Abstract
Objective
This study aimed to evaluate the incidence and severity of immediate hypersensitivity reactions (HSRs) in the first exposure to rituximab with the adoption of skin testing screening and desensitization and investigate the value of skin testing as a predictive tool for immediate HSR to rituximab.
Methods
This was a prospective intervention study. Patients with hematological malignancies who required rituximab were recruited. Skin testing screening with rituximab was conducted before the first infusion. Patients with positive skin testing results underwent desensitization, while those with negative results received rituximab at a standard infusion rate. All immediate HSRs were recorded, and the predictive value of positive skin testing results for immediate HSRs to rituximab was analyzed.
Results
In the 19 patients who adopted the novel protocol, six patients (31.6%) had immediate HSRs during the first infusion, with three mild reactions (15.8%), two moderate reactions (10.5%), and only one severe reaction (5.3%). The positive predictive value of intradermal test (IDT) with 1 mg/mL rituximab solution for immediate HSR was 100%, and the negative predictive value was 84.6%.
Conclusion
The protocol of skin testing screening and desensitization might have some potential to control the incidence and severity of immediate HSRs to rituximab during the first exposure. IDT result before the first infusion could become a useful predictor for immediate HSR to rituximab.
Plain Language Summary
Skin Testing Screening and Desensitization of Rituximab Before the First Infusion
Hypersensitivity reactions (HSRs) to rituximab are common and interfere with the administration of this first-line therapy for lymphoma patients. In order to develop novel protocols to reduce the incidence of immediate HSRs during the first infusion of rituximab, we designed this study.
We recruited patients with hematological malignancies who needed rituximab treatment and performed skin testing with rituximab before the first infusion. If the patients showed positive skin testing results, we adopted desensitization procedure to administer the first infusion of rituximab, and if the skin testing results were negative, we used the standard infusion rate following the manufacturer’s instructions.
Through this research, we found that in the 19 patients who underwent skin testing screening, six (31.6%) patients showed positive skin testing results, and later six patients (31.6%) had immediate HSRs during the first infusion, with three mild reactions (15.8%), two moderate reactions (10.5%), and only one severe reaction (5.3%). However, in the literature reports, incidence rate of immediate HSRs during the first infusion of rituximab could be as high as 70–78%, with severe reactions accounting for 30–37%, indicating that the protocol used in the current study might have some potential to control the incidence and severity of immediate HSRs to rituximab during the first exposure. We also found that intradermal skin testing screening had remarkably high accuracy for the prediction of immediate HSR to rituximab. The positive predictive value was 100%, and the negative predictive value was 84.6%.
Unfortunately, we have no control group in this study, thus further investigations through prospective randomized controlled studies with larger sample size are necessary.
Introduction
Drug hypersensitivity reactions (HSRs) are a crucial concern for clinicians and patients. HSRs affect more than 7% of the general population and cause a significant public health problem.Citation1 As an important monoclonal antibody directed against the CD20 antigen, rituximab is an irreplaceable weapon to treat B-cell lymphomas/lymphoproliferative diseases and autoimmune disorders.Citation2,Citation3 Due to its nature of chimeric murine/human mAb, immediate HSRs to rituximab are common, and often occur during the first infusion. Incidence rate of immediate HSRs as high as 70–78% has been reported without pretreatment with high-dose corticosteroids.Citation4 To prevent the recurrence of HSR, a slow infusion rate would be used and the process would be elongated to 10–20 h, representing an increase in medical resources and duration of treatment. Even such a slow infusion rate might not solve the problem of HSR in some patients, which compels them to abandon this first-line therapy and exerts adverse influence over their prognosis. Therefore, novel protocols are urgently needed to reduce the incidence of immediate HSRs during the first infusion of rituximab.
Skin testing is a valuable tool to evaluate Immunoglobulin E (IgE)-mediated hypersensitivity to drugs. Their sensitivity and predictive values are especially good for immediate HSRs to β-lactam antibiotics, neuromuscular-blocking agents (NMBA), and platin salts.Citation5 It has been reported that some drug cause type I hypersensitivity in the first exposure, possibly due to the pre-existence of drug-reactive IgE antibody in the sera of patients. Immediate HSRs to paclitaxel and docetaxel used to occur in 50% of patients, often during the first lifetime exposure.Citation6 It was speculated that some of these HSRs were IgE-mediated due to positive skin test results and immunoblot assays, and the preexistent IgE to taxane was possibly induced by the cross-sensitivity to yew tree pollen which had sensitized the patient before his exposure to taxane.Citation7 Anti-cetuximab IgE antibodies were also found in pretreatment samples of some patients who experienced anaphylaxis during their first exposure to cetuximab.Citation8 Similarly, the fact that many subjects react to rituximab on first exposure leads to speculation that specific IgE to rituximab might already exist in some high-risk patients before the first infusion, who could be recognized by skin testing.
Drug desensitization is a therapeutic process that involves gradually increasing the rate and concentration of drug administration over several hours,Citation9 and could induce a temporary state of tolerance to the drug responsible for a proven immediate HSR.Citation10 According to the literature, through the treatment of desensitization, the HSR symptoms to rituximab could be significantly alleviated, and the whole incidence of anaphylaxis was reduced by 80%.Citation4
The aim of the study was to evaluate the incidence and severity of immediate HSRs to rituximab with the adoption of skin testing screening before the first exposure and following desensitization based on the results of skin testing. Meanwhile, the value of skin testing as a predictive marker for immediate HSR to rituximab would be investigated through calculating the sensitivity, specificity, and positive and negative predictive value of skin testing for HSR.
Materials and Methods
Subjects
All the subjects were recruited from the Hematology Department of Peking Union Medical College Hospital. The inclusion criteria were as follows: patients with hematological malignancies; patients who needed the rituximab treatment as first-line therapy; age older than 14 years, men or women. The exclusion criteria included patients who used rituximab before; pregnant or lactating women; patients taking antihistamines in the 3 days before the skin testing; long-term use of systemic corticosteroids; patients with skin lesions including infection, dermatitis, trauma, or scarring in both arms; patients with acute attack of asthma; and other conditions that the researchers considered inappropriate for participation in the study.
Study Design
This was a prospective intervention study conducted for 6 months (from September 2020 to February 2021). The patients underwent skin testing screening with rituximab before the first rituximab infusion. In case of a positive skin testing result, the patient would receive a desensitization procedure. If the result of the skin testing was negative, the standard infusion way following the manufacturer’s instructions would be administered. Before both the desensitization and standard infusion, diphenhydramine (20 mg administered intramuscularly) and dexamethasone (5 mg administered intravenously) were administered to the patients. The incidence and severity of immediate HSRs to rituximab during the first infusion was recorded. The predictive value of skin testing for immediate HSR to rituximab, especially for the reaction with symptoms suggesting type I hypersensitivity, was also evaluated through the comparison between the skin testing results and HSR manifestations during the first infusion. The ethics committee of Peking Union Medical College Hospital approved this study (ZS-2515), and this study was conducted in accordance with the Declaration of Helsinki. The informed consent was obtained from all subjects. The parents of the 16-year-old patient also provided informed consent.
Skin Testing
Patients underwent skin testing for rituximab, with histamine (10 mg/mL for skin prick test and 0.01 mg/mL for intradermal test) as the positive control and saline solution as the negative control.
For skin prick test (SPT), a rituximab solution of 1 mg/mL was applied on the volar aspect of the forearm. A positive SPT was defined as a wheal size of at least 3 mm greater than that of the negative control at 15 min.Citation5 Where the result of the SPT was negative, intradermal test (IDT) was performed.
For IDT, 0.02 mL of a 1:100 dilution (0.1 mg/mL) of full-strength solution (10 mg/mL) and a 1:10 dilution (1 mg/mL) were used.Citation11 IDT results were defined according to the mean diameter of the wheal reaction, and a mean diameter ≥5 mm at 15 min was considered a positive result.Citation5 All wheals were accompanied by itching and surrounding flares.
SPT and IDT with rituximab dilution of 1 mg/mL were performed on 12 subjects not known for any drug allergy labels and showed negative results, indicating a low risk of irritant false-positive results in our cohort.
Desensitization Procedure
As shown in , three solutions with different concentrations were delivered in 12 consecutive steps; each step increased the rate of drug administration by 2- to 2.5-fold. Solution 1 was a 100-fold dilution (0.01 mg/mL) of the final target concentration, solution 2 was a 10-fold dilution (0.1 mg/mL) of the final target concentration, and the concentration of solution 3 was the target concentration (1 mg/mL). Each step took 15 min until the target rate of 200 mL/h was reached, and the final step was prolonged to complete the target dose. The whole procedure took approximately 5.5 h.
Table 1 Desensitization Protocol for Intravenous Rituximab (600 mg)
During the process of desensitization, in case of any breakthrough symptoms, the infusion was held and therapy was administered immediately. Local or generalized hives were treated with intramuscular antihistamines. Anaphylaxis was treated with intramuscular epinephrine, intravenous glucocorticoids, and intramuscular antihistamines. Other symptoms were treated accordingly. Bronchospasm was treated with inhaled β-agonists, and fever was treated with oral or intravenous non-steroidal anti-inflammatory drugs. Intravenous fluid resuscitation and infusion of pressor agents were used if indicated. Once symptoms were controlled and the patient was asymptomatic, the infusion was resumed at the step preceding that where it was suspended.
Severity Classification of HSRs
The severity of immediate HSRs was classified according to Brown's classification.Citation12 Some symptoms of infusion-related reactions including fever and chills, which were not included in Brown’s classification, were classified based on the Clinical Trials Classification of Adverse Events.Citation13
Positive Criteria of Immediate HSR to Rituximab and Different Phenotypes
Immediate HSR to rituximab was considered positive if one or more typical hypersensitivity symptoms occurred during the standard infusion or desensitization procedure of rituximab. Typical symptoms include cutaneous symptoms (skin itch, flush, and rash), respiratory symptoms (cough, dyspnea, wheezing, and asphyxia), cardiovascular symptoms (palpitation, dizziness, and hypertension or hypotension), gastrointestinal symptoms (vomiting, diarrhea, and abdominal pain), and fever/chills and rigor. If the patient adopted desensitization procedure due to positive skin testing results and experienced no HSR attack during the infusion, he would be excluded from the evaluation of predictive value of IDT for immediate HSR because we did not know whether he would have HSR if the standard infusion was administered.
The phenotype of cytokine release reaction was defined as fever/chill, nausea, pain, headache, and rigors. The phenotype of type I hypersensitivity was defined as flushing, pruritus, urticaria, shortness of breath, wheezing, and hypotension.Citation14
Statistics
Statistical analyses were performed using SPSS software (ver. 23; SPSS Inc., Chicago, IL, USA). Qualitative data are described as percentages. Quantitative data are presented as mean and standard deviation if they conformed to a normal distribution, or shown to be in median and interquartile range if they did not follow a normal distribution. The normality of the indices was examined using the Kolmogorov–Smirnov test. The predictive value of skin testing was evaluated using a receiver operating characteristic (ROC) curve. Differences were considered significant when the P-value was less than 0.05.
Results
Patient Characteristics
Nineteen patients were recruited in the study without any dropout, including 8 women and 11 men with a mean age of 60 ± 15 years (range, 16–77 years). Clinical features, skin testing results, and HSR manifestations are shown in . Most patients were treated for B-cell non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (13/19, 68.4%), follicular lymphoma (2/19, 10.5%), high-grade B-cell lymphoma (2/19, 10.5%), and Burkitt lymphoma (1/19, 5.3%). The other case was diagnosed as Waldenstrom macroglobulinemia. Eighteen lymphoma patients were graded with Ann Arbor stages, including eight stage I, two stage II, and eight stage IV.
Table 2 Clinical Features, Skin Testing Results, and HSR Manifestations of the Patients
The rate of self-reported atopy, defined as a history of allergic rhinitis, allergic conjunctivitis, allergic asthma, food allergy, or other drug HSR, was 21.1% (4/19), with 3 patients (15.8%) reporting past history of HSRs to other drugs (1 to penicillin, 2 to cephalosporin). 21.1% (4/19) of patients had a positive family history of allergic disorders.
Immediate HSRs During the First Infusion of Rituximab
As shown in , in the 19 patients, six patients underwent desensitization infusion due to positive skin testing, and four of them experienced breakthrough reactions, with two mild reactions (skin itch, flush, or rash), one moderate (rigors, hypertension, and palpitation), and one severe reaction (hypotension), according to Brown’s classification. Three breakthrough reactions occurred during the infusion of the final solution of 1mg/mL. Only the moderate reaction with rigor and hypertension was onset during the infusion of the first solution of 0.01mg/mL. The other 13 patients received routine infusion based on negative skin testing, and two of them experienced immediate HSRs, including one mild (red macula on the right arm) and one moderate reaction (fever 37.3°C, rigors, and hypertension).
Figure 1 The management algorithm of the patients in this study. The phenotype of type I hypersensitivity reaction was defined as flushing, pruritus, urticaria, shortness of breath, wheezing, and hypotension, and the phenotype of cytokine release reaction was defined as fever/chills, nausea, pain, headache, and rigors.
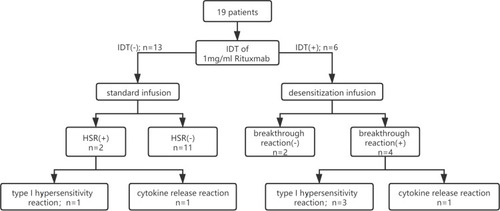
In total, in the 19 patients who underwent skin testing screening and desensitization, six patients (31.6%) had immediate HSRs during the first infusion, with three mild reactions (15.8%), two moderate reactions (10.5%), and one severe reaction (5.3%). The infusion of rituximab continued once the HSR symptoms were controlled, and all the patients completed the first infusion successfully.
Predictive Value and Safety of the Skin Testing
SPT: The SPT results for all patients were negative.
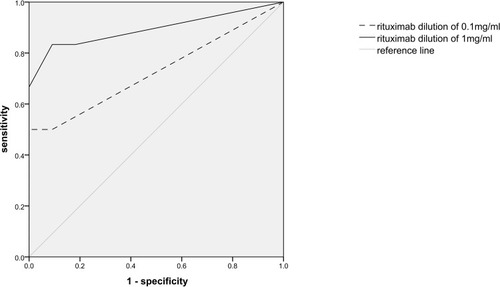
IDT: The rituximab dilutions of 0.1 mg/mL and 1 mg/mL were both used in IDT, and the mean diameter of the wheal reaction was used to draw ROC curve to evaluate the predictive value of IDT for immediate HSRs to rituximab during the first infusion. As shown in , the area under the curve (AUC) corresponding to 0.1 mg/mL solution (0.727, P = 0.132) was smaller than that of the 1 mg/mL solution (0.894, P = 0.009). According to the data in , the sensitivity of 1 mg/mL solution IDT for immediate HSRs with the cutoff value of 5 mm was 66.7% (4/6), and the specificity was 100% (11/11). The positive predictive value of 1 mg/mL solution IDT was 100% (4/4), and the negative predictive value was 84.6% (11/13), with the whole diagnose accordance rate of 88.2% (15/17). Two patients who had no HSR attack during the desensitization infusion were excluded from the calculation because we did not know whether they would have HSR if the standard infusion was administered, thus here the total number of subjects was 17.
Table 3 The Predictive Value of IDT Results Before the First Infusion of Rituximab for Immediate HSR During the Infusion
Figure 2 Receiver operating characteristic (ROC) curve analysis of the intradermal tests with two dilutions of rituximab (0.1mg/mL and 1mg/mL). The ROC curve was produced using the SPSS statistics software, with the IDT wheal diameter of each patient as the test variable and his reaction to rituximab (positive or negative immediate HSR) as the state variable.
As mentioned above, the HSR symptoms of two patients were mainly rigors and hypertension, indicating the phenotype of cytokine release reaction other than IgE-mediated type I hypersensitivity. If these two cases were excluded, the positive predictive value of 1 mg/mL solution IDT for the phenotype of type I hypersensitivity was still 100% (3/3), and the negative predictive value could be as high as 91.7% (11/12), as shown in .
In order to evaluate the risk of irritant false-positive results in skin testing with rituximab dilution, both SPT and IDT with 1 mg/mL rituximab dilution were performed in 12 healthy controls without any known history of allergy, and all the results were negative, suggesting rituximab dilutions of 1 mg/mL used in this study was non-irritant. Positive control testing with histamine and negative control testing with normal saline were performed in the meantime, and all the positive control testing showed positive results, indicating a low risk of false-negative results in the skin testing with rituximab dilution.
Safety: Both the SPT and IDT displayed a high level of safety in this study and caused no large local reaction or systemic adverse reactions in our patients.
Discussion
The hypothesis of this study design was that skin testing screening before the first infusion of rituximab might identify the high-risk subgroup for type I hypersensitivity, and subsequent desensitization in this subgroup might reduce the whole incidence and severity of immediate HSRs during the first infusion. In our study, this scheme was implemented in 19 patients, and one-third of them experienced immediate HSRs in the first exposure, with half of reactions mild. Unfortunately, this study was single-armed and had no control group, which used the standard infusion way to compare with. Thus, we can only use data from the literature as references. According to the literature report, incidence rates of immediate HSRs as high as 70–78% during the first infusion have been reported,Citation4 and moderate reactions usually make up 50–60% of the HSRs, with severe reactions accounting for 30–37%,Citation14 indicating that the protocol used in the current study might have some potential to control the incidence and severity of immediate HSRs to rituximab during the first exposure. Except for the lack of control group, the small sample size also limited the reliability of the results in this study, and further investigations through prospective randomized controlled studies with larger sample size are necessary.
The immediate HSRs to rituximab have been classified as infusion related, cytokine release, and type I reactions in the literature according to the clinical manifestations of patients.Citation14 Infusion-related reactions and cytokine release reactions usually present with symptoms of flushing, chill, fever, tachycardia, hypertension, dyspnea, nausea, vomiting, and syncope, while the typical symptoms of type I reactions include flushing, pruritus, urticaria, dyspnea, and life-threatening shock.Citation15 Our results show that the boundary between different phenotypes is not very clear due to overlapping symptoms, and HSRs with mixed phenotypes often occur in clinical practice. In addition, the absence of biomarker measurement (increased IL-6 and TNF-α indicating infusion-related reactions or cytokine release reactions, increased tryptase indicating type I hypersensitivity) at the time of HSRs onset makes the definite classification more difficult to complete. Isabwe et al reported that in the whole initial HSRs to therapeutic mAbs, type I reactions accounted for 63% and cytokine release reactions accounted for 13%.Citation15 In this study, we also classified the possible phenotypes of immediate HSRs according to the clinical symptoms, and found the percentage of type I reactions 66.7% (4/6) and the percentage of cytokine release reactions 33.3% (2/6), which were similar with the literature reports. In future studies, we planned to investigate more accurate percentages of different phenotypes in a larger patient group through combining consideration of symptoms and representative biomarker levels.
Consistent with the results of previous studies,Citation13 this study observed positive skin testing results mainly with IDT, and all the SPT results were negative. In previous researches evaluating HSRs to mAbs, positive results of IDT were observed in 52–67% of patients with past history of HSRs to rituximab,Citation13,Citation15 which was in agreement with our finding that the sensitivity of 1 mg/mL solution IDT for immediate HSRs to rituximab was 66.7%. The screening IDT results with 1mg/mL solution of rituximab turned out to be highly consistent with the occurrence of immediate HSRs during the first infusion, especially those reactions with the phenotype of type I hypersensitivity, with the positive predictive value of 100% and the negative predictive of 91.7% in the current study. This result suggested that IDT result could become a useful predictor for type I hypersensitivity to rituximab during the first exposure. High-risk patients with positive IDT, especially those with advanced malignancy, old age, or severe complications who could hardly bear the impact of HSRs, could choose desensitization during the first infusion. The likelihood of false-positive IDT was low, because the dilutions for skin testing were chosen based on published nonirritating concentrationsCitation4,Citation11,Citation13,Citation15 and 12 healthy controls not known for any drug allergy labels were non-reactive to these dilutions in our study.
On the other hand, the positive IDT results before the first exposure of rituximab observed in this study also indicated the possibility of pre-existence of anti-rituximab IgE in these patients. However, this hypothesis requires lots of further investigations. First, the serum assay for specific IgE against rituximab should be performed in patients before the first exposure to rituximab. If positive IgE to rituximab could be found, whether the IgE antibodies are specific to the Fab portion or Fc portion should be investigated. After that, the relevant epitopes on the specific portion could be tested, respectively, using sera of patients with phenotype of type I hypersensitivity to rituximab. If relevant epitope could be identified, then possible allergens in daily life that might have cross-reactivity with this epitope and induce pre-existing IgE to rituximab could be analyzed and further confirmed through cross-inhibition tests.
The major limitation of this study was the lack of control group, which could provide data to be compared with the incidence of HSRs to rituximab in intervention group adopting the protocol of skin testing screening and desensitization. Another limitation was the small sample size, and further prospective randomized controlled trials with larger sample size are needed. Also, the phenotypes of immediate HSRs were judged totally based on the symptoms of patients, while the serum levels of IL-6, TNF-α, and tryptase, which were not measured in this study could provide stronger evidences for the classification of phenotypes, with increased IL-6 and TNF-α indicating infusion-related reactions or cytokine release reactions and increased tryptase indicating type I hypersensitivity. Polysorbate 80 contained in the rituximab formulation has been reported to be able to cause immediate HSRs due to complement activating ability.Citation7 Polysorbate 80 has high degree of cross-reactivity with polyethylene glycol (PEG), and a review analyzing 37 cases of immediate HSRs to PEG suggested that most reactions might be IgE-mediated, supported by positive results from skin testing and basophil activating tests.Citation16 Recently, Stone et al first detected specific IgE to PEG in two cases with immediate HSRs caused by PEG.Citation17 Thus, the risk of hypersensitivity to polysorbate 80 in rituximab is supposed to be evaluated in our study, but unfortunately, we could not perform the skin testing of polysorbate 80 due to lack of this organic reagent, which is another limitation. Also, findings in this study could not be directly extrapolated to other patient groups with disorders other than lymphomas requiring rituximab treatment and other anti-CD20 therapies causing fewer HSRs, both of which were not involved in this study.
Conclusions
The current study found that in 19 patients who underwent skin testing screening and following desensitization of rituximab, 31.6% (6/19) had immediate HSRs during the first infusion, with four of the six patients (66.7%) showed positive IDT results before the first exposure. The study also indicated that IDT with 1 mg/mL rituximab dilution had a good sensitivity of 66.7% (4/6) and a high specificity of 100% (11/11) for the prediction of immediate HSRs, with the occurrence of typical HSR symptoms during the standard infusion or desensitization procedure with rituximab as the gold standard for comparison. However, due to a small sample size and lack of control group in this study, multicenter prospective controlled studies are required to confirm the applicable value of this novel protocol in clinical practice.
Abbreviations
AUC, Area under the curve; HSRs, Hypersensitivity reactions; IDT, Intradermal test; IgE, Immunoglobulin E; IPI, International prognostic index; mAbs, Monoclonal antibodies; NMBA, Neuromuscular-blocking agents; PEG, Polyethylene glycol; ROC, Receiver operating characteristic; SPT, Skin prick tests.
Data Sharing Statement
All the data of this study have been included in this manuscript.
Disclosure
The authors declare that there is no conflict of interest.
References
- Demoly P, Castells M. Important questions in drug allergy and hypersensitivity: consensus papers from the 2018 AAAAI/WAO international drug allergy symposium. World Allergy Organ J. 2018;11(1):42. doi:10.1186/s40413-018-0224-130598723
- Berghen N, Vulsteke JB, Westhovens R, Lenaerts J, De Langhe E. Rituximab in systemic autoimmune rheumatic diseases: indications and practical use. Acta Clin Belg. 2019;74(4):272–279. doi:10.1080/17843286.2018.152190430253707
- Mohammed R, Milne A, Kayani K, Ojha U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J Blood Med. 2019;10:71–84. doi:10.2147/JBM.S19078430881167
- Wong JT, Long A. Rituximab hypersensitivity: evaluation, desensitization, and potential mechanisms. J Allergy Clin Immunol Pract. 2017;5(6):1564–1571. doi:10.1016/j.jaip.2017.08.00429122155
- Demoly P, Adkinson NF, Brockow K, et al. International consensus on drug allergy. Allergy. 2014;69(4):420–437. doi:10.1111/all.1235024697291
- Tsao LR, Young FD, Otani IM, Castells MC. Hypersensitivity reactions to platinum agents and taxanes. Clin Rev Allergy Immunol. 2021. doi:10.1007/s12016-021-08877-y
- Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177–191. doi:10.1007/s12016-014-8416-024740483
- Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi:10.1056/NEJMoa07494318337601
- Vultaggio A, Matucci A, Nencini F, et al. Mechanisms of drug desensitization: not only mast cells. Front Pharmacol. 2020;11:590991. doi:10.3389/fphar.2020.59099133424601
- Kepil Ozdemir S, Gorgulu B, Doganay Erdogan B, et al. Effect of drug desensitization on drug hypersensitivity-related quality of life. J Allergy Clin Immunol Pract. 2020;9(4):1738–1741.e1. doi:10.1016/j.jaip.2020.10.05533186768
- Broyles AD, Banerji A, Barmettler S, et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8(9S):S16–S116. doi:10.1016/j.jaip.2020.08.00633039007
- Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. doi:10.1016/j.jaci.2004.04.02915316518
- Brennan PJ, Rodriguez Bouza T, Hsu FI, Sloane DE, Castells MC. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. 2009;124(6):1259–1266. doi:10.1016/j.jaci.2009.09.00919910036
- Fouda GE, Bavbek S. Rituximab hypersensitivity: from clinical presentation to management. Front Pharmacol. 2020;11:572863. doi:10.3389/fphar.2020.57286333013416
- Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol. 2018;142(1):159–170 e152. doi:10.1016/j.jaci.2018.02.01829518427
- Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi:10.1111/cea.1276027196817
- Stone CA Jr., Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540 e1538. doi:10.1016/j.jaip.2018.12.00330557713
