:10.1200/JCO.2005.11.12416192593
- Asaoka M, Narui K, Suganuma N, et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur J Surg Oncol. 2019;45(12):2289–2294. doi:10.1016/j.ejso.2019.08.00131787153
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.998424101045
- Asselain B, Barlow W, Bartlett J. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. doi:10.1016/S1470-2045(17)30777-529242041
- Wei W, Kurita T, Hess KR, et al. Comparison of residual risk-based eligibility vs tumor size and nodal status for power estimates in adjuvant trials of breast cancer therapies. JAMA Oncol. 2018;4(4):e175092. doi:10.1001/jamaoncol.2017.509229372234
- Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009;16(10):2705–2710. doi:10.1245/s10434-009-0606-219593632
- Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised Phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599–2612. doi:10.1016/S0140-6736(19)30650-631178152
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. doi:10.1016/S1470-2045(13)70225-023764181
- Joensuu H, Fraser J, Wildiers H, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4(9):1199–1206. doi:10.1001/jamaoncol.2018.138029852043
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. doi:10.1016/S1470-2045(17)30717-929146401
- Tanioka M, Shimizu C, Yonemori K, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010;103(3):297–302. doi:10.1038/sj.bjc.660576920606681
- Yan H, Xiao H, Zhu J, Zhang J, Liu Z. Association between the HER2 protein expression level and the efficacy of neoadjuvant chemotherapy in HER2-positive breast cancer. Cancer Manag Res. 2020;12:12715–12722. doi:10.2147/CMAR.S27869433328766
- Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–2848. doi:10.1158/1078-0432.CCR-19-349232046998
- von Minckwitz G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125(1):145–156. doi:10.1007/s10549-010-1228-x21042932
- Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi:10.1200/JCO.20.0077532468955