Abstract
The standard of care for advanced non-small cell lung cancer (NSCLC) without known driver oncogenes is immune checkpoint inhibitor (ICI) therapy combined with platinum-based chemotherapy. About 20% of patients with advanced NSCLC have brain metastases, which are related to poor prognosis. However, the effect of ICI therapy combined with platinum-based chemotherapy on untreated brain metastases derived from NSCLC remains unclear. The primary endpoint of this study is intracranial response rate as determined by modified Response Evaluation Criteria in Solid Tumors (RECIST) for brain metastases of ≥5 mm as target lesions. Eligible patients are 20 years of age or older with chemotherapy-naïve advanced NSCLC and at least one brain metastasis ≥5 mm in size that has not been previously treated. Patients receive nivolumab plus ipilimumab intravenously combined with histology-based platinum doublet chemotherapy (two cycles). Individuals with known genetic driver alterations such as those affecting EGFR or ALK are excluded. Planned enrollment is 30 patients over 2.5 years at 27 oncology facilities in Japan. This is the first prospective study to focus on the intracranial response to ICI therapy combined with platinum-based chemotherapy in patients with untreated brain metastases derived from NSCLC. If the study demonstrates intracranial efficacy for this patient population, then this regimen has the potential to become a new treatment option for such individuals.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality in many countries.Citation1 At diagnosis, ~20% of individuals with metastatic NSCLC have brain metastases, which are related to poor prognosis and a declining quality of life.Citation2–Citation4 Radiotherapy is the main treatment for brain metastases but can cause complications such as cognitive decline and symptomatic radiation necrosis.Citation5,Citation6
Substantial progress has been made in the treatment of NSCLC with immune checkpoint inhibitors (ICIs),Citation7,Citation8 with the current standard of care for advanced NSCLC with no known driver oncogenes being ICI therapy combined with platinum-based chemotherapy.Citation9,Citation10 However, no prospective data have been available with regard to the intracranial efficacy of such combination therapy for NSCLC patients with untreated brain metastases. Monotherapy with pembrolizumab, an antibody to programmed cell death–1 (PD-1), has shown intracranial efficacy with an intracranial response rate of 29.7% (95% confidence interval, 15.9–47.0%) in NSCLC patients with a PD-1 ligand 1 (PD-L1) expression level of ≥1%, but no intracranial responses were seen in those with a PD-L1 expression level of <1%.Citation11,Citation12 Dual immunotherapy with nivolumab plus ipilimumab was found to have an intracranial efficacy superior to that of nivolumab monotherapy, with an intracranial response rate of 51% versus 21%, for melanoma patients with untreated brain metastases,Citation13 suggesting that the combination of ipilimumab, an antibody to cytotoxic T lymphocyte antigen–4 (CTLA-4), with an antibody specific for PD-1 is more effective than the latter alone for brain metastases.
In the CheckMate 227 trial, nivolumab plus ipilimumab conferred a longer overall survival (OS) than did platinum-based chemotherapy in advanced NSCLC patients regardless of PD-L1 expression level.Citation14 In the CheckMate 9LA trial, the combination of two cycles of platinum-based chemotherapy with nivolumab-ipilimumab resulted in a significant improvement in OS compared with platinum-based chemotherapy alone in patients with advanced NSCLC.Citation15 Although patients with untreated brain metastases were excluded from these trials, those with adequately treated brain metastases derived a survival benefit from treatment with nivolumab plus ipilimumab, suggesting that the ICI combination might be effective against brain metastases. An intracranial response to systemic treatment can improve the quality of life and avoid the necessity for radiotherapy in patients with brain metastases. It is therefore important to assess the intracranial efficacy of such treatment in NSCLC patients with untreated brain metastases. Given that the addition of two cycles of platinum-based chemotherapy to nivolumab plus ipilimumab provided earlier disease control relative to nivolumab-ipilimumab alone by reducing the rate of primary disease progression,Citation14,Citation15 this combination therapy appears to be a reasonable approach to the treatment of previously untreated brain metastases derived from NSCLC.
With this background, we designed a single-arm study to evaluate the efficacy of nivolumab-ipilimumab combined with platinum-based chemotherapy for untreated brain metastases in patients with NSCLC.
Materials and Methods
Study Design and Objective
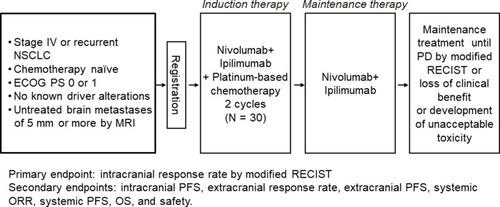
This study (NIke, LOGiK2004) was designed as a multicenter, single-arm phase II trial to evaluate the efficacy and safety of nivolumab plus ipilimumab together with platinum combination chemotherapy with the primary endpoint of response rate for brain metastases in chemotherapy-naïve patients with advanced NSCLC ().
Endpoints
The primary endpoint of the trial is the proportion of patients achieving an intracranial response (partial response or complete response) as defined by the modified Response Evaluation Criteria in Solid Tumors (modified RECIST).Citation12
The secondary endpoints are intracranial progression free survival (PFS), extracranial response rate (RR), extracranial PFS, systemic RR, systemic PFS, OS, and safety.
Treatment
Patients receive nivolumab (360 mg intravenously every 3 weeks) followed by NSCLC-optimized ipilimumab (1 mg/kg intravenously every 6 weeks) combined with histology-based platinum doublet chemotherapy (intravenously every 3 weeks for two cycles). The intravenous chemotherapy regimens consist of carboplatin (area under the concentration-time curve [AUC] of 6 mg mL–1 min) plus paclitaxel (200 mg/m2) for patients with squamous histology, and carboplatin (AUC of 5 mg mL–1 min) plus pemetrexed (500 mg/m2) for those with nonsquamous histology. After the two cycles of induction therapy, treatment with nivolumab plus ipilimumab continues until disease progression, loss of clinical benefit, development of unacceptable toxicity, or completion per protocol (2 years).
Key Eligibility Criteria
Patients 20 years of age or older with histologically or cytologically confirmed NSCLC and at least one brain metastasis measuring ≥5 mm that has not been treated previously are eligible for the study. Symptomatic brain metastases are also eligible, but brain metastases that require urgent radiation or emergency surgery are excluded. Each patient is required to have stage IV or postoperative recurrent disease (clinical stage is determined in accordance with the tumor, node, metastasis classification system, 8th edition) that is not curable by radiation and has not been previously treated with cytotoxic chemotherapy. Eligibility stipulates an Eastern Cooperative Oncology Group performance status of 0 or 1 as well as adequate lung, bone marrow, liver, and kidney function. Patients with sensitizing driver genetic alterations of EGFR, ALK, ROS1, BRAF, MET, RET, or NTRK are ineligible. The expression level for PD-L1 on tumor cells is not a determinant of eligibility.
Individuals who meet any of the following criteria are not eligible to participate in the study: drainage is required for symptom management; diagnosis of autoimmune disease; major surgery within 7 days or local palliative radiation therapy within 7 days before registration; a history of cancer within the previous 2 years; serious psychosis or psychotic symptoms; serious uncontrolled medical conditions including diabetes; concurrent unstable angina or a history of myocardial infarction within the last 1 year; and positive status for active hepatitis B or hepatitis C. Patients with conditions requiring systemic corticosteroids (>10 mg daily prednisone or equivalent) or immunosuppressive medication are also not eligible.
Study Assessments
The primary endpoint of the trial is the proportion of patients achieving an intracranial response (partial response or complete response) as defined by the modified RECIST,Citation12 consisting of RECIST (version 1.1) modified to allow up to five target brain metastases of ≥5 mm in maximum diameter (and more than twice the slice thickness of a magnetic resonance imaging (MRI) scan). Key secondary endpoints are the proportion of patients achieving an overall response, defined as those who show a partial response or complete response for extracranial and systemic disease as determined by modified RECIST; progression-free survival, defined as the time from start of treatment to disease progression (based on modified RECIST for the brain and systemic disease) or death, whichever occurs first; OS, defined as the time from start of treatment to death; and safety and toxicity as measured by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).
A magnetic resonance imaging (MRI) scan of the brain, computed tomography (CT) scans of the chest and abdomen, a bone scan or positron emission tomography scan, and an electrocardiogram are required before initiation of study treatment. Patients undergo tumor assessment with an MRI scan of the brain and CT scans at baseline, every 6 weeks during the first 24 weeks, every 9 weeks during the next 27 weeks, and every 12 weeks thereafter.
Statistical Analysis
The expected intracranial response rate was assumed to be 38% on the basis that the study treatment would result in a response in the brain similar to the systemic response observed in the CheckMate 9LA trial.Citation15 On the basis of the lower limit of the 95% confidence interval for the intracranial response rate in a previous trial of pembrolizumab,Citation12 we set a threshold value for the intracranial response rate of 16%. Given this assumption, the study was designed to have a power of 80% and a one-sided level of alpha error of 0.025, resulting in a requirement for 27 patients. Allowing for ineligibility of patients for analysis, we plan on enrolling 30 patients.
Ethical Considerations
This study is being conducted in accordance with the principles of the Declaration of Helsinki and has been approved by the central review board of Clinical Research Network Fukuoka. The study is registered in the Japan Registry of Clinical Trials (jRCTs071210019). All participants in this trial will provide informed consent.
Discussion
As far as we are aware, this is the first prospective study to focus on the intracranial response to ICI therapy combined with platinum-based chemotherapy in patients with untreated brain metastases derived from NSCLC. The addition of ipilimumab, an antibody to CTLA-4, to a PD-1–targeting antibody is expected to increase treatment efficacy for brain metastases in this study. If the study demonstrates intracranial activity for nivolumab-ipilimumab combined with platinum-based chemotherapy in this patient population, then this regimen has the potential to become a new treatment option for such individuals. Study enrollment began in May 2021 and is to continue for 2.5 years.
Conclusion
The phase II study will be able to evaluate the intracranial efficacy of ICI therapy combined with platinum-based chemotherapy in patients with untreated brain metastases derived from NSCLC.
Disclosure
Dr.Tsuchiya-Kawano has received personal fees from Taiho Pharmaceutical, Chugai Pharma, Ono Pharmaceutical and Kyowa Kirin, all outside the submitted work. Dr.Shiraishi has received honoraria and research grants from Chugai Pharma as well as honoraria from Eli Lilly and Co., Ono Pharmaceutical, AstraZeneca, and Taiho Pharmaceutical, all outside the submitted work. Dr.Okamoto has received research grants and personal fees from AstraZeneca, Taiho Pharmaceutical, Chugai Pharma, Boehringer-Ingelheim, Ono Pharmaceutical, MSD Oncology, Eli Lilly and Co., and Bristol-Myers Squibb; research grants from Astellas Pharma, Novartis, and AbbVie; and personal fees from Pfizer, all outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
We thank the patients, their families, and all of the investigators who participate in the study (Supplementary table). This study is a collaboration of the Lung Oncology Group in Kyushu (LOGiK). Data management and monitoring for the study are conducted by Clinical Research Support Center Kyushu under a funding contract with Ono Pharmaceutical Co. Ltd. and Bristol-Myers Squibb K.K.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.2126225651787
- Waqar SN, Samson PP, Robinson CG, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. 2018;19:373–379. doi:10.1016/j.cllc.2018.01.007
- Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. 2016;45:139–162. doi:10.1016/j.ctrv.2016.03.00927019457
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi:10.1200/JCO.2011.38.052722203767
- Alomari A, Rauch PJ, Orsaria M, et al. Radiologic and histologic consequences of radiosurgery for brain tumors. J Neurooncol. 2014;117:33–42. doi:10.1007/s11060-014-1359-824442402
- Khan AJ, Dicker AP. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;1:11–13.
- Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, Phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi:10.1200/JCO.20.0160533449799
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi:10.1056/NEJMoa160677427718847
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa180100529658856
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi:10.1056/NEJMoa181086530280635
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, Phase 2 trial. Lancet Oncol. 2016;17:976–983. doi:10.1016/S1470-2045(16)30053-527267608
- Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:655–663. doi:10.1016/S1470-2045(20)30111-X32251621
- Long GV, Atkinson V, Lo S, et al. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): randomized phase 2 study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets). J Clin Oncol. 2021;39(15_suppl):9508. doi:10.1200/JCO.2021.39.15_suppl.9508
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi:10.1056/NEJMoa191023131562796
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, Phase 3 trial. Lancet Oncol. 2021;22:198–211. doi:10.1016/S1470-2045(20)30641-033476593